Vapor-Liquid Equilibrium Prediction of Ammonia-Ionic Liquid Working Pairs of Absorption Cycle Using UNIFAC Model*
2014-07-18SUNGuangming孙光明HUANGWeijia黄维佳ZHENGDanxing郑丹星DONGLi董丽andWUXianghong武向红
SUN Guangming (孙光明), HUANG Weijia (黄维佳), ZHENG Danxing (郑丹星)**, DONG Li (董丽) and WU Xianghong (武向红)
College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
Vapor-Liquid Equilibrium Prediction of Ammonia-Ionic Liquid Working Pairs of Absorption Cycle Using UNIFAC Model*
SUN Guangming (孙光明), HUANG Weijia (黄维佳), ZHENG Danxing (郑丹星)**, DONG Li (董丽) and WU Xianghong (武向红)
College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
On the basis of reported experimental vapor-liquid equilibrium (VLE) data of NH3-1-ethyl-3-methylimidazolium acetate (NH3-[Emim]Ac), NH3-1-butyl-3-methylimidazolium tetrafluoroborate (NH3-[Bmim][BF4]), NH3-1,3-dimethylimidazolium dimethyl phosphate (NH3-[Mmim]DMP) and NH3-1-ethyl-3-methylimidazolium ethylsulfate (NH3-[Emim]EtOSO3) binary systems, the interaction parameters of 14 new groups have been regressed by means of the UNIFAC model. To validate the reliability of the method, these parameters have been used to calculate the VLE data with the average relative deviation of pressures of less than 9.35%. The infinite dilution activity coefficient (1γ∞) and the absorption potential (1Ψ) are important evaluation criterions of the affinity between working pair species of the absorption cycle. The UNIFAC model is implemented to predict the values of1γ∞and1Ψ of 16 sets of NH3-ionic liquid (IL) systems. The work found that the1Ψ gradually increases following the impact order: Ψ1([Cnmim][BF4])<Ψ1([Cnmim]EtOSO3)<Ψ1([Cnmim]DMP)<Ψ1([Cnmim]Ac) (n=1, 2, 3, …) at a given cation of IL species and constant temperature, and Ψ1([Mmim]X)<Ψ1([Emim]X)<Ψ1([Pmim]X)<Ψ1([Bmim]X) (X=Ac, [BF4], DMP or EtOSO3) at a given anion of IL species and constant temperature. Furthermore, the Ψ1gradually increases with increasing temperature. Then, it could be concluded that the working pair NH3-[Bmim]Ac has the best potential research value relatively.
absorption cycle working pairs, vapor-liquid equilibrium, UNIFAC model, ammonia, ionic liquid
1 INTRODUCTION
With the continuous increasing of energy consumption, low-grade heat, such as waste heat, solar energy, biomass and etc., has been attracting more and more attentions. One efficient method of using low-grade heat is the absorption heat pump technology [1]. Presently, NH3-H2O is one of the most common working pairs for low-temperature refrigeration system and air-conditioning system respectively [2]. However, one problem researchers faced with is the strong affinity between NH3and H2O, which costs great heat consumption to separate NH3and H2O [3]. One solution is to develop novel absorbent species instead of H2O.
In the last decade, the room temperature ionic liquids (ILs) have aroused considerable interest because of their perfect feature, e.g. ignorable vapor pressure and environment-friendly characteristic [4].
Many researchers have investigated NH3-IL systems including 1-ethyl-3-methylimidazolium acetate ([Emim]Ac) [5], 1-ethyl-3-methylimidazolium ethylsulfate ([Emim]EtOSO3) [5], 1-ethyl-3-methylimidazolium thiocyanate ([Emim][SCN]) [5], N,N-dimethylethanolammonium acetate ([DMEA]Ac) [5], 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]) [6], 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4]) [6], 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Emim][Tf2N]) [6], 1-hexyl-3-methylimidazolium chloride ([Hmim]Cl) [6], ([Cnmim][BF4]) (n=2, 4, 6, 8) [7] and 1,3-dimethylimidazolium dimethyl phosphate ([Mmim]DMP) [8]. Hu et al. reported their work [9] on molecular characteristics dominating the solubility of gases in ionic liquids, in which the interaction between gases and ionic liquids was summarized and explicated at a deeper level. By assessing literatures, it can be found that there are just few sets of experimental data about vapor-liquid equilibrium (VLE) of NH3-IL binary systems. Furthermore, the reported working pairs are not always to cover the research requirements. Therefore, it will do researchers a big favor in selecting IL, if there is an approach to predict the affinity between NH3and IL with a sufficient accuracy.
