Relative Chemical Shifts for Obtaining Accurate Chemical Shifts of Hydrogen Atoms in CH Groups
2014-07-11RongZhngJunLiWenjunWu
Rong Zhng,Jun Li,Wen-jun Wu
a.College of Pharmacy,Guangdong Pharmaceutical University,Guangzhou 510006,China
b.Department of Chemistry,Guangdong University of Education,Guangzhou 510303,China (Dated:Received on September 4,2013;Accepted on November 12,2013)
Relative Chemical Shifts for Obtaining Accurate Chemical Shifts of Hydrogen Atoms in CH Groups
Rong Zhanga∗,Jun Lib,Wen-juan Wua
a.College of Pharmacy,Guangdong Pharmaceutical University,Guangzhou 510006,China
b.Department of Chemistry,Guangdong University of Education,Guangzhou 510303,China (Dated:Received on September 4,2013;Accepted on November 12,2013)
Accurate chemical shifts of hydrogen atoms in CH groups are difficult to obtain.To solve this problem,relative chemical shifts are introduced.Internal and external standard methods were used to measure the chemical shifts in a whole-concentration of N-methylacetamidewater system.Determination of the chemical shifts of hydrogen atoms,especially those of CH groups,according to the two methods yielded signif i cant dif f erences.Relative chemical shifts were proven to be independent of the reference and may be applied to other systems.
Key words:Relative chemical shift,CH group,Internal and external method
I.INTRODUCTION
Nuclear magnetic resonance(NMR)spectroscopy is a highly powerful technique to investigate structures and interactions in mixtures[1-10].However,spectral data over the entire composition range of binary mixtures, especially in aqueous mixtures,are limited.Obtaining accurate concentration-and temperature-dependent chemical shifts is difficult[11-13].NMR measurements are usually obtained by internal and external standard methods.The chemical shifts of some aqueous solutions have been measured by the external reference method [14-17].This method,especially the external double reference method,can obtain generally accurate chemical shifts.In this method,the reference is separated from the sample solution;thus,no interactions occur between the sample and the reference.However,the values of the chemical shifts must be corrected for magnetic susceptibility[18].The method also requires special devices and experimental skills.The internal reference method is often adopted to qualitatively measure the concentration-and temperature-dependence of chemical shifts[19-22].In this method,the reference is added into the sample solution,and the values of the chemical shifts do not need to be corrected.However, the reference can interact with the sample,which will probably af f ect the chemical shifts.Thus,neither the internal nor the external reference methods can determine accurate chemical shifts.
The chemical shifts of hydrogen atoms,which can form traditional strong hydrogen bonds,are greatly affected by temperature or concentration.These changes are not signif i cantly af f ected by dif f erent measurement methods.For hydrogen atoms in CH groups,changes in chemical shifts with temperature or concentration are minimal.These changes are signif i cantly af f ected by the used method.Accurate chemical shifts of alkyl protons are difficult to obtain.Thus,an approach to obtain accurate chemical shifts in CH groups is necessary.
In the present work,we use the internal and external reference methods to measure the chemical shifts of a whole-concentration N-methylacetamide(NMA)-water mixture.This mixture is extensively investigated as a model of peptide bonds at dif f erent temperatures [23-25].
II.EXPERIMENTS
Chemical shifts were measured using a Bruker DMX 500 spectrometer operating at 500 MHz at dif f erent temperatures and with an accuracy of±0.1 K.Both internal and external methods were used in the NMR experiment.In the internal reference method,a 2 mm capillary tube,in which deuterated dimethyl sulfoxide (DMSO-d6)was sealed,was placed at the center of a 5 mm sample tube f i lled with the chemical shift reference,sodium 2,2-dimethyl-2-silapentane-5-sulfonate, and the sample solution.In the external reference method,a 2 mm capillary tube,in which DMSO-d6and tetramethylsilane were sealed,was placed inside the 5 mm tube f i lled with the sample solution.The1H NMR spectra of the NMA-water mixture at temperatures of 308,323,and 338 K were measured using the internal and external reference methods.
