Kinetic Study on Extraction of Red Pepper Seed Oil with Supercritical
2014-07-18WANGJinyan王金艳WANGYuqi王玉琪ZHENGLan郑岚NIShifeng倪士峰FANZhaoyun范召运YAORuiqing姚瑞清andCHENKaixun陈开勋SchoolofChemicalEngineeringNorthwestUniversityXian70069China
WANG Jinyan (王金艳), WANG Yuqi (王玉琪),**, ZHENG Lan (郑岚), NI Shifeng (倪士峰), FAN Zhaoyun (范召运), YAO Ruiqing (姚瑞清)and CHEN Kaixun (陈开勋)School of Chemical Engineering, Northwest University, Xi’an 70069, China
2Key Laboratory of Resource Biology and Biotechnology in Western China, Northwest University, Xi’an 710069, China
Kinetic Study on Extraction of Red Pepper Seed Oil with Supercritical
WANG Jinyan (王金艳)1,2, WANG Yuqi (王玉琪)1,2,**, ZHENG Lan (郑岚)1, NI Shifeng (倪士峰)2, FAN Zhaoyun (范召运)1,2, YAO Ruiqing (姚瑞清)1and CHEN Kaixun (陈开勋)21School of Chemical Engineering, Northwest University, Xi’an 710069, China
2Key Laboratory of Resource Biology and Biotechnology in Western China, Northwest University, Xi’an 710069, China
A mathematical model for extraction of red pepper seed oil with supercritical CO2was proposed. Some factors influencing the process were investigated, including operation pressure, temperature and extraction yield Xe(%). The model was solved by the method of weighted residuals and used to simulate the process numerically. The kinetic equation is expressed as Xe=−16.8606exp(−t/9.98177)+16.95457 and the simulation results are in excellent agreement with the experimental data. The optimal operating parameters are 30 MPa, 318 K and 60 min.
supercritical CO2, red pepper seed, mathematical model
1 INTRODUCTION
China is rich in red pepper resources [1]. Capsaicin and capsanthin are important ingredients of red pepper, which are widely used in the fields of food, pharmacy, cosmetics and so on [2]. Red pepper seeds account for 45%-50% of the total pepper weight and are often ignored [3]. Red pepper seeds contain 25% oil and fatty acids, which mainly consists of linoleic acid, oleic acid, hexadecanoic acid, stearic acid and linolenic acid [4]. The contents of linoleic acid and oleic acid can reach over 90% among unsaturated fatty acids, which are fundamental to human and can reduce low density lipoprotein cholesterol [5]. Some medical investigators consider that red pepper seeds can take precautions against cardiovascular disease and regulate the central nervous system. Red pepper seeds also contain various minerals and vitamins A, D, E and K, and its high level of vitamin E is especially helpful to health care and anti-aging.
Although there are some researches about red pepper seeds, most of them are focused on extraction technology, property analysis and oxidation resistance. Few reports are concentrated on the theoretical analysis on red pepper seed extraction. Seed extraction technologies mainly include mechanical pressing, organic solvent extraction and supercritical CO2(SC-CO2) extraction [6]. Mechanical pressing process consumes large amounts of energy and the extraction yield is low. Organic solvent extraction has an advantage of high extraction yield, but organic solvent residues in the product will be harmful to human health [7]. SC-CO2has favorable solubility, which can attain incredible effects on extraction and separation [8]. The supercritical fluids are essentially limited to SC-CO2in food industry, since it has the advantages such as cheap, nontoxic, zero residual organic solvent and operation convenience. Thus we choose SC-CO2to extract red pepper seed oil. Red pepper seed oil mainly contains linoleic acid, oleic acid, hexadecanoic acid and so on, and the total amount of seed oil is the target in this study.
The objective of this study is to establish appropriate conditions for extraction of red pepper seed oil using SC-CO2, and different temperatures and pressures are tested by the weighted residuals method. A mathematical model is built based on the mass balance between solid and SC-CO2fluid to predict the seed extraction kinetics. Experimental data are used to verify the model parameters. The sensitive analysis is performed to evaluate the contribution of dimensionless parameters in the kinetic model.
2 MATERIALS AND METHODS
2.1 Instruments and materials
Figure 1 depicts the main devices for supercritical fluid extraction, which was made by Huaan Supercritical Extraction Co., Ltd. CO2was used as the supercritical solvent with a purity of 99.9% produced by Shaanxi Xinghua Chemical Co., Ltd. Red pepper seeds in this work were grown and produced in Shaanxi province.
