Role of La2O3as Promoter and Support in Ni/γ-Al2O3Catalysts for Dry Reforming of Methane
2014-07-18AhmedAlFateshMuhammadNaeemAnisFakeehaandAhmedAbasaeed
Ahmed S. Al-Fatesh*, Muhammad A. Naeem, Anis H. Fakeeha and Ahmed E. Abasaeed
Chemical Engineering Department, College of Engineering, King Saud University, P.O. Box 800, Riyadh 11421, Kingdom of Saudi Arabia
Role of La2O3as Promoter and Support in Ni/γ-Al2O3Catalysts for Dry Reforming of Methane
Ahmed S. Al-Fatesh*, Muhammad A. Naeem, Anis H. Fakeeha and Ahmed E. Abasaeed
Chemical Engineering Department, College of Engineering, King Saud University, P.O. Box 800, Riyadh 11421, Kingdom of Saudi Arabia
The nature of support and type of active metal affect catalytic performance. In this work, the effect of using La2O3as promoter and support for Ni/γ-Al2O3catalysts in dry reforming of methane was investigated. The reforming reactions were carried out at atmospheric pressure in the temperature range of 500-700 °C. The activity and stability of the catalyst, carbon formation, and syngas (H2/CO) ratio were determined. Various techniques were applied for characterization of both fresh and used catalysts. Addition of La2O3to the catalyst matrix improved the dispersion of Ni and adsorption of CO2, thus its activity and stability enhanced.
CO2, methane reforming, Ni/γ-Al2O3, La2O3, carbon deposition, H2/CO
1 INTRODUCTION
Global climatic variations and environmental safety requires mitigation of greenhouse gases, such as methane and carbon dioxide into the atmosphere. The CO2(or dry) reforming of methane (DRM) can convert these two gases into more valuable syngas (H2and CO). This process yields syngas with H2/CO ratio closer to unity which is more appropriate for several industrial processes such as oxo- and Fischer-Tropsch synthesis [1-5]. DRM suffers from high endothermicity and carbon deposition on the catalyst surface. On the basis of thermodynamic investigations, under typical reaction conditions, generally, there are four feasible side reactions which give rise to carbon formation during DRM process, including methane decomposition/cracking reaction (1), CO disproportionation, i.e., Boudouard reaction (2), CO/H2reduction reaction (3) and CO2hydrogenation reaction (4):

Also, the severe reaction conditions could lead to sintering of the active metal component to result in catalyst deactivation [6]. Many research efforts have been concentrated on developing metal catalysts with high catalytic performances towards syngas production and high resistance to carbon deposition for stable long-standing operations. Noble metal based catalysts have high catalytic performance in terms of methane conversion and selectivity to syngas, and are less sensitive to carbon formation [7].
However, transition metals, such as Ni, Fe and Co, are often favored due to their low cost and availability compared to noble metals. Among these metals, nickel is very promising because it is highly active and relatively cheaper than the others; however, during dry reforming reaction nickel-based catalysts tend to deactivate due to coke formation and sintering of nickel particles, which is closely related to catalyst structure and composition [8]. Therefore, various researchers have studied numerous approaches to minimize carbon formation on Ni catalysts. For Ni-based catalysts, the addition of alkaline earth and/or rare earth oxides to the catalyst was shown to decrease coke formation by the creation of carbonates or oxycarbonates during the reaction [9-11].
It has been proposed that carbon deposition can be inhibited when nickel is used with supports having strong Lewis basicity. The increase in Lewis basicity enhances the ability of the catalyst to chemisorb CO2and thus, decreases carbon formation via reverse Boudouard reaction (2) by shifting the equilibrium concentration. In addition, during reforming reaction, metal-support interaction and metal particle size play important roles in carbon deposition over supported metal catalysts [12]. It has been suggested that the addition of basic promoters such as MgO, CaO, K2O and lanthanum oxide La2O3can enhance both catalyst performance and coke resistance [13]. Roh and Jun [14] revealed that the addition of La2O3to Ni/α-Al2O3can increase the dispersion of Ni particles on the supports and reduce the agglomeration of Ni particles during the reforming reaction. Also, La2O3can adsorb and react with CO2to form La2O2CO3species on the surface of catalyst which can speed up the conversion of surface CHχspecies (χ=0-3). A recent study by Gao et al. [15] revealed the advantages of modifying the SiO2supported Ni catalysts by La2O3. It has been shown that the presence of La2O3improved the dispersion of Ni, enhanced Ni interaction with the support and promoted the activation of CO2.
Zhang et al. [16] claimed that doping of rare earth ion (La3+) in the Ni/ZSM-5 improved the stability andthe catalyst activity in DRM. Pour et al. [17] has shown that although the addition of La2O3on NiO/MgO/α-Al2O3has no effect on activity, it can suppress coke deposition on the catalyst surface due to the enhanced mobility of lattice oxygen anions. Verykios and co-workers [18, 19] proposed that the collaboration between nickel and lanthanum species produces a unique type of collegial active sites at the Ni-La2O3interface that boosts the activity and stability of the catalyst. Martinez et al. [20] reported that addition of La within a certain bound can increase the Ni dispersion in catalyst which in turn improves the conversion level and enhances the catalyst stability.
In this work the effect of lanthanum as promoter and support for Ni/γ-Al2O3catalysts used in dry reforming of methane was experimentally studied at different calcination and testing temperatures with different loadings of lanthanum.