III.RESULTS AND DISCUSSION
A.Chemical shifts using two methods
Dif f erent types of hydrogen atoms are present in an NMA molecule.NMA molecules contain amide hydro-gen atoms and CH groups.Figure 1 shows the structure of an NMA molecule,the def i nitions of the hydrogen atom symbols are as follows:HN is the amide hydrogen atom in NMA,HC1is hydrogen atoms in CH group connected with C=O,and HC2is hydrogen atoms in CH group connected with amide.Hydrogen atoms in the amide group can form traditional hydrogen bonds, and hydrogen atoms in the CH group can form weak C-H···O bonds[26-30].Hydrogen bonding interactions are sensitive to temperature and concentration. The chemical shifts of good donors,such as amide protons,change greatly with the concentration,and the ef f ect of the reference may be ignored.However,the change in chemical shifts of alkyl protons is minimal over the entire concentration measured.Strong hydrogen bonds lead to greater shifts than weak hydrogen bonds.
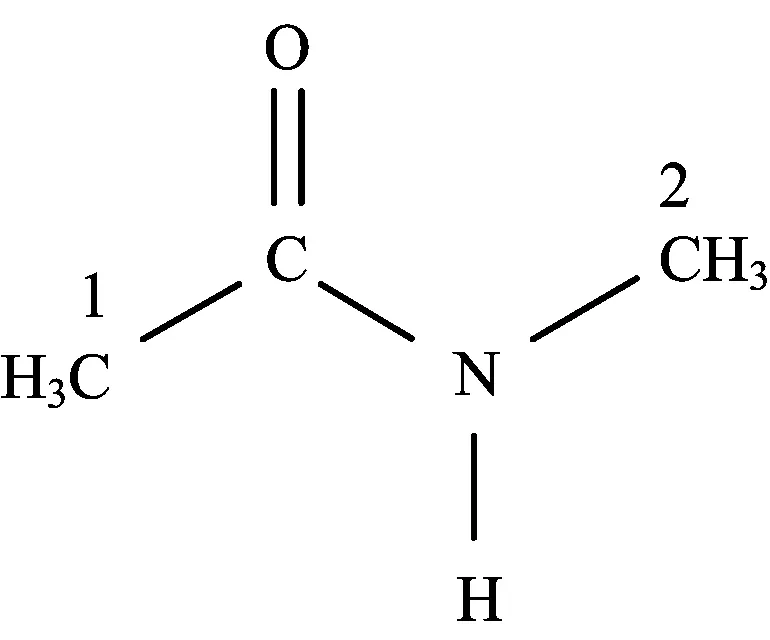
FIG.1 Structure of NMA molecule.
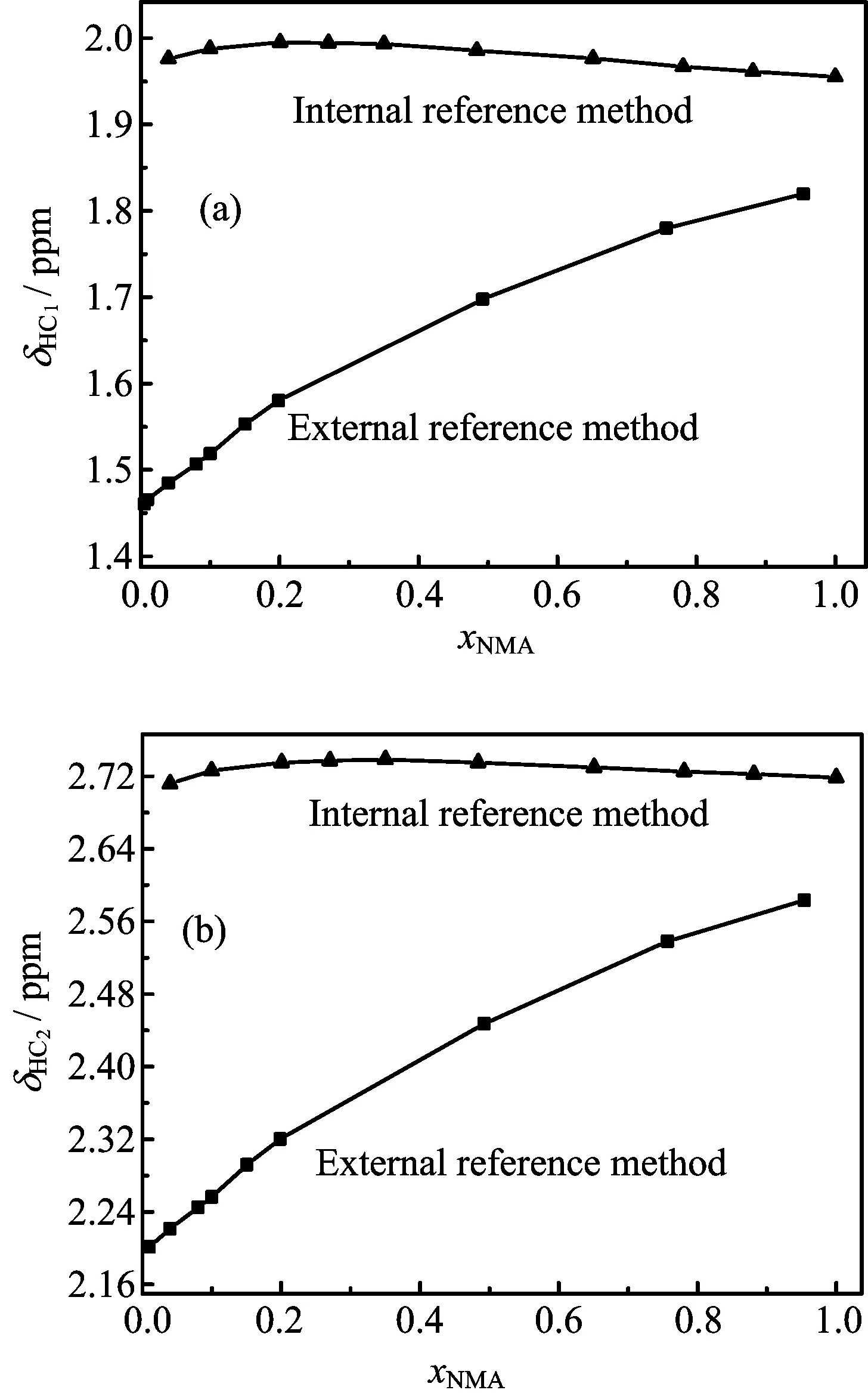