2.2 Mathematical model
The kinetic model is based on differential massbalance. The fixed bed extractor with pepper seeds is the stationary phase and SC-CO2is the mobile phase [9-11]. The overall extraction process is described by partial differential equations (PDEs). Fig. 2 presents the supercritical fluid extraction bed and differential element. Following assumptions are used to derive the extraction model. (1) SC-CO2goes through the extractor uniformly and temperature or pressure difference inside the extractor is negligible. (2) Although several components are present, their mass transfer behavior is similar and the extracted products are considered as a single component. (3) At certain temperature and pressure, extraction concentration is a function of time and extractor height Z. (4) The density of SC-CO2is constant in extraction process. (5) Most particles are spherical and quite small (<1 mm), so the internal diffusion is negligible and mass transfer is the rate-controlling step.
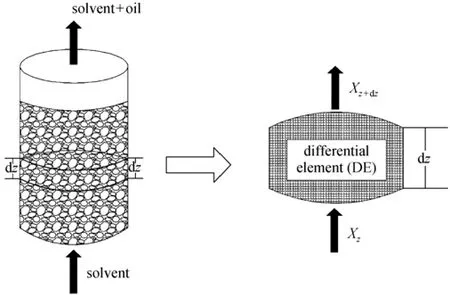
Figure 2 Schematic of the extraction and mass transfer in the differential element
The following PDEs can be used to describe the mass conservation of differential units in the extractor:
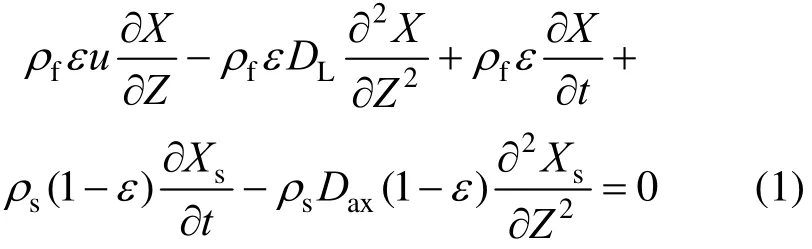
The radial concentration gradient of oil is negligible, soax0D≈ [12] and Eq. (1) can be simplified to

The mass transfer rate of solute from solid phase to fluid phase is [13]

The initial and boundary conditions are as follows:
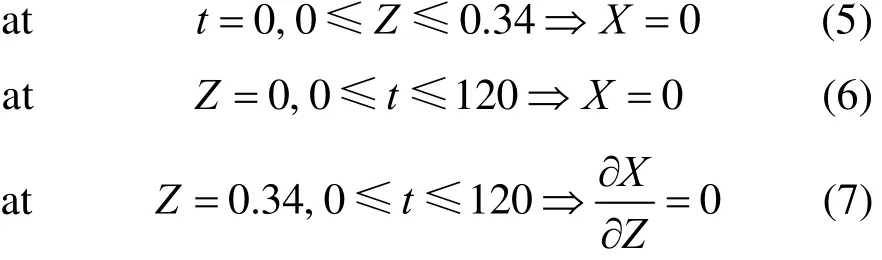
Equation (2) is solved with Eqs. (5)-(7).
2.3 Model parameters
The density of SC-CO2is calculated by Peng-Robinson Eq. [14],

with a=acα(Tr,w), b=0.077796RTc/Pcwhere
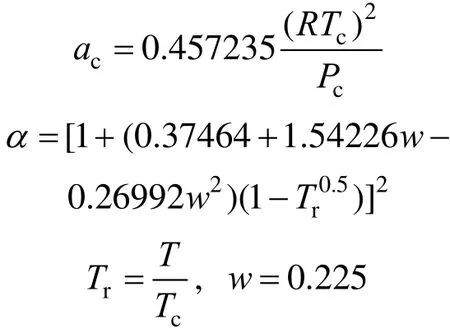
The equilibrium solubility of vegetable oil in SC-CO2has been proposed by Del Valle et al [15, 16].

where k=10.724, a=−18708, d=2186840, b= 40.316.
The concentration of red pepper seed oil in SC-CO2is zero at the inlet of extractor and yiis the solubility of red pepper seed oil in SC-CO2at the outlet of extractor at different time ti[17]. Based on the mass conservation of differential units, we have

From Eq. (10),
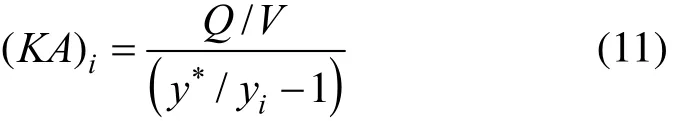
The axial diffusion coefficient (DL) is calculated through injecting a pulse of methane into SC-CO2[18, 19].