2 EXPERIMENTAL
2.1 Catalyst preparation
High surface area alumina (γ-Al2O3) SA6175 (purity 99.99%, particle size 25 nm, BET (Brunauer-Emmett-Teller): 230 m2·g−1) (Norton Co., USA) was used as the catalyst support throughout this investigation. La2O3powder (purity 99.99%, particle size <90 nm, BET: <40 m2·g−1) was from MKnano, Canada. The nitrate salts of nickel [Ni(NO3)2·6H2O] and lanthanum [La(NO3)3·6H2O] from Sigma Aldrich were used as precursors for active metal and promoter respectively. Nickel catalysts [5% (by mass) loading] were prepared on γ-Al2O3, La2O3and mixed La2O3-γ-Al2O3supports by the wet impregnation. Solutions containing known amounts of nickel nitrate were prepared in distilled water; then γ-Al2O3, La2O3and mixed supports (La2O3+ γ-Al2O3) having different amounts of La2O3range from 10%-30% were impregnated with the previously prepared solution of active metal. The catalysts were dried for 10 h at 120 °C and calcined at 500 °C and then 700 °C for 2 h.
The 5% (by mass) Ni-promoted catalysts with La mass content ranging from 0-4% (i.e., for a typical catalyst that contains 5% Ni, 4% La and Al2O3, the mass ratio of Ni︰La︰Al2O3is 5︰4︰100) were prepared by co-impregnation of lanthanum nitrate and nickel nitrate with γ-Al2O3support using the same procedure mentioned above. For simplicity these catalysts are abbreviated as Ni-Laχ-Al where χ=0-4% (by mass) e.g., for 2% (by mass) La promoted catalyst the short name is Ni-La4-Al.
2.2 Catalytic reaction
Catalytic activity measurements were carried out in a stainless steel fixed-bed micro tubular reactor (9.4 mm i.d. and 48 cm long, Zeton Altimira 2000) under atmospheric pressure. The reactor was electrically heated by a furnace having four independently heated zones while the reaction temperature was measured using a thermocouple placed in an axial thermowell centered in the catalyst bed. Prior to the reaction, the catalysts were reduced inside the reactor at 650 °C by passing hydrogen at a rate of 40 ml·min−1for 2 h followed by 20 min of N2at a rate of 30 ml·min−1. The molar ratio of the feed gas mixture CH4/CO2/N2was fixed at 5︰5︰1 and the flow rate per gram catalyst at 60 ml·min−1·(g cat)−1. The activity of the prepared catalyst was tested in the temperature range of 500-700 °C. Catalytic stability was evaluated at 700 °C for 540 min. The products were analyzed using an on-line Varian gas chromatography equipped with a thermal conductivity detector. The details of the experimental setup and procedure have been given in previous publications [21, 22].
2.3 Catalyst characterization
The specific surface area, pore volume, average pore diameter and nano-particle size of the fresh and used catalyst was measured in Micrometrics Tristar II 3020 surface area and porosity analyzer by N2adsorption data at 77 K according to the standard BET procedure. For each run a 0.3 g of catalyst was used. Degassing of the samples was done at 300 °C for 3 h to remove the moisture content and other adsorbed gases from the surface of catalyst. The pore size distribution was calculated from desorption branch of the corresponding nitrogen isotherm by applying the Barrett, Joyner, and Halenda (BJH) method.
The powder X-ray diffraction (XRD) analysis of fresh and used catalyst was carried out using Cu-Kαradiation operated at 40 kV and 40 mA. The scanning step and range of 2θ for analysis were 0.01° and 10°-85° respectively.
Temperature programmed desorption (CO2-TPD) and temperature programmed oxidation (TPO) measurements were carried out on Micromeritics Auto Chem II 2920 apparatus. Prior to experiments, physically adsorbed and/or weakly bound species were removed from catalysts by pretreating it under He flow at 150 °C for 1 h. The CO2adsorption was carried out at 50 °C for 30 min by using CO2/He mixture gas (volume ratio, 10/90) at a flow rate of 25 ml·min−1, while for TPO experiments O2/He mixture gas (volume ratio, 10/90) at a flow rate of 30 ml·min−1was used.
In order to study the morphology of the catalyst and to elucidate carbon deposition on the used catalysts, Scanning Electron Microscopy (SEM) interpretations of the fresh and used catalyst samples were performed using a JSM-6360A (JEOL Ltd., Japan) scanning electron microscope.
Carbon formation on the surface of used catalysts was estimated by thermogravimetric analysis (TGA) and differential thermal analysis (DTA) in air atmosphere using EXSTAR SII TG/DTA 7300 analyzer. The used catalyst sample 10-20 mg in quantity was heated from room temperature to 800 °C at a heating rate 20 °C·min−1.
3 RESULTS AND DISCUSSION
3.1 Textural properties
Nitrogen (N2) adsorption-desorption isotherms of fresh catalysts, prepared at calcination temperature 500 and 700 °C, such as 5% (by mass) Ni/γ-Al2O3, 5% (by mass) Ni/(100% La2O3), 5% (by mass) Ni/(10% La2O3+ 90% γ-Al2O3), 5% (by mass) Ni/(20% La2O3+ 80% γ-Al2O3) and 5% (by mass) Ni/(30% La2O3+ 70% γ-Al2O3) are shown in Figs. 1 (a) and 1 (b), respectively. For simplicity, these catalysts here and after will be denoted as Ni-Al, Ni-La, Ni-10LaAl, Ni-20LaAl and Ni-30LaAl respectively. It is clear from these figures that N2isotherms are similar to type IV, showing a hysteresis loop of the H2type escorting with the existence of mesopores. Pore size distribution of fresh catalysts calcined at 500 and 700 °C are presented in Figs. 1 (c) and 1 (d) respectively. In comparison to Ni-Al catalyst, the mixed supported catalysts showed smaller pore size distribution for both calcination temperatures.