FIG.2 Chemical shifts of dif f erent alkyl hydrogen atoms in the NMA-water mixtures against xNMAat 308 K by two dif f erent methods.(a)Chemical shifts of HC1atom(δHC1). (b)Chemical shifts of HC2atom(δHC2).
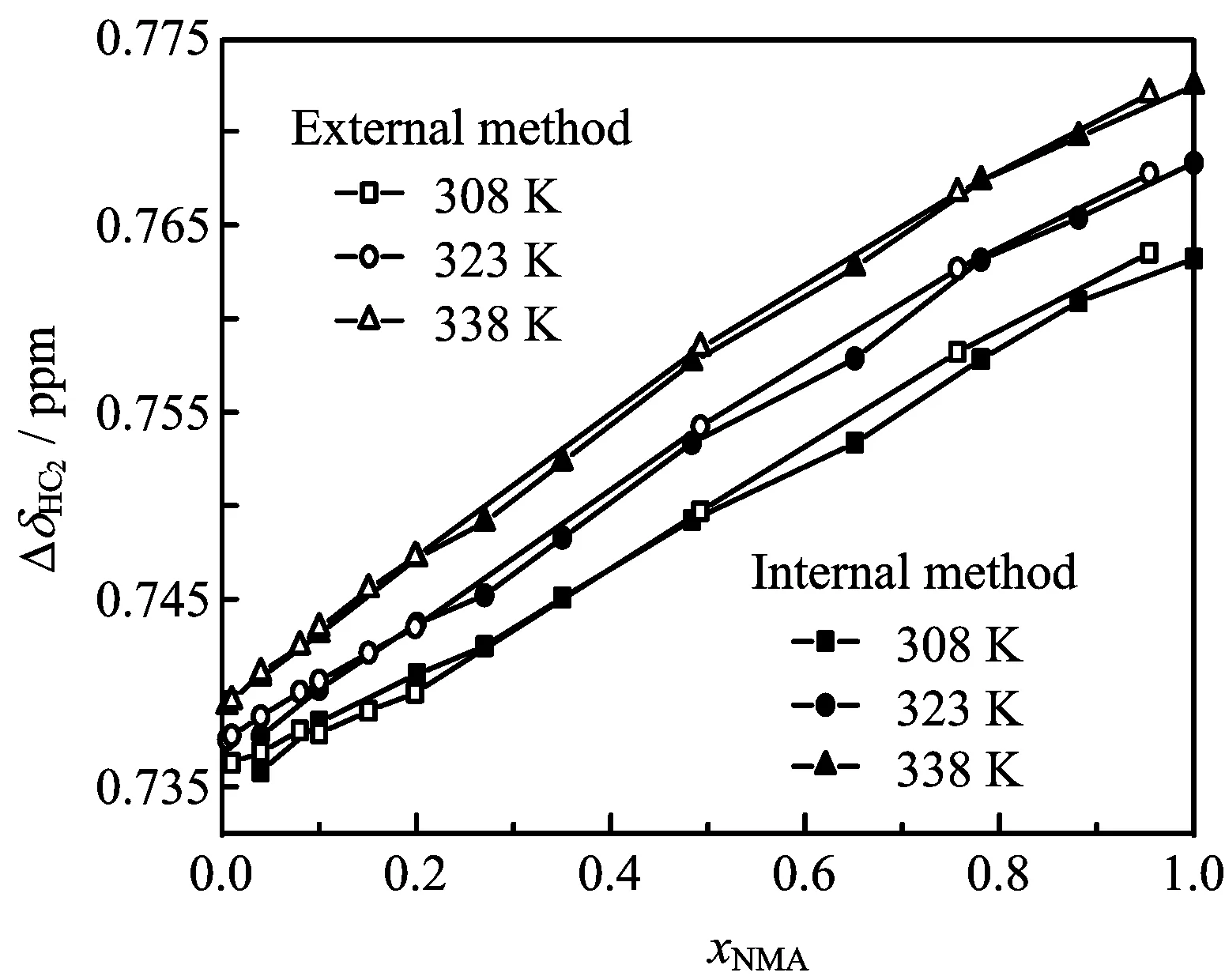
FIG.3 Relative chemical shifts with temperature dependence of the HC2atom(∆δHC2)by the two methods.
Figure 2 shows the chemical shifts of alkyl protons obtained using the internal and external reference methods against concentration at a temperature of 308 K. The chemical shifts obtained using the external reference methods are not corrected for magnetic susceptibility.The chemical shifts of alkyl protons(δHC2and δHC1)show signif i cant variations in values and tendencies.These results imply that the used method greatly af f ects the value of chemical shifts in CH groups.Finding an approach to solve this problem is thus necessary.
B.Relative chemical shifts in NMR
To avoid variations in chemical shifts obtained using dif f erent methods,the relative chemical shift is used. This shift is def i ned as follows:

where∆δ is the relative chemical shift,δ is the direct value of the chemical shifts obtained in the NMR experiment,and δrefdenotes the chemical shifts of the reference standard.The chemical shifts of alkyl protons can be easily af f ected by changes in the reference. Compared with the chemical shifts of the HC1atom, the relative chemical shifts(∆δHC1)are independent of the reference because the HC1,HC2,and amide hydrogen atoms are in the same NMA molecule.Thus,δHC1was used as a reference standard in this work.
Figure 3 presents the ef f ects of concentration and temperature on the relative chemical shifts of alkyl protons(∆δHC2)determined using the external and internal reference methods.Values of∆δHC2obtained using the two methods against concentration with temperature dependence are close.The relative chemical shifts of amide hydrogen atoms(∆δHN)are shown in Fig.4. The deviations between the two methods of both∆δHC2and∆δHNare less than 0.005 ppm,and the precision of the NMR apparatus is±0.005 ppm.Thus,variations in the relative chemical shifts of the HC2atoms are independent of the reference.
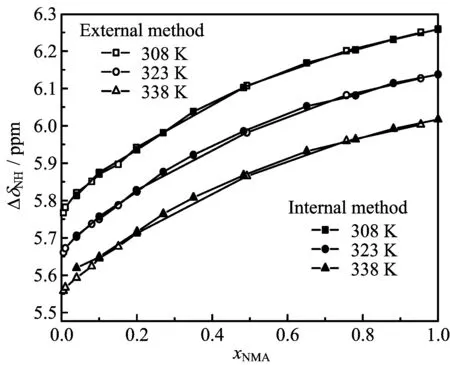
FIG.4 Relative chemical shifts with temperature dependence of the amide hydrogen atom(∆δNH)by the two methods.