SC-CO2viscosity is calculated using the correlation proposed by Heidaryan et al [20]. The temperature and pressure range from 310K to 900K and 7.5 MPa to 101.4 MPa, respectively.
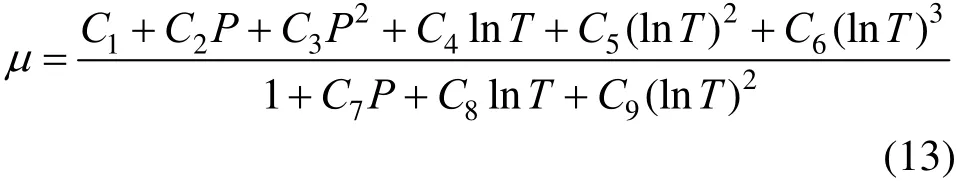
where C1-C9are all constants.
Other model parameters are
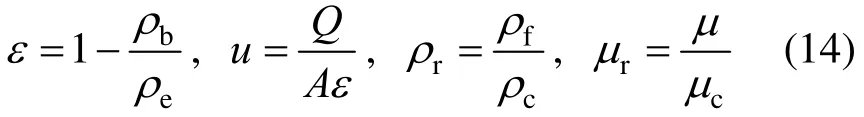
The extraction yield is
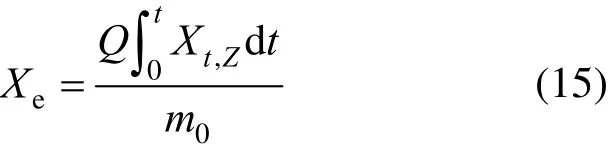
2.4 Method of weighted residuals
Method of weighted residuals is an effective method for solving PDEs. In this paper, it is applied to determine the optimal extractor operating parameters (temperature and pressure). The procedures are as follows.
(1) Define the function of PDEs by comparing Eq. (2) with regular PDEs. Characteristic parameters (symmetry: 0; diagonal matrix: B; flux: /XZ∂∂, source: C; where B and C are temperature and pressure functions) are obtained.
(2) Define the initial and boundary conditions, and arrange them to standard format.
(3) Grids are divided into n×m, where n and m denote the number of radial and axial grids, respectively.
(4) Calling pdepe() function. Solve the linear equations with MATLAB.
3 RESULTS AND DISCUSSION
3.1 Calculated density of SC-CO2
The density of SC-CO2under operation conditions are obtained by PRO II. The data for density training in literature are compared with calculated values at different temperatures and pressures in Table 1 [14, 21]. The average relative error (R) is 0.53% and acceptable. The calculated SC-CO2density with PRO II is highlyeffective, including the density apart from supercritical point. Table 2 gives some density data of SC-CO2calculated by PRO II.

Table 1 Comparison of trained data with calculated values of SC-CO2density
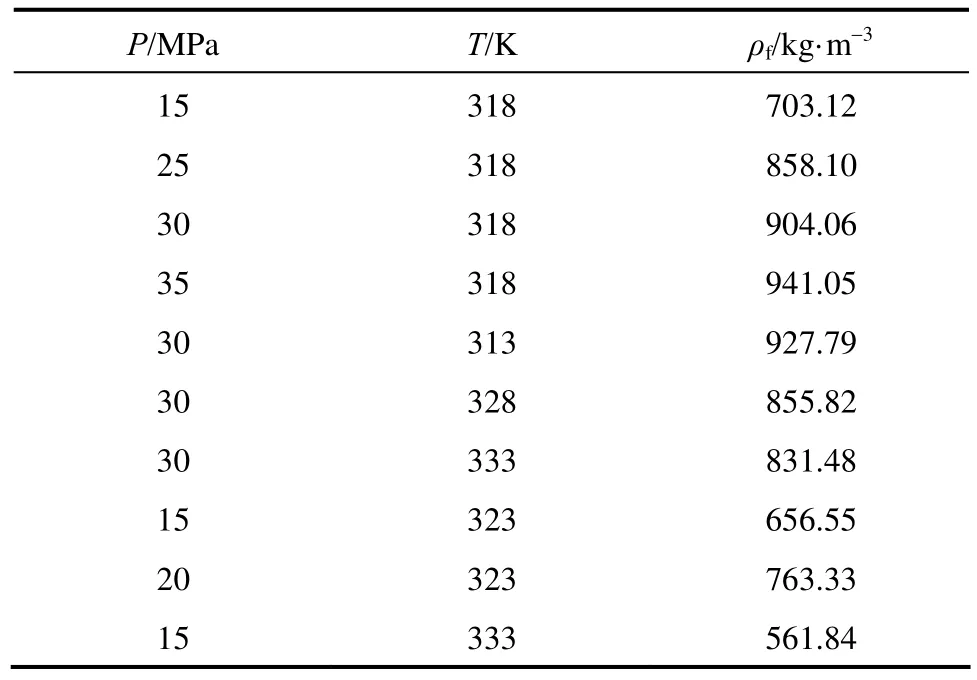
Table 2 The density of SC-CO2under operation conditions
3.2 Kinetic modeling results
The experimental operation (Exp.) conditions and the calculated values of parameters used in kinetic model are shown in Table 3.