Fortunately, the group contribution methods [10, 11] based on the essential information of working pairs species have been developed to get more information about the systems, which have not been determined experimentally before, by using the known VLE data of other related systems. Among these methods, the most frequently used one is the UNIFAC model. Dong et al. [12] had predicted the activity coefficients of hydrofluorocarbon-IL systems. Wang et al. [13] had calculated benzene-IL, water-IL, acetone-IL, ethylene- IL and methanol-IL systems, and the infinite dilution activity coefficient (γ∞) of mixtures had been obtained.Kato et al. [14] had forecasted the thermodynamic behavior of some mixtures containing IL species.
In order to select potential working pairs, as an affinity criterion between the absorbent and absorbate, the absorption potential(Ψ1) of a binary system had been proposed by Wu et al. [15] on the basis of selectivity factor defined as the reciprocal of γ∞presented by Kolbe et al [16]. The bigger the Ψ1of the absorbent species is, the better the absorption effect will be [15]. The UNIFAC model is a rigorous method to calculate the activity coefficient upon the group-contribution concept, in which the mixtures are treated as consisting of functional groups instead of molecules. Hence, the desired value of the activity coefficient of a binary system can be predicted reasonably based on the strength of the known interactions of the limited number of functional groups, even if the related experimental data are limited.
This paper aims at striving to predict the VLE data of the NH3-IL systems through the UNIFAC method, and assessing alternative working pairs of absorption heat pump cycle using the evaluation criteria of1γ∞and1Ψ. Four binary systems, NH3-[Mmim]Ac, NH3-[Mmim][BF4], NH3-[Mmim]DMP and NH3-[Mmim]EtOSO3, were determined as research objects and investigated, respectively.
2 VAPOR-LIQUID EQUILIBRIUM METHOD OF NH3-ILS SYSTEMS
2.1 VLE modeling of NH3-IL binary system
For binary system, the activity coefficient can be written as Eq. (1) [17].

where yiand χiare the mole fractionsof i species invapor and liquid phases, respectively, stands for the fugacity coefficient of i species in vapor phase, p is thesystempressure, γi expresses the activitycoefficient ofispeciesanddenotes the fugacity ofispeciesat the saturated vapor pressure and system temperature.
The absorption potential is defined as [15]

For NH3(1)-IL (2) system, owing to the concentration of the IL species in the vapor phase can be neglected [17, 18], the activity coefficient can be described as [18]
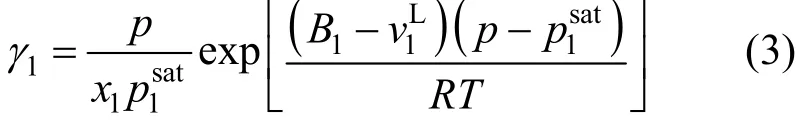
2.2 UNIFAC model
It is Fredenslund [20] that came up with the UNIFAC model in 1975 for the first time. The UNIQUAC model was built on the basis of the group-contribution method. In the theory, the definition of the activity coefficient can be written as follows [21].

The combinatorial term thinks about the different sizes and shapes of the molecules. The residual term embodies the intermolecular attractive force.
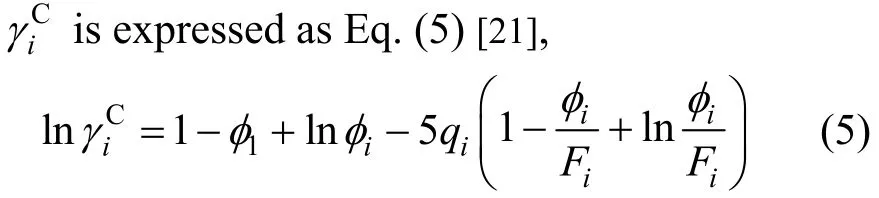
where the parameters φiand Fiare defined asThe parameters riand qiare the volume and surface area of i species, respectively. They are calculated by the van der Waals volumes Rkand surface areas Qkof the individual functional group k using equations ofandwhere()ikν is the number of group k in i species. The parameters rjand qjcan be obtained by the same way as like as riand qi.