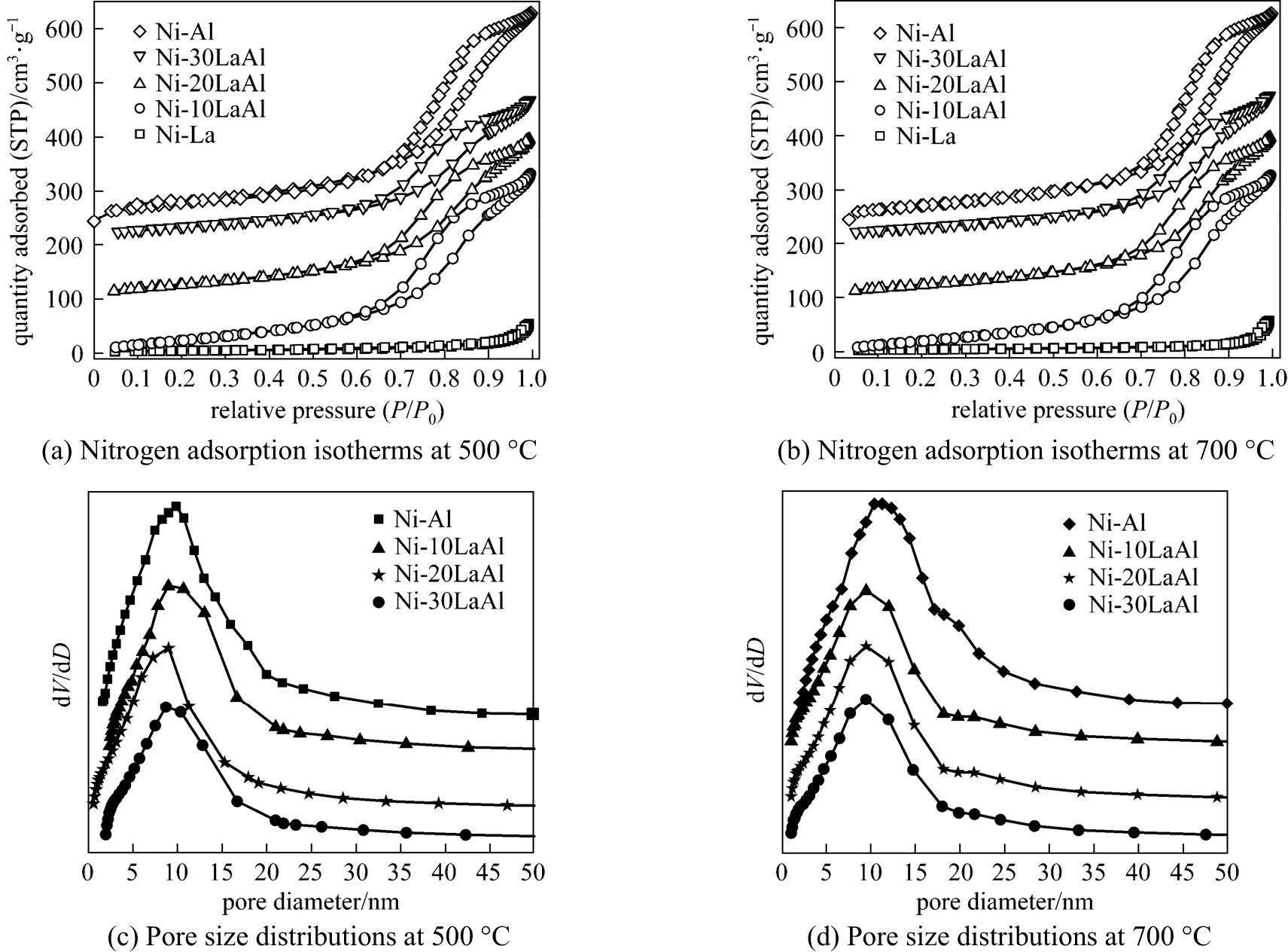
Figure 1 Nitrogen adsorption-desorption isotherms and pore size distributions for catalysts with different supports calcined at 500 °C and 700 °C
The textural properties of the fresh and used catalysts are listed in Tables 1 and 2. The catalysts with mixed supports acquired higher surface area, larger pore volume, and more prominent mesoporosity as compared to La2O3supported catalysts. However, upon increasing the La2O3content in the mixed catalyst, the specific surface area of the catalyst decreased. The catalysts which were calcined at 500 °C possessed larger specific surface areas as compared to catalysts calcined at 700 °C. These catalytically advantageoustextural properties can improve the dispersion of nickel, diminish the carbon deposition, and increase the catalyst activity and stability.