The influences of extractor height and extraction time on the solution concentration of SC-CO2in the whole extraction process are shown in Fig. 3 (Exp. 3 data) and Fig. 4 (Exp. 10 data). The seed oil concentration of red pepper increases rapidly with extractor height and extraction time, then becomes slowly and tends to be steady, in accordance with that mass transfer is the rate-controlling step [22].
Figures 5 and 6 show that the model prediction is in good agreement with experiment data. The kinetic model will provide guidance to large scale productions.
3.3 Numerical simulation result
The main operation conditions are determined to maximize the extraction yield. Temperature and pressure have direct effects on the extraction yield. The effect of pressure on Xeat different temperatures is shown in Fig. 7. Xeincreases with pressure at certain temperature, similar with that the seed oil solubility in SC-CO2increases with pressure [23]. However, the extraction yield at 35 MPa is slightly lower than that at 30 MPa. Higher pressure can improve the solubility and density of SC-CO2, and the diffusion coefficient of SC-CO2decreases. The optimal extraction pressure for red pepper seed oil is considered as 30 MPa. The effect of temperature on Xeis similar, as shown in Fig. 8. Although higher temperature can improve the volatility and diffusion coefficient of red pepper seed oil inthe extraction process, the density of SC-CO2decreases as a result of expansion effect [24, 25]. Based on economic and other factors, the optimal extraction temperature for red pepper seed oil is 318K.
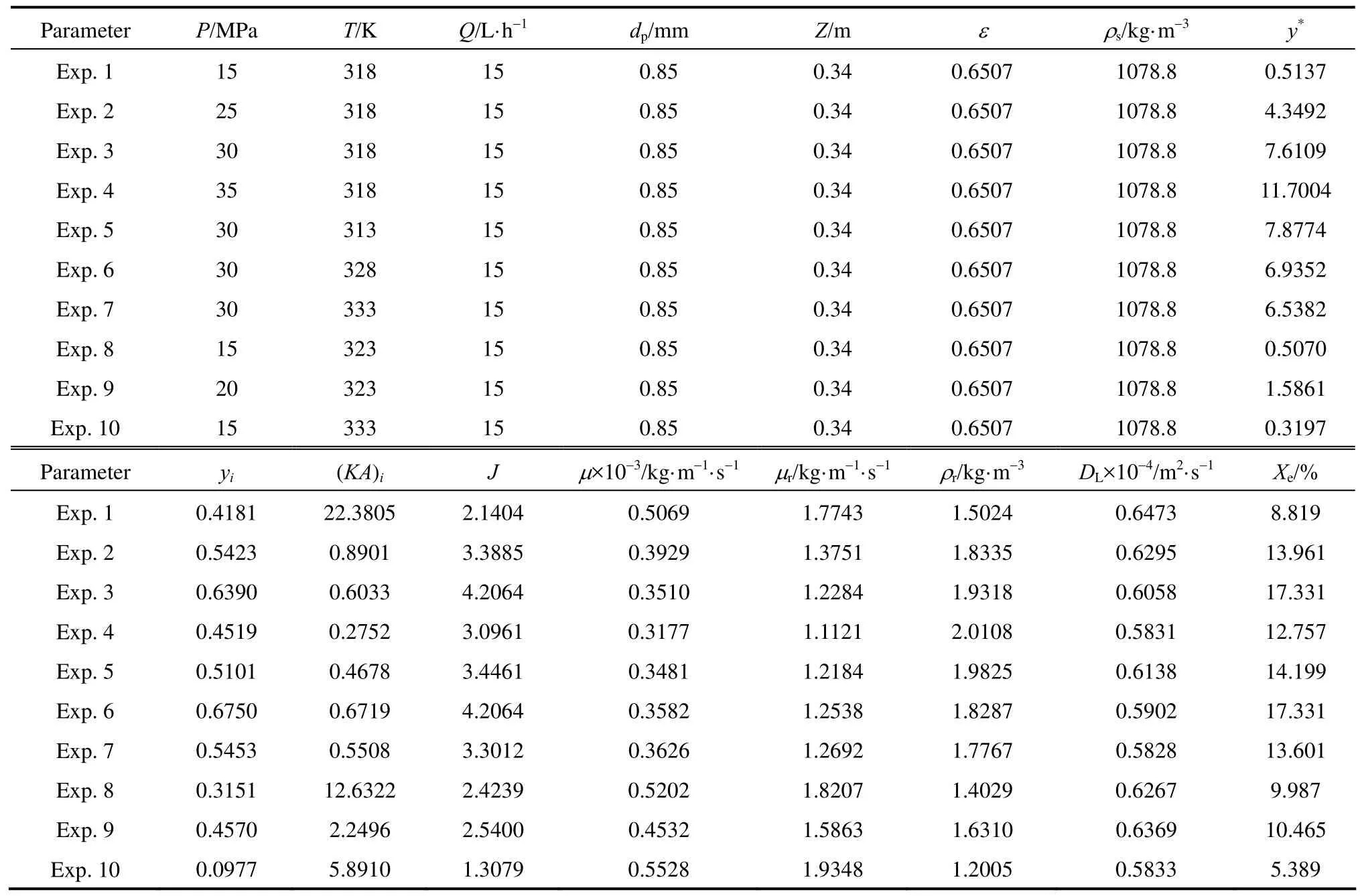
Table 3 Experimental parameters for SC-CO2extraction of red pepper seeds

Figure 3 Influence of extractor length and extraction time on SC-CO2concentration at 318K and 30 MPa
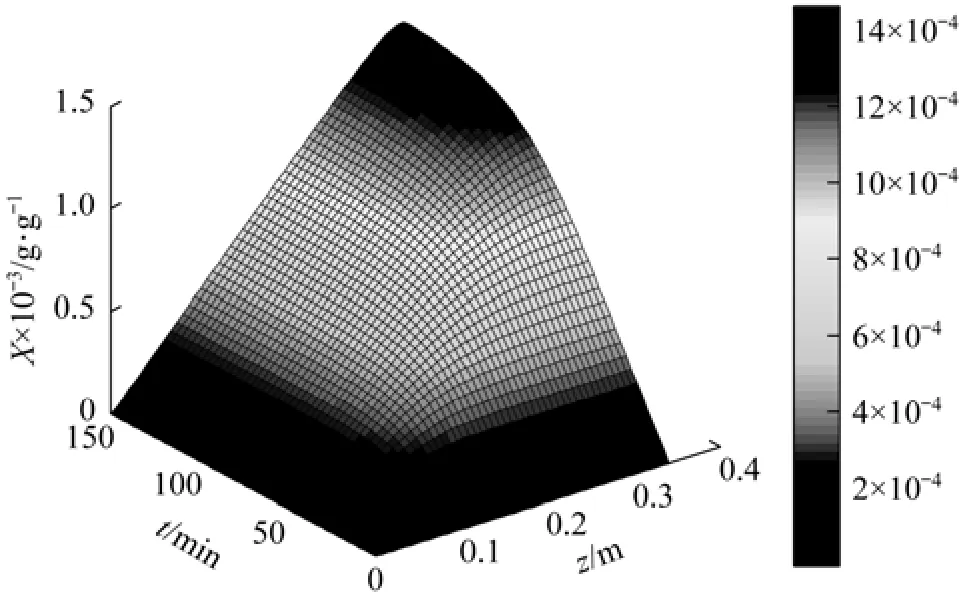
Figure 4 Influence of extractor length and extraction time on SC-CO2concentration at 333K and 15 MPa
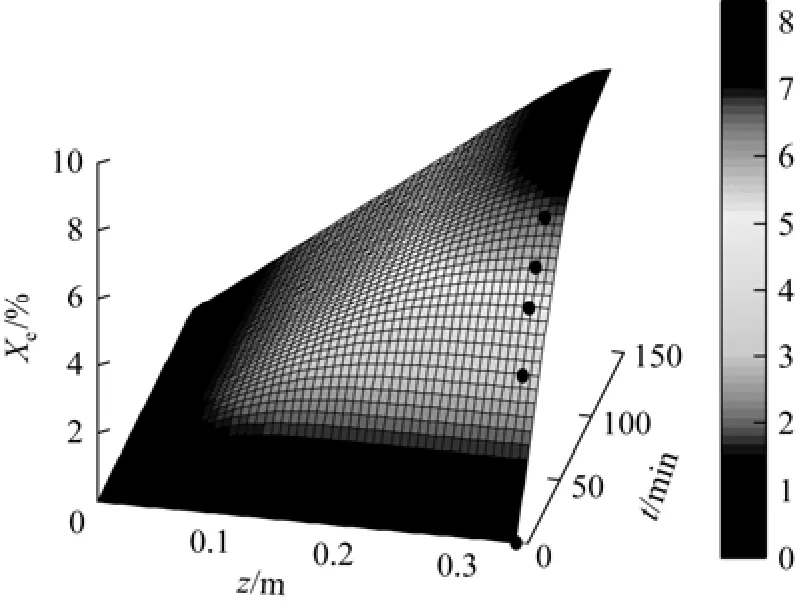
Figure 5 Influence of extractor length and extraction time on the extraction rate at 318 K and 15 MPa● Exp. 1