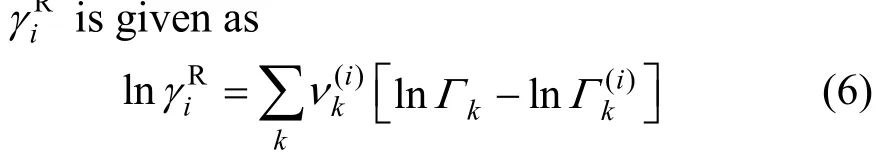
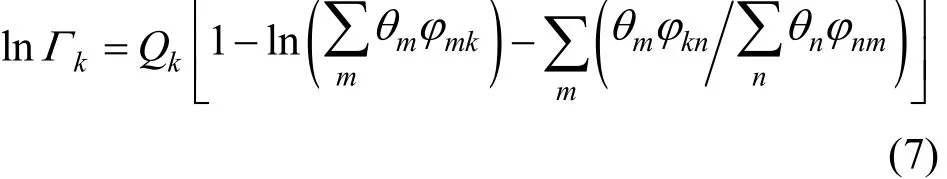

where anm(K−1) is the adjustable group interaction parameter of the difference of short range interaction between group-group. For each group-group interaction, the two parameters exhibit relation of anm≠amn.
2.3 Group parameters of ammonia-ILs
The ILs have to be decomposed to functional group when using the UNIFAC model. In this work, the method of division is the same as Kim et al. [22], which is listed in Table 1.

Table 1 Group division of ILs
The group volume parameters (Rk) and group surface area parameters (Qk) of CH3, CH2, [mim]DMP, [mim][BF4], NH3, [mim]Ac and [mim]EtOSO3used in this work are listed in Table 2, which were cited from references [13, 23-25], respecitively.
The new group interaction parameters anmand amnof CH2-[mim][BF4] and CH2-[mim]DMP can be obtained from reference [13, 23]. The new anmand amnof other groups were determined by means of correlating literature data. The objective function F is
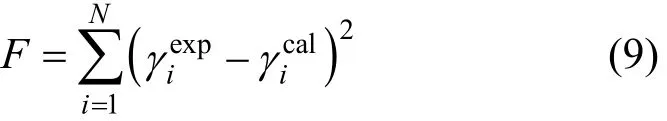

Table 2 Parameters of Rkand Qk
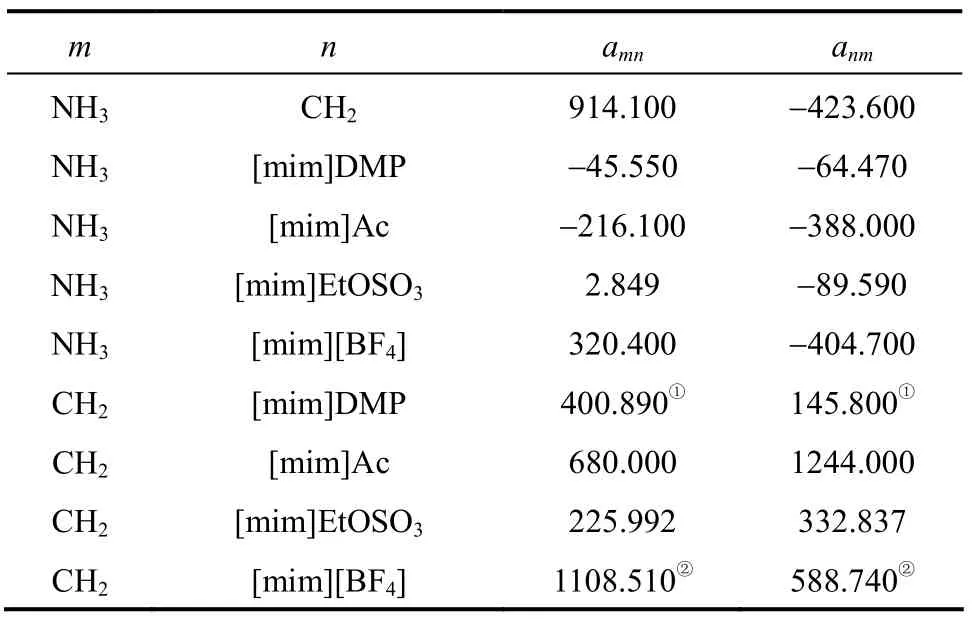
Table 3 Group interaction parameters amn
Thus, the VLE data of NH3-[Emim]Ac [5], NH3-[Bmim][BF4] [6], NH3-[Mmim]DMP [8] and NH3-[Emim]EtOSO3[5] systems were predicted by the UNIFAC model. For the four systems, the comparisons of experimental data and predicted data plotted as several p-χ1curves at different temperatures are illustrated in Figs. 1-4. For example, Fig. 1 shows the comparison for NH3(1) in [Emim]Ac (2). The diagrams exhibit that the results predicted by the UNIFAC interaction parameters have good agreement with experimental data of NH3-IL binary systems generally.