IV.CONCLUSION
External and internal reference methods are used to obtain the chemical shifts of the hydrogen atoms in an NMA-water system.The chemical shifts obtained using the two methods exhibit very dif f erent values and tendencies.Relative chemical shifts are thus introduced to solve these variations,and the chemical shifts of HC1atoms are used as a reference standard.The deviations of the relative chemical shifts between the two methods are less than 0.005 ppm.Changes in the relative chemical shifts of the HC2atoms are independent of the reference.If the chemical shifts of one hydrogen atom are chosen as a reference standard,the relative chemical shifts of other hydrogen atoms in the same molecule will be independent of this reference.Relative chemical shifts solve the problem of variations in chemical shifts obtained using dif f erent measurement methods.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.20903026),the Talents Introduction Foundation for Universities of Guangdong Province(No.2011),Scientif i c Research Foundation of the Natural Science Foundation of Guangdong Province,China(No.S2011010002483),and Science and Technology Planning Project of Guangzhou (No.2013J4100071).
[1]J.Czernek,T.Pawlak,and M.J.Potrzebowski,Chem. Phys.Lett.527,31(2012).
[2]A.Bornet,P.Ahuja,R.Sarkar,L.Fernandes,S.Hadji, S.Y.Lee,A.Haririnia,D.Fushman,G.Bodenhausen, and P.R.Vasos,ChemPhysChem 12,2677(2011).
[3]R.Dringen,Y.Koehler,L.Derr,G.Tomba,M.M. Schmidt,L.Treccani,L.C.Ciacchi,and K.Rezwan, Langmuir 27,9449(2011).
[4]L.A.Odell and I.L.Moudrakovski,Chem.Phys.Lett. 565,56(2013).
[5]D.S.Kummli,H.M.Frey,and S.Leutwyler,Chem. Phys.367,36(2010).
[6]A.Krezel,J.W´ojcik,M.Maciejczyk,and W.Bal,Inorg. Chem.50,72(2011).
[7]P.Maes,Y.B.Monakhova,T.Kuballa,H.Reusch,and D.W.Lachenmeier,J.Agric.Food Chem.60,2778 (2012).
[8]J.W.Peng,J.Phys.Chem.Lett.3,1039(2012).
[9]K.Jackowski,M.Jaszun´nski,and M.Wilczek,J.Phys. Chem.A 114,2471(2010).
[10]G.F.Pauli,T.G¨odecke,B.U.Jaki,and D.C.Lankin, J.Nat.Prod.75,834(2012).
包括宏观经济因素和微观经济因素。宏观经济因素主要指人口、国民收入等,而微观经济因素主要指企业所在地的居民收入水平和消费偏好及就业程度等。我国涉农企业发展环境中经济方面的因素主要有利率变动、税率、贷款的难易程度等。人民币利率的变动,极大地影响着涉农企业的发展。利率升高,在吸引居民增加储蓄的同时,间接增加了居民的利息收入,增加了消费需求和投资需求,促进了涉农企业的发展。
[11]K.Mizuno,S.Imafuji,T.Ochi,T.Ohta,and S.Maeda, J.Phys.Chem.B 104,11001(2000).
[12]G.Manley,I.Rivalta,and J.P.Loria,J.Phys.Chem. B 117,3063(2013).
[13]A.Piserchio,M.Warthaka,A.K.Devkota,T.S. Kaoud,S.Lee,O.Abramczyk,P.Ren,K.N.Dalby, and R.Ghose,Biochemistry 50,3660(2011).
[14]Y.Lei,H.Li,H.Pan,and S.Han,J.Phys.Chem.A 107,1574(2003).
[15]Y.Han,J.Ahn,J.Concel,I.L.Byeon,A.M.Gronenborn,J.Yang,and T.Polenova,J.Am.Chem.Soc. 132,1976(2010).
[16]K.Mizuno,Y.Kimura,H.Morichika,Y.Nishimura,Y. Shimada,S.Maeda,S.Imafuji,and T.Ochi,J.Mol. Liq.85,139(2000).
[17]K.Mizuno,Y.Masuda,T.Yamamura,J.Kitamura,H. Ogata,I.Bako,Y.Tamai,and T.Yagasaki,J.Phys. Chem.B 113,906(2009).
[19]R.Zhang,H.Li,Y.Lei,and S.Han,J.Phys.Chem.B 109,7482(2005).
[20]R.Novoa-Carballal,E.Fernandez-Megia,andR. Riguera,Biomacromolecules 11,2079(2010).
[21]R.Zhang,W.Zeng,X.Meng,J.Huang,and W.Wen, Chem.Phys.402,130(2012)
[22]S.A.Nu˜nez and M.A.Hickner,ACS Macro.Lett.2, 49(