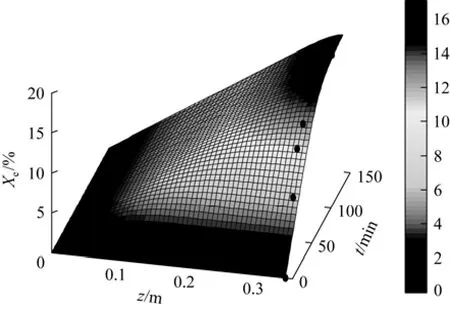
Figure 6 Influence of extractor length and extraction time on the extraction rate at 328 K and 30 MPa● Exp. 6
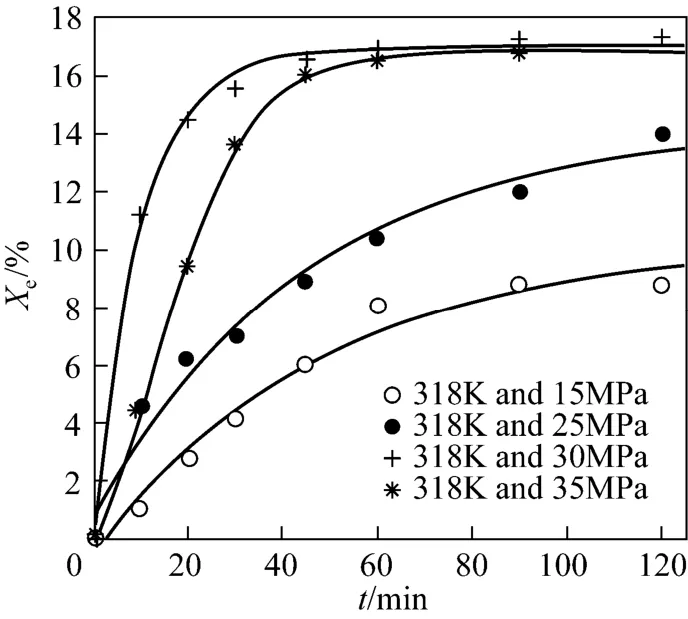
Figure 7 Effect of pressure on extraction yield calculation; ○ Exp. 1; ● Exp. 2;Exp. 3;Exp. 4

Figure 8 Effect of temperature on extraction yield calculation; ○ Exp. 5; ● Exp. 3;Exp. 6;Exp. 7
Figures 7 and 8 show that the extraction yield Xeincreases with time and then tends to be steady. As revealed in Figs. 9 and 10, the extraction rate (Er) increases sharply at the beginning in the extraction, and then decreases due to gradual decrease of mass transfer driving force (concentration difference). Therefore, the optimal extraction time for red pepper seed oil is 60 min.
The optimum conditions for red pepper seed oil extraction by SC-CO2are 30 MPa and 318 K. The kinetic correlation between Xeand time is obtained as
Xe=−16.8606exp(−t/9.98177)+16.95457 (16) It is verified by experimental study, as shown in Fig. 8.