In addition, the average relative deviation (ΔARD) values of pressure between the predicted and experimental data of the four systems are listed in Table 4. It shows that the results are in essential agreement with the cited experimental data. Because the ΔARDvalues of less than 3% indicate data of a high degree of consistency; the ΔARDvalues of less than 10% are probablyacceptable [16]. The distribution of deviation of all points in the four NH3-IL systems is presented in Fig. 5. The maximal value of the ΔARDof pressure is 9.35%, which proves that the approach adopted in this work , i.e. the UNIFAC model, may be an appropriate tool to predict the VLE data for the NH3-[Emim]Ac, NH3-[Bmim][BF4], NH3-[Mmim]DMP and NH3-[Emim]EtOSO3systems.
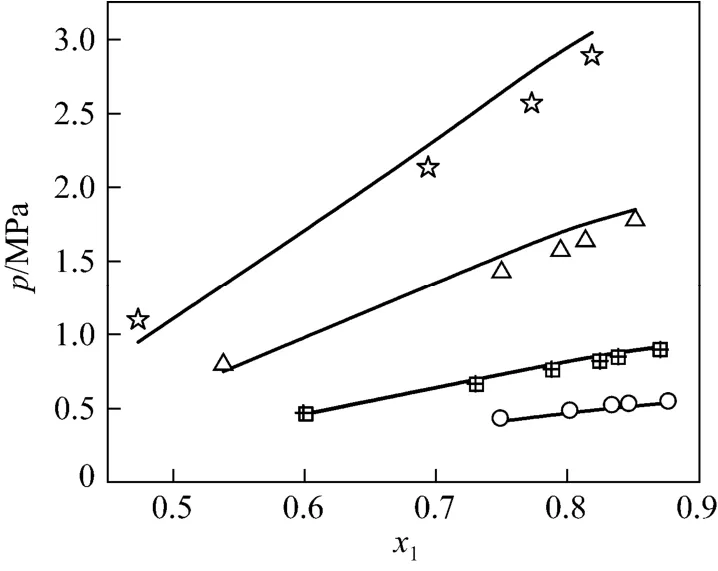
Figure 1 p-x1diagram of comparison of experimental and predicted data for NH3(1)-[Emim]Ac (2) systemsymbols: experimental data; lines: data calculated by UNIFAC model; ☆ 348.5 K; △ 324.5 K; 298.3 K; □ 298.2 K;○ 282.5 K
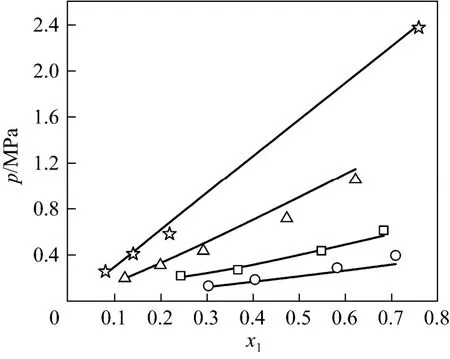
Figure 2 p-x1diagram of comparison of experimental and predicted data for NH3(1)-[Bmim][BF4] (2) systemsymbols: experimental data; lines: data calculated by UNIFAC model; ☆ 347.5 K; △ 323.6 K; □ 298.4 K; ○ 282.2 K

Figure 4 p-x1diagram of comparison of experimental and predicted data for NH3(1)-[Emim]EtOSO3(2) systemsymbols: experimental data; lines: data calculated by UNIFAC model; ☆ 372.3 K;347.5K; △ 322.3 K; 298.1 K;□ 297.6 K; ○ 282.7 K
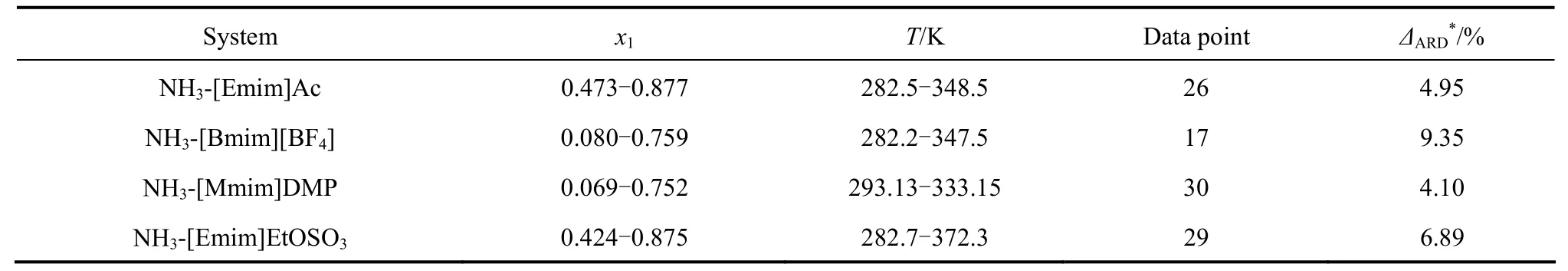
Table 4 Comparison of experimental and predicted values
3 RESULTS AND DISCUSSION

Figure 5 Distribution of deviation of all points in the four NH3-IL systems■ NH3-[Emim]Ac; □ NH3-[Bmim][BF4];▲ NH3-[Mmim]DMP ; △ NH3-[Emim]EtOSO3
Wang et al. pointed that the affinity between H2O and IL species is influenced by the structural variations such as changing the length of the alkyl chain in the cation and replacing the anion of IL species [13]. Similarly, changing the length of the alkyl chain in the cation of IL species can influence the affinity between NH3and IL species. The1γ∞of NH3in IL species can be derived by the UNIFAC model through setting χ1→0 and χ2→1. The parameter Ψ1can be used as an evaluation criterion for absorption cycle working pairs [15]. In this work, the Ψ1values of NH3in 16 IL species have been predicted and listed in Table 5.