Table 1 The textural properties of fresh and used catalysts calcined at 500 °C①
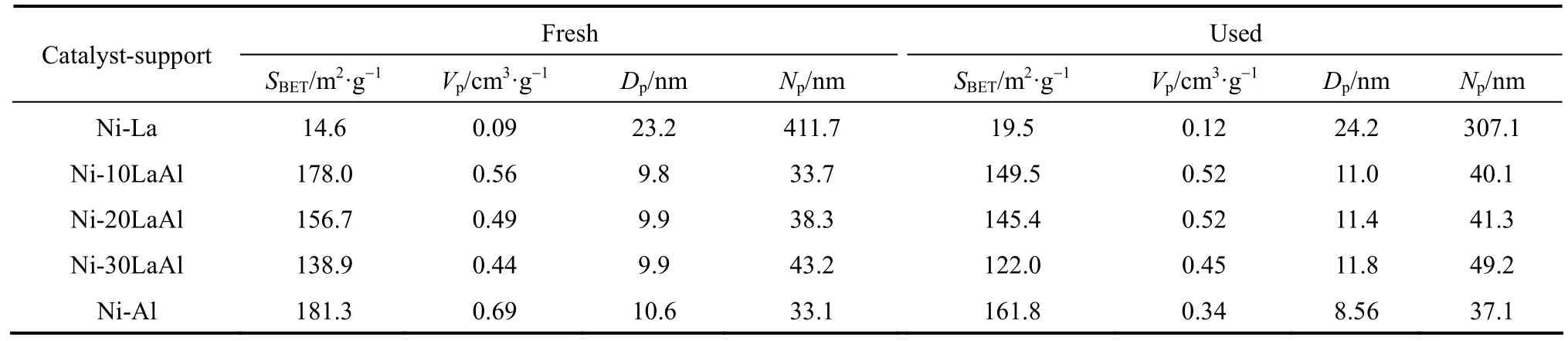
Table 2 The textural properties of fresh and used catalysts calcined at 700 °C①
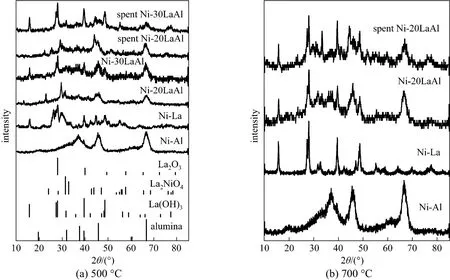
Figure 2 XRD of fresh and used catalysts at calcination temperatures of 500 °C and 700 °C
3.2 X-ray diffraction (XRD)
Figures 2 (a) and 2 (b) show X-ray diffraction (XRD) patterns of fresh and spent catalysts, prepared at calcination temperature 500 and 700 °C respectively, supported on γ-Al2O3, La2O3and mixed supports. At both calcination temperatures, the figures show three diffraction peaks for the catalyst supported on γ-Al2O3at 2θ=37°, 45.6° and 66.7° (JCPDS: 00-029-0063) which were assigned to Al2O3. The peaks for NiO phase appeared at 2θ=37.2°, 43.3° and 63° (JCPDS; 01-073-1519). Moreover, for the diffraction patterns of La2O3supported and mixed supported catalysts it is hard to distinguish between La2O3and La(OH)3due to the peak broadening and/or overlapping of La2O3and La(OH)3. According to reported JCPDS, the peaks appeared at 2θ=28° and 49° may be attributed to La2O3(JCPDS: 01-089-4016) while peaks at 2θ=16°, 27.3° and 48.6° are assigned to La(OH)3(JCPDS: 00-006-0585). The other detected peaks are due to La-Ni aluminate (La2NiO4) (JCPDS: 00-027-1180). For spent catalysts the diffraction peaks observed at 2θ= 44.5°, 52° and 76.4° may be attributed to metallic Ni (JCPDS: 00-004-0850) while other small diffraction peaks observed at 2θ=30.4°, and 54.7° are ascribed to La2O2CO3(JCPDS: 00-037-0804). This specie is very beneficial to remove coke deposits.
3.3 CO2TPD
The basicity of pure and mixed supported catalysts was measured by TPD using CO2as a probe gas. Generally, in TPD, the strengths of the basic sites are reported in terms of temperature range where the adsorbed CO2on the basic sites is desorbed. Normally, the Lewis alkaline sites are categorized into weak (<200 °C) medium (200-400 °C), strong (400-600 °C) and very strong (>600 °C) depending on the desorption temperature of CO2[23]. The CO2-TPD spectra of catalysts calcined at 500 °C and 700 °C, pure and mixed supported, are presented in Figs. 3 (a) and 3 (b) respectively. In both cases the Ni-Al catalyst, i.e., withpure alumina support, shows the smallest capacity to adsorb CO2with two desorption peaks centered at 100 °C and 275 °C. These desorption peaks are assigned to the low and medium strength basic sites respectively. In contrast, all mixed supported catalysts showed more than two distinct desorption peaks which indicates the addition of La2O3significantly improves the basicity of catalyst. In fact this improvement favors the gasification of coke from catalyst surface by supplying oxygen for gasification, thus increasing the stability of catalyst. Comparing to Ni-Al catalyst, the Ni-30LaAl catalyst, at 700 °C calcination, displayed three distinct CO2desorption peaks centered at 115 °C, 335 °C and 675 °C respectively, while at 500 °C calcination temperature, this catalyst showed desorption peaks centered at 120 °C, 275 °C and 730 °C respectively. The low temperature peaks at 115 °C and 120 °C are assigned to weaker Lewis alkaline sites while peaks at 335 °C and 275 °C are allotted to medium Lewis alkaline sites. Moreover, the desorption peaks at 675 °C and 730 °C, in Ni-30LaAl catalyst, indicate the presence of very strong Lewis alkaline sites in catalyst.
In comparison, for Ni-30LaAl catalyst, the biggest peak of very strong basic sites was observed at 500 °C calcination temperature [Fig. 3 (a)] which assured its better coking resistance than catalyst calcined at 700 °C [Fig. 3 (b)].
3.4 Catalytic activity and stability
The catalyst performance of Ni-Al catalyst modified with La2O3in various proportions and calcined at different temperatures (500 and 700 °C) is shown in Tables 3 and 4. The results revealed that the conversionof both CH4and CO2increased with the reaction temperature, thus affirming the endothermicity of reforming reaction. The conversions of CH4, CO2and synthesis gas ratio for the Ni-Al catalyst calcined at 700 °C were 10.2%, 16.6% and 0.4, respectively, at 500 °C reaction temperature. On the other hand, by increasing the reaction temperature to 700 °C the conversions and synthesis gas ratio increased to 80.7%, 86.2% and 0.94 respectively (Table 4).