Figure 9 Oil extraction rate as a function of extraction time for Exps. 1, 2, 3 and 4

Figure 10 Oil extraction rate as a function of extraction time for Exps. 5, 3, 6 and 7
4 CONCLUSIONS
For the kinetic model on the red pepper seed oil extraction by SC-CO2, the density of SC-CO2and other parameters were determined. The mathematical model was solved with method of weighted residuals and the results for the influence of extractor height and extraction time on solution concentration of SC-CO2and extraction yield Xewere in accordance with the experimental data. The extraction yield Xeof oil was determined by numerical simulation. The correlated kinetic equation is Xe=−16.8606exp(−t/9.97177)+ 16.95457. It is in good agreement with the experimental data. The optimal pressure, temperature and time for red pepper seed oil extraction are 30 MPa, 318 K and 60 min, respectively.
NOMENCLATURE
A cross-sectional area of extractor, m2
Daxradial diffusion coefficient, m2·s−1
DLaxial diffusion coefficient, m2·s−1
dpparticle diameter, m
Erextraction rate, g·min−1
J mass transfer rate of solute from solid phase to fluid phase
K mass transfer coefficient
m0mass of extraction raw material, g
P pressure, MPa
Q supercritical fluid flow rate, L·h−1
q mass fraction of seed oil in pepper seeds, g·g−1
qsraw mass fraction of seed oil in pepper seeds, g·g−1
T temperature, K
t time, min
u supercritical fluid velocity, m·s−1
V extractor volume, m3
X mass fraction of solute in fluid phase, g·g−1
Xeextraction yield, %
Xsmass fraction of solute in solid particle, g·g−1
y∗equilibrium solubility of solute in SC-CO2
yisolubility of red pepper seed oil in SC-CO2at the outlet of extractor
Z extractor height, m
ρbstacking density of red pepper seed, kg·m−3
ρcdensity of SC-CO2calculated by PROⅡ, kg·m−3
ρLdensity of SC-CO2in literature, kg·m−3
ε void fraction of extractor
μ viscosity, kg·m−1·s−1
ρ density, kg·m−3
NOMENCLATURE
c critical
f fluid
r contrast
s solid particle
REFERENCES
1 Wang, Y., Chen, K., Yao, R., Zheng, L., “Preparation of capsanthin by supercritical extraction”, Chemical Engineering, 36 (8), 9-12 (2008). (in Chinese)
2 Sun, Y., Yan, J., Jia, N., Zhong, M., Zhai, C., “Visual analysis of extraction process of paprika seeds oil with supercritical carbon dioxide”, Food Science and Technology, 37 (6), 209-214 (2012). (in Chinese)
3 Wang, Z., Li, D., Ding, Z., Deng, S., Zhang, D., “The nutrition ingredient analysis to the main varieties of pepper seeds in Guizhou”, China Condiment, 35 (5), 93-96 (2010). (in Chinese)
4 Gnayfeed, M.H., Daood, H.G., Illes, V., Biacs, P.A., “Supercritical CO2and subcritical propane extraction of pungent paprika and quantification of carotenoids, tocopherols and capsaicinoids”, J. Agric. Food Chem., 49, 2761-2766 (2001).
5 Chang, C., Shen, S., Song, Y., “Research on composition of fatty acids in paprika oil”, Journal of Anhui Agri Sci, 38 (6), 3133-3134 (2010). (in Chinese)
6 Li, D., Wang, Z., Ding, Z., Deng, S., “Study on quality analysis and flavonoids extraction technology of capsicum seed”, China Condiment, 35 (4), 48-51 (2010). (in Chinese)
7 Karabegovi, I., Nikolova, M., Veli kovi, D., Stoji, S., Veljkovi, V., Miodrag, L., “Comparison of antioxidant and antimicrobial activities of methanolic extracts of the Artemisia sp. Recovered by different extraction techniques”, Chin. J. Chem. Eng., 19 (3), 504-511 (2011).
8 Jarén-Galán, M., Nienaber, U., Steven, J.S., “Paprika (capsicum annuum) oleoresin extraction with supercritical carbon dioxide”, J. Agric. Food Chem, 47, 3558-3564 (1999).
9 Hatami, T., Meireles, M.A.A., Zahedic, G., “Mathematical modeling and genetic algorithm optimization of clove oil extraction with supercritical carbon dioxide”, J. of Supercritical Fluids, 51, 331-338 (2010).