Figure 6 has been plotted to express the impact of different anion with the same cation [Mmim] visually in a working pair combination [Mmim]X. It can be found that the Ψ1gradually increases following the impact order: Ψ1([Mmim][BF4])<Ψ1([Mmim]EtOSO3)< Ψ1([Mmim]DMP)<Ψ1([Mmim]Ac) at the same temperature, meanwhile the Ψ1gradually decreases with increasing temperature. According to the scaled particle theory used by Hu and co-workers [9], the partial molar Gibbs free energy of cavity formation and solute-solvent interaction may increase in the order of [BF4] Generally, Table 5 shows that the Ψ1gradually increases with increasing length of the alkyl chain inthe cation of IL species at a given anion of IL species and constant temperature. However, the increase degree of Ψ1is much smaller than that of alternation in anion type. In other words, the solubility of NH3in [Cnmim]X (n=1, 2, 3, 4, X=Ac, [BF4], DMP or EtOSO3) increases slightly with the cation chain length, which is essential in agreement with conclusion of Hu and co-workers [9]. Additionally, the Ψ1gradually decreases with increasing temperature at a given IL species. Table 5 Ψ1of NH3(1)-ILs (2) Figure 6 Ψ1of NH3in [Mmim]X□ [Mmim]Ac; ▲ [Mmim]DMP; ☆ [Mmim]EtOSO3;● [Mmim][BF4] Finally, it can be found that Ψ1([Bmim]Ac) exhibits the biggest value of absorption potential among the 16 NH3-IL binary systems. Therefore, it could be concluded that the working pair of NH3-[Bmim]Ac possesses potential for putting relevant research forward relatively. In this work, 14 new group interaction parameters were obtained by the UNIFAC model on the basis of the available data of NH3-[Emim]Ac, NH3-[Bmim][BF4], NH3-[Mmim]DMP, and NH3-[Emim]EtOSO3systems cited from the literatures, and then were used to predict the VLE data of these four systems. The maximal value of the average relative deviation of pressure between the predicted and experimental data for the four systems was 9.35%, which proved that the UNIFAC model might be used to predict the VLE data of NH3-ionic liquid (IL) systems. The UNIFAC model was implemented to predict the values of infinite dilution activity coefficient and the absorption potential Ψ1of 16 sets of NH3-IL systems. It