Table 3 Catalytic activity of the 5% (by mass) Ni/support catalysts calcined at 500 °C

Table 4 Catalytic activity of the 5% (by mass) Ni/support catalysts calcined at 700 °C

Figure 4 Conversions of CH4and CO2versus time on stream for different supports at reaction 700 °C for catalysts calcined at 500 °C
The effect of using mixed supports and calcination temperature on activity and stability of 5% (by mass) Ni supported catalysts for dry reforming of methane was conducted for 9 h at 700 °C as presented in Figs. 4 and 5. In this study Ni-Al and Ni-La catalyst were taken as reference catalysts. Fig. 4 show the conversions of CH4[Fig. 4 (a)] and CO2[Fig. 4 (b)] versus time-on-stream (TOS) for the catalysts calcined at 500 °C. Fig. 4 (a) shows the initial CH4conversion at 700 °C for Ni-La catalyst was the highest followed by mixed catalysts with (30% and 20% La, i.e., Ni-30LaAL and Ni-20LaAl) and catalyst with 10% La while for Ni-Al was the lowest. Additionally from Fig. 4 (a) it is revealed that methane conversion remained stable for mixed supported catalysts, while for Ni-La and Ni-Al conversion decreased continuously.
Regarding the H2/CO ratios Ni-La catalyst, calcined at 500 °C, showed ratio ≥1 while for all other catalysts the ratio was <1. The higher ratio for Ni-La catalyst is actually resulted due to higher conversion of CH4(side reaction 1) than CO2, (Table 3). The occurrence of methane cracking reaction (1) was confirmed by TGA results. In case of CO2conversion [Fig. 4 (b)], Ni-10LaAl showed the highest conversion amongst all. Fig. 5 shows the initial methane conversions for mixed catalysts calcined at 700 °C were higher than that obtained for Ni-Al and/or Ni-La but the methane conversion for Ni-La catalyst increased with time on stream from 81% at 30 min to 86% after 300 min probably due to side reaction (1) i.e., methane decomposition/cracking.
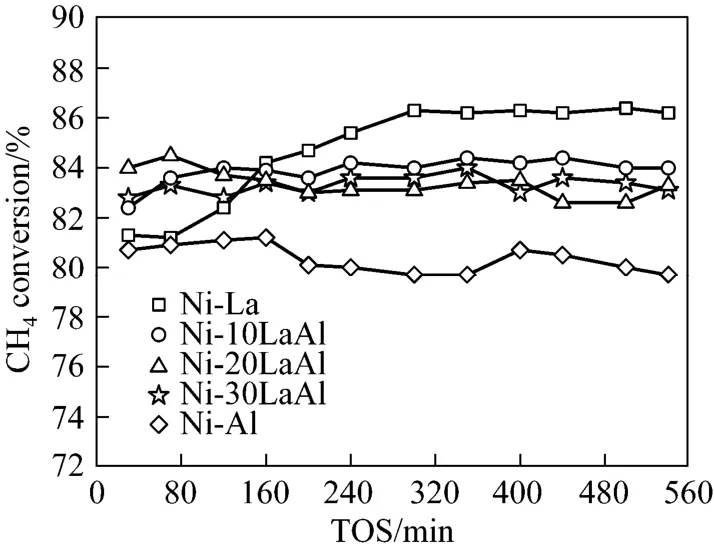
Figure 5 CH4conversion versus time on stream for different supports at reaction 700 °C for catalysts calcined at 700 °C
The real time catalytic activity and stability (before and after regeneration) of Ni-La and Ni-30LaAl catalysts, calcined at 500 °C, were studied by testing these catalysts at 700 °C for 30 h of reaction. Regeneration was carried out under O2atmosphere at 700 °C, but prior to regeneration both of these catalysts were tested for 24 h. After regeneration the catalytic activity was again analyzed for 6 h under the same operating conditions i.e., at 700 °C. The results of catalytic activity, in terms of methane conversion, for 30 h of operation before and after regeneration are presented in Fig. 6. It depicts that overall the CH4conversion is very high for Ni-La catalyst as compared to Ni-30LaAl. In fact that higher CH4conversion was responsible for huge coke deposition over catalyst surface. Additionally the results revealed that for Ni-La catalyst, the CH4conversion decreased until 9 h. This decrease in conversion was probably due to thermal sintering of active metal component. After 9 h, CH4conversion increased again until catalyst regenerated. The increased conversion was attributed to methane cracking side reaction (1). In comparison to Ni-La the Ni-30LaAl catalyst showed a very stable behavior. After regeneration, i.e., gasification of coke deposition, the Ni-30LaAl catalyst almost regained its initial activity while Ni-La could not, which confirms the role of sintering in deactivating Ni-La catalyst. To compare the stability after regeneration the experiments were further continued for 6 h i.e., up to 30 h. After 30 h of operationthere was a big drop in CH4conversion for Ni-La catalyst while only a slight decrease was observed for Ni-30LaAl catalyst (Fig. 6), which assured that Ni-30LaAl is better catalyst as compared to Ni-La.
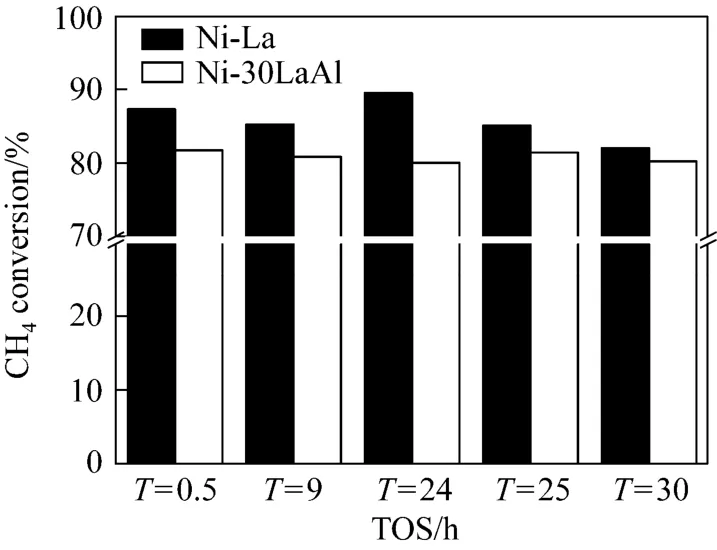
Figure 6 Long term activity and effect of regeneration vs. time on stream for catalysts calcined at 500 °C and reaction at 700 °C
3.5 Effect of La loading
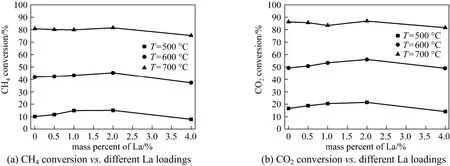
Figure 7 Variations of (a) CH4and (b) CO2conversions with La loadings at different temperatures
The results of adding La as promoter to the Ni-Al catalyst in amount range 0-4% (by mass) using different reaction temperatures (500, 600 and 700 °C) are shown in Fig. 7. As it was expected the conversions of CH4[Fig. 7 (a)] and CO2[Fig. 7 (b)] increased with rise in reaction temperature. This increase is obviously due to nature of reaction i.e., endothermic. Moreover the results revealed that the CO2conversion was higher than CH4conversion which indicates the simultaneous occurrence of reverse water gas shift (RWGS) reaction. The presence of RWGS reactionresulting in smaller H2/CO ratio (<1) (Table 5) because it consumes the slight amount of formed H2.
Additionally, according to the literature, the higher CO2conversion or adsorption over catalyst surface can also favor gasification of coke deposition. Compared to all other La promoted catalysts Ni-La2-Al showed relatively higher H2/CO ratio and the higher conversions of both CH4and CO2(Table 5).
The carbon deposition, which is an ultimate challenge in Ni based catalysts, could be formed over the catalyst in different ways depending on the reaction conditions and the properties of catalyst. In principle, the amount of carbon deposition depends on the nickel quantity present at the catalyst surface and on its particle size. Usually, bigger particles of an active metal are more prone to coking. The pyrolytic carbon is produced over Ni-alumina catalysts, depending on the surface acidity and the components of the support [24, 25]. The promotion of La2O3, in Ni-Al catalyst, actually played dual role in preventing the carbon deposition during dry reforming of methane: (1) it decreased the acidity of the support, thereby stopped pyrolytic carbon formation, and the basic La2O3favored the chemisorption and dissociation of CO2andsubsequently accelerated the carbon elimination by reverse Boudouard reaction [reaction (2)] and (2) the La2O3dispersed over the Al2O3and Ni crystallites prevented the Ni grains from excessive growth at high temperatures, i.e., minimized catalyst sintering due to formation of La2O2CO3[26].

Table 5 Catalytic activity of x% (by mass) La + 5% (by mass) Ni/γ-Al2O3(Ni-Lax-Al catalysts)

Figure 8 O2-TPO profiles for spent catalysts at calcination temperatures of 500 °C and 700 °C Ni-Al; Ni-La; Ni-10LaAl; Ni-20LaAl; Ni-30LaAl
The O2-TPO experiments were executed in order to find the nature and/or type of coke deposits over spent catalysts. It is revealed after experiments that the nature of carbon deposition is remarkably influenced by La2O3loading. Normally, several types of carbon deposition could occur over Ni-based catalysts, for instance atomic carbon, amorphous carbon and graphitic carbon. These carbons can be gasified to CO and/or CO2, under the oxidative atmosphere, at different temperatures: atomic carbon (Cα)<250 °C, filamentous carbon (Cβ) 250-600 °C and graphitic carbon (Cγ)> 600 °C. The first two types are active as compared to graphitic, moreover atomic and filamentous carbon has slower deactivation rate while graphitic, being more inactive, has a rapid deactivation rate [27]. The TPO patterns of spent catalysts, with pure and mixed supports, calcined at 500 °C and 700 °C are shown in Figs. 8 (a) and Fig. 8 (b) respectively. In both cases the formation of graphitic carbon (inactive) was observed for the Ni-Al spent catalyst that is why it showed severe deactivation [Figs. 4 (a) and 5] even though the amount was not very big as compared to others. For all the mixed supported catalysts small amounts of filamentous type carbon deposition were detected, which were also verified by SEM and TGA results (Figs. 9 and 10). In case of Ni-La catalysts both the filamentous as well as graphitic carbons were deposited when calcined at 700 °C [Fig. 8 (b)], while only a huge amount of filamentous carbon was deposited when calcined at 500 °C [Fig. 8 (a)]. The smallest coke deposition, after 9 h of reaction, was observed for Ni-30LaAl catalyst, calcined at 500 °C, which conforms its overall better and stable performance during long term process.
The SEM micrographs of fresh and used catalysts calcined at 500 °C and 700 °C with and without addition of La2O3in γ-Al2O3are shown in Fig. 9. From the micrographs it is clear that the used catalyst with La2O3alone as support [Figs. 9 (b) and 9 (c)] experienced considerable amounts of carbon deposition with filamentous structure.
The La supported catalyst which is calcined at 500 °C showed slightly higher amount of filamentous carbon deposition [Fig. 9 (c)] as compared to catalyst calcined at 700 °C [Fig. 9 (b)]. However, the spent catalysts with the mixed support [Figs. 9 (e) to 9 (f)] were similar to the fresh catalyst [Fig. 9 (d)] in terms of carbon formation as compared to pure La supported catalysts [Figs. 9 (b) and 9 (c)], assuring better performance of mixed supported catalysts. The carbon formation on spent catalyst was, further, quantified using thermogravimetric analysis (TGA) and results are shown in Fig. 10.
Catalysts, with pure supports i.e., Ni-La and Ni-Al, showed highest mass losses due to carbon gasification. Carbon deposition mainly results from any of the four side reactions (1) to (4), among which three reactions [reactions (2) to (4)] are exothermic and favorable at low temperature (<575 °C). So methane cracking reaction (1) is the only responsible one for large amount of carbon deposition over Ni-La catalyst because of its endothermic nature which makes this reaction favorable at high temperature (>575 °C) [28]. On the other hand by combining La2O3and γ-Al2O3, a significant decrease in carbon deposition was observed. It is thus clear that catalysts calcined at 500 °C are less prone to carbon formation as compared to those at 700 °C. Among all the tested catalysts, Ni-30LaAl showed the least mass loss of 3.88%. These results confirmed the advantages of using a mixed support (La2O3and γ-Al2O3).
4 CONCLUSIONS

Figure 9 SEM micrographs of (a) fresh Ni-La calcined at 700 °C; (b) used Ni-La calcined at 700 °C; (c) used Ni-La calcined at 500 °C; (d) fresh Ni-20LaAl calcined at 500 °C (e) used Ni-20LaAl calcined at 500 °C; (f) used Ni-20LaAl calcined at 700 °C
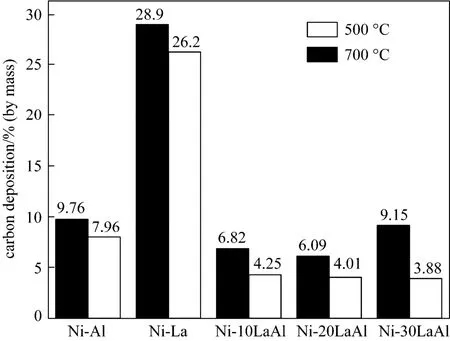
Figure 10 TGA data for carbon deposited over spent catalysts calcined at 500 °C and 700 °C
The effect of using La2O3as a promoter and as a support in dry reforming of methane to produce syngas over Ni-Al catalyst was investigated. In both cases, the CH4and CO2conversions increased with the reaction temperature. The addition of controlled amounts of La2O3as a support with γ-Al2O3and use of La as promoter for Ni/γ-Al2O3not only increased the catalytic activity but also suppressed carbon formation. It also facilitated the adsorption of CO2and Ni sintering through formation of La2O2CO3. The minimum carbon formation and the best stability were attained for the 2% (by mass) La-promoted catalyst, Ni-La2-Al, whereas in case of mixed supported catalyst Ni-30LaAl calcined at 500 °C showed the lowest carbon formation among others.
ACKNOWLEDGEMENTS
The authors eχtend their appreciation to the Deanship of Scientific Research at KSU for funding the work through the research group Project # RGP-VPP-119.
REFERENCES
1 Fan, M.S., Abdullah, A.Z., Bhatia, S., “Utilization of greenhouse gases through carbon dioxide reforming of methane over Ni-Co/MgO-ZrO2: Preparation, characterization and activity studies”, Appl. Catal. B Envir., 100, 365-377 (2010).
2 Atashi, H., Siami, F., Mirzaei, A.A., Sarkari, M., “Kinetic study of Fischer-Tropsch process on titania-supported cobalt-manganese catalyst”, J. Ind. Eng. Chem., 16, 952-961 (2010).
3 Yan, Z., Wang, Z., Bukur, D.B., Goodman, D.W., “Fischer-Tropsch synthesis on a model Co/SiO2catalyst”, J. Catal., 268, 196-200 (2009).
4 Hertwicha, E.G., Aabergb, M., Singha, B., Strømmana, A.H.,“Life-cycle assessment of carbon dioxide capture for enhanced oil recovery”, Chin. J. Chem. Eng., 16, 343-353 (2008).
5 Zhang, X., Wang, B., Liu, Y., Xu, G., “Conversion of methane by steam reforming using dielectric-barrier discharge”, Chin. J. Chem. Eng., 17, 625-629 (2009).
6 Haag, S., Burgard, M., Ernst, B., “Beneficial effects of the use of a nickel membrane reactor for the dry reforming of methane: Comparison with thermodynamic predictions”, J. Catal., 252, 190-204 (2007).
7 Wang, H.Y., Au, C.T., “Carbon dioxide reforming of methane to syngas over SiO2-supported rhodium catalysts”, Appl. Catal. A Gen., 155, 239-252 (1997).
8 Wang, S., Lu, G.Q., “Reforming of methane with carbon dioxide over Ni/Al2O3catalysts: Effect of nickel precursor”, Appl. Catal. A Gen., 169, 271-280 (1998).
9 Nandini, A., Pant, K.K., Dhingra, S.C., “K-, CeO2-, and Mn-promoted Ni/Al2O3catalysts for stable CO2reforming of methane”, Appl. Catal. A Gen., 290, 166-174 (2005).
10 Sutthiumporn, K., Kawi, S., “Promotional effect of alkaline earth over Ni-La2O3catalyst for CO2reforming of CH4: Role of surface oxygen species on H2production and carbon suppression”, Int. J. Hydrogen Energy, 36, 14435-14446 (2011).
11 Pholjaroen, B., Laosiripojana, N., Praserthdam, P., Assabumrungrat, S., “Reactivity of Ni/SiO2·MgO toward carbon dioxide reforming of methane under steady state and periodic operations”, J. Ind. Eng. Chem., 15, 488-497 (2009).
12 Hu, Y.H., Ruckenstein, E., “Catalytic conversion of methane to synthesis gas by partial oxidation and CO2reforming”, Adv. Catal., 48, 297-345 (2004).
13 Xu, J.K., Zhou, W., Wang, J.H., Li, Z.J., Ma, J.X., “Characterization and analysis of carbon deposited during the dry reforming of methane over Ni/La2O3/Al2O3catalysts”, Chin. J. Catal., 30, 1076-1084 (2009).
14 Roh, H.S., Jun, K.W., “Carbon dioxide reforming of methane over Ni catalysts supported on Al2O3modified with La2O3, MgO, and CaO”, Catal. Surv. Asia, 12, 239-252 (2008).
15 Gao, J., Hou, Z., Guo, J., Zhu, Y., Zheng, X., “Catalytic conversion of methane and CO2to synthesis gas over a La2O3-modified SiO2supported Ni catalyst in fluidized-bed reactor”, Catal. Today, 131, 278-284 (2008).
16 Zhang, W.D., Liu, B.S., Zhu, C., Tian, Y.L., “Preparation of La2NiO4/ZSM-5 catalyst and catalytic performance in CO2/CH4reforming to syngas”, Appl. Catal. A Gen., 292, 138-143 (2005).
17 Pour, A.N., Kheirolah, Y.Z., Jozani, J.K., Mehr, J.Y., “The influence of La2O3and TiO2on NiO/MgO/α-Al2O3”, React. Kinet. Catal. Lett., 86, 157-162 (2005).
18 Verykios, X.E., “Catalytic dry reforming of natural gas for the production of chemicals and hydrogen”, Int. J. Hydrogen Energy, 28, 1045-1063 (2003).
19 Tsipouriari, V.A.,Verykios, X.E., “Kinetic study of the catalytic reforming of methane with carbon dioxide to synthesis gas over Ni/La2O3catalyst”, Catal. Today, 64, 83-90 (2001).
20 Martinez, R., Romero, E., Guimon, C., Bilbao, R., “CO2reforming of methane over coprecipitated Ni-Al catalysts modified with lanthanum”, Appl. Catal. A Gen., 274, 139-149 (2004).
21 Al-Fatesh, A.S., Fakeeha, A.H., Abasaeed, A.E., “Effects of selected promoters on Ni/γ-Al2O3catalyst performance in methane dry reforming”, Chin. J. Catal., 32, 1604-1609 (2011).
22 Al-Fatish, A.S.A., Ibrahim, A.A., Fakeeha, A.H., Soliman, M.A., Siddiqui, M.R., Abasaeed, A.E., “Coke formation during CO2reforming of CH4over alumina-supported nickel catalysts”, Appl. Catal. A Gen., 364, 150-155 (2009).
23 Yan, J., Yu, D., Sun, P., Huang, H., “Alkaline earth metal modified NaY for lactic acid dehydration to acrylic acid: Effect of basic sites on the catalytic performance original research article”, Chin. J. Catal., 32, 405-411 (2011).
24 Chen, D., Lødeng, L., Anundskås, A., Olsvik, O., Holmen, A., “Deactivation during carbon dioxide reforming of methane over Ni catalyst: Microkinetic analysis”, Chem. Eng. Sci., 56, 1371-1377 (2001).
25 Horiuchia, T., Hidakab, H., Fukuib, T., Kubob, Y., Horioa, M., Suzukia, K., Moria, T., “Effect of added basic metal oxides on CO2adsorption on alumina at elevated temperatures”, Appl. Catal. A Gen., 167, 195-202 (1998).
26 Gallego, G.S., Mondrago, F., Tatibouët, J.M., Barrault, J., Batiot-Dupeyrat, C., “Carbon dioxide reforming of methane over La2NiO4as catalyst precursor—Characterization of carbon deposition”, Catal. Today, 133-135, 200-209 (2008).
27 Hao, Z., Zhu, Q., Jiang, Z., Hou, B., Li, H., “Characterization of aerogel Ni/Al2O3catalysts and investigation on their stability for CH4-CO2reforming in a fluidized bed”, Fuel Process. Technol., 90, 113-121 (2009).
28 Nikoo, M.K., Amin, N.A.S., “Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation”, Fuel Process. Technol., 92, 678-691 (2011).
10.1016/S1004-9541(14)60029-X
2012-11-08, accepted 2013-04-26.
* To whom correspondence should be addressed. E-mail: aalfatesh@ksu.edu.sa
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Influence of Design Margin on Operation Optimization and Control Performance of Chemical Processes*
- Measurement and Modeling for the Solubility of Hydrogen Sulfide in Primene JM-T*
- Enhancing Structural Stability and Pervaporation Performance of Composite Membranes by Coating Gelatin onto Hydrophilically Modified Support Layer*
- Photocatalytical Inactivation of Enterococcus faecalis from Water Using Functional Materials Based on Natural Zeolite and Titanium Dioxide*
- A Group Contribution Method for the Correlation of Static Dielectric Constant of Ionic Liquids*
- Interaction Analysis and Decomposition Principle for Control Structure Design of Large-scale Systems*