10 Jia, D., Li, S., Wu, X., Liu, N., Liu, T., “Mass transfer models for supercritical CO2extraction of volatile oils”, Journal of Chemical Industry and Engineering, 59 (3), 537-543 (2008). (in Chinese)
11 José, M., del Valle, R.O., Mattea, M., “Supercritical CO2processing of pretreated rosehip seeds: Effect of process scale on oil extraction kinetics”, J. of Supercritical Fluids, 31, 159-174 (2004).
12 Zheng, J., Xu, M., Lu, X., Lin, C., “Measurement and correlation of solubilities of CI disperse red 73, CI disperse blue 183 and their mixture in supercritical carbon dioxide”, Chin. J. Chem. Eng., 18 (4), 648-653 (2010).
13 Sovová, H., “Mathematical model for supercritical fluid extraction of natural products and extraction curve evaluation”, J. of Supercritical Fluids, 33, 35-52 (2005).
14 Yuan, H., Yin, J., Ding, X., “Artificial neural networks application to the density calculations for supercritical fluids”, Computer and Applied Chemistry, 20 (6), 848-850 (2003). (in Chinese)
15 Del Valle, J.M., Aguiilera, J.M., “An improved equation for predicting the solubility of vegetable oils in supercritical CO2”, Ind. Eng. Chem. Res., 27, 1551-1553 (1988).
16 Luo, N., Lu, Y., Jiang, Y., “Solubility of paclitaxel in mixtures of dichloromethane and supercritical carbon dioxide”, Chin. J. Chem. Eng., 19 (4), 558-564 (2011).
17 Saldana, M.D.A., Tomberli, B., Guigard, S.E., Goldman, S., Gray, C.G., Temelli, F., “Determination of vapor pressure and solubility correlation of phenolic compounds in supercritical CO2”, J. of Supercritical Fluids, 40, 7-19 (2007).
18 Tan, C.S., Liou, D.C., “Axial dispersion of supercritical carbon dioxide in packed beds”, Ind. Eng. Chem. Res., 28, 1246-1250 (1989).
19 Raspo, I., Nicolas, C., Neau, E., “Diffusion coefficients of solids in supercritical carbon dioxide: Modelling of near critical behaviour”, Fluid Phase Equilibria, 263, 214-222 (2008).
20 Heidaryan, E., Hatami, T., Rahimi, M., Moghadasi, J., “Viscosity of pure carbon dioxide at supercritical region: Measurement and correlation approach”, J. of Supercritical Fluids, 56, 144-151 (2011).
21 Hatamia, T., Sandra, B, “Modelling and optimization of supercritical CO2extraction of St. John’s Wort using genetic algorithm”, J. of Supercritical Fluids, 62, 102-108 (2012).
22 Sovová, H., “Steps of supercritical fluid extraction of natural products and their characteristic times”, J. of Supercritical Fluids, 66, 73-79 (2012).
23 Varona, S., Martin, A., Cocero, M.J., “Supercritical carbon dioxide fractionation of Lavandin essential oil: Experiments and modeling”, J. of Supercritical Fluids, 45, 181-188 (2008).
24 Melrelesa, M.A.A., Zahedi, G., Hatamib, T., “Mathematical modeling of supercritical fluid extraction for obtaining extracts from vetiver root”, J. of Supercritical Fluids, 49, 23-31 (2009).
25 Rahimia, E., Pradob, J.M., Zahedic, G., “Meirelesb chamomile extraction with supercritical carbon dioxide: Mathematical modeling and optimization”, J. of Supercritical Fluids, 56, 80-88 (2011).
10.1016/S1004-9541(14)60003-3
2013-03-04, accepted 2013-05-15.
* Supported by the Opening Foundation of Key Laboratory of Resource Biology and Biotechnology in Western China (ZS12015), the Ministry of Education and Natural Science Foundation of Education Committee of Shaanxi Province (11JK0598), and the 2012 Xi’an Modern Agricultural Promotion Project (NC1207).
** To whom correspondence should be addressed. E-mail: wangyuqi@nwu.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Influence of Design Margin on Operation Optimization and Control Performance of Chemical Processes*
- Measurement and Modeling for the Solubility of Hydrogen Sulfide in Primene JM-T*
- Enhancing Structural Stability and Pervaporation Performance of Composite Membranes by Coating Gelatin onto Hydrophilically Modified Support Layer*
- Photocatalytical Inactivation of Enterococcus faecalis from Water Using Functional Materials Based on Natural Zeolite and Titanium Dioxide*
- A Group Contribution Method for the Correlation of Static Dielectric Constant of Ionic Liquids*
- Interaction Analysis and Decomposition Principle for Control Structure Design of Large-scale Systems*