was found that Ψ gradually increases with increasing the length of the alkyl chain in the cation of IL species at a given anion and constant temperature. The order is Ψ1([Mmim]X)<Ψ1([Emim]X)<Ψ1([Pmim]X)< Ψ1([Bmim]X), which is similar to the viewpoint of Hu and co-workers [9]. Also, Ψ gradually increases following the impact order: Ψ1([Cnmim][BF4])< Ψ1([Cnmim]EtOSO3)<Ψ1([Cnmim]DMP)<Ψ1([Cnmim]Ac) at a given cation of IL species and constant temperature. It might be due to the charge density, size and steric effects of the anion [9]. Meanwhile, Ψ1gradually increases with increasing temperature at a given IL species. In addition, this work proposed that the working pair of NH3-[Bmim]Ac has the best potential research value relatively. Definitely, it is easier to separate NH3from ionic liquids instead of water. However, the viscosity of ionic liquids is much higher than that of water, leading to a certain impact on the absorption kinetics of NH3, and thereby the efficiency of the heat pump will be influenced. Furthermore, ionic liquids are more expensive than water. The two cases above have to be taken into account if engineers want to improve a heat pump performance. REFERENCES 1 Zheng, D.X., Meng, X.L., “Ultimate refrigerating conditions, behavior turning and a thermodynamic analysis for absorption-compression hybrid refrigeration cycle”, Energy Convers. Manage., 56, 166-174 (2012). 2 Balamuru, V.G., Ibrahim, O.M., Barnett, S.M., “Simulation of ternary ammonia-water-salt absorption refrigeration cycles”, Int. J. Refrigeration., 23 (1), 31-42 (2000). 3 Mclinden, M.O., Radermacher, R., “Experimental comparison of ammonia-water and ammonia-water lithium bromide mixtures in an absorption heat pump”, http://fire.nist.gov/bfrlpubs/build85/art005.html. 4 Dong, L., Zheng, D.X., Wei, Z., Wu, X.H., “Synthesis of 1, 3-dimethylimidazolium chloride and volumetric property investigations of its aqueous solution”, Int. J. Thermophys., 30 (5), 1480-1490 (2009). 5 Yokozeki, A., Shiflett, M.B., “Vapor-liquid equilibria of ammonia + ionic liquid mixtures”, Appl. Energy., 84 (12), 1258-1273 (2007). 6 Yokozeki, A., Shiflett, M. B., “Ammonia solubilities in roomtemperature ionic liquids”, Ind. Eng. Chem. Res., 46 (5), 1605-1610 (2007). 7 Li, G.H., Zhou, Q., Zhang, X.P., Wang, L., Zhang, S.J., Li, J.W.,“Solubilities of ammonia in basic imidazolium ionic liquids”, Fluid Phase Equilib., 297 (1), 34-39 (2010). 8 Sun, G.M., Zheng, D.X., Huang, W.J., Dong, L., “The measurement of ammonia solubility in ionic liquid [Dmim]DMP”, Journal of Beijing University of Chemical Techchnology (Natural Science)., 39 (4), 17-21 (2012). (in Chinese) 9 Hu, Y. F., Liu, Z. C., Xu, C. M., Zhang, X. M., “The molecular characteristics dominating the solubility of gases in ionic liquids”, Chem. Soc. Rev., 40, 3802-3823 (2011). 10 Klamt, A., “Conductor-like screening model for real solvents: A new approach to the quantitative calculation of solvation phenomena”, J. Phys. Chem., 99 (7), 2224-2235 (1995). 11 Langmir, I., “The distribution and orientation of molecules”, In: Third Colloid Symposium Monogragh, Chemical Catalog Company, New York, 48 (1925). 12 Dong, L., Zheng, D.X., Wu, X.H., “Working pair selection of compression and absorption hybrid cycles through predicting the activity coefficients of hydrofluorocarbon + ionic liquid systems by the UNIFAC model”, Ind. Eng. Chem. Res., 51 (12), 4741-4747 (2012). 13 Wang, J.F., Sun, W., Li, C.X. Wang, Z.H., “Correlation of infinite dilution activity coefficient of solute in ionic liquid using UNIFAC model”, Fluid Phase Equilib., 264 (1-2), 235-241 (2008). 14 Kato, R., Gmehling, J., “Systems with ionic liquids: Measurement of VLE and γ∞data and prediction of their thermodynamic behavior using original UNIFAC, mod. UNIFAC(Do) and COSMO-RS(OL)”,J. Chem. Thermodyn., 37 (6), 603-619 (2005). 15 Wu, X.H., Zheng, D.X., “A new approach for appropriate absorbent selection with excess Gibbs function”, In: 18th European Conference on Thermophysical Properties, Pau, France, 413 (2008). 16 Kolbe, B., Gmehling, J., Onken, U., “Auswahl von Loesungsmitteln fuer die Extraktiv-Rektifikation mittels vorrausberechneter Gleichgewichtsdaten”, Ber. Bunsenges. Phys. Chem., 83 (11), 1133-1136 (1979). 17 Smith, J.M., Ness, H.C., Abbott, M.M., Chemical Engineering Thermodynamics, Chemical Industry Press, Beijing (2002). 18 Dong, L., Zheng, D.X., Sun, G.M., Wu, X.H., “Vapor-liquid equilibrium measurements of difluoromethane + EmimOTf, difluoromethane + BmimOTf, difluoroethane + EmimOTf, and difluoroethane + BmimOTf systems”, J. Chem. Eng. Data, 56 (9), 3663-3668 (2011). 19 Lemmon, E.W., Huber, M.L., McLinden, M.O., National Institute of Standards and Technology, Gaithersburg, Maryland, 2007. 20 Fredenslund, A., Jones, R.L., Prausnitz, J.M., “Group-contribution estimation of activity coefficients in nonideal liquid mixtures”, AIChE J., 21 (6), 1086-1099 (1975). 21 Wittig, R., Lohmann, J., Gmehling, J., “Vapor-liquid equilibria by UNIFAC group contribution, revision and extension”, Ind. Eng. Chem. Res., 42 (1), 183-188 (2003). 22 Kim, Y.S., Choi, W.Y., Jang, J.H., Yoo, K.P., Lee, C.S., “Solubility measurement and prediction of carbon dioxide in ionic liquids”, Fluid Phase Equilib., 228-229, 439-445 (2005). 23 Lei, Z.G., Zhang, J.G., Li, Q.S., Chen, B.H., “UNIFAC model for ionic liquids”, Ind. Eng. Chem. Res., 48 (5), 2697-2704 (2009). 24 Thomsen, K., Rasmussen, P., “Modeling of vapor-liquid-solid equilibrium in gas-aqueous electrolyte systems”, Chem. Eng. Sci., 54 (12), 1787-1802 (1999). 25 Bondi, A., “Physical properties of molecular crystals, liquids and glasses”, Science, 163 (3868), 666-666 (1969). 26 Fredenslund, A., Gmehling, J., Rasmussen, P., “Vapor-liquid equilibria using UNIFAC a group contribution method”, J. Chem. Thermodyn., 10 (9), 901-902 (1978). 10.1016/S1004-9541(14)60008-2 2012-09-17, accepted 2013-02-07. * Supported by the National Natural Science Foundation of China (50890184, 51276010) and the National Basic Research Program of China (2010CB227304). ** To whom correspondence should be addressed. E-mail: dxzh@mail.buct.edu.cn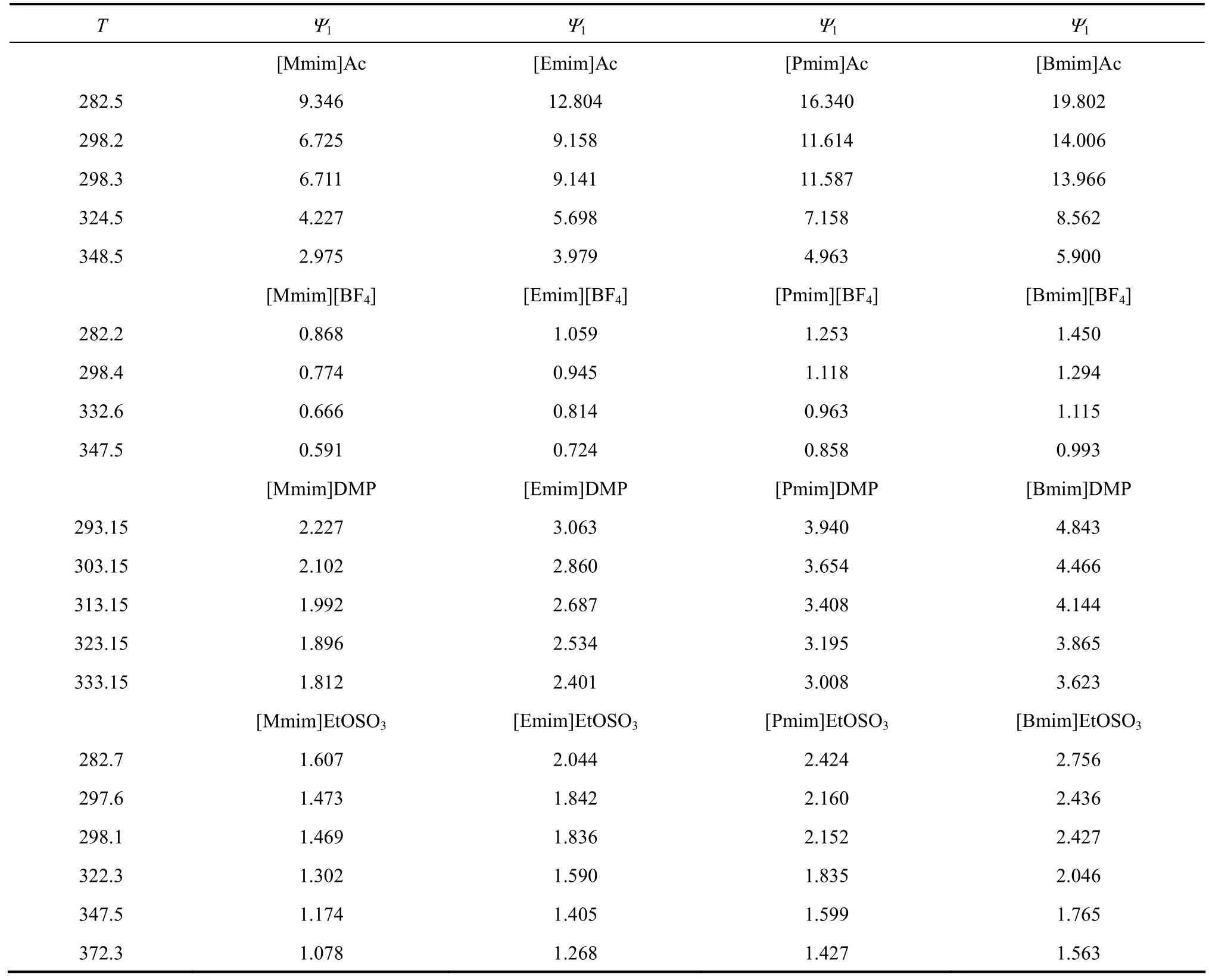

4 CONCLUSIONS
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Influence of Design Margin on Operation Optimization and Control Performance of Chemical Processes*
- Measurement and Modeling for the Solubility of Hydrogen Sulfide in Primene JM-T*
- Enhancing Structural Stability and Pervaporation Performance of Composite Membranes by Coating Gelatin onto Hydrophilically Modified Support Layer*
- Photocatalytical Inactivation of Enterococcus faecalis from Water Using Functional Materials Based on Natural Zeolite and Titanium Dioxide*
- A Group Contribution Method for the Correlation of Static Dielectric Constant of Ionic Liquids*
- Interaction Analysis and Decomposition Principle for Control Structure Design of Large-scale Systems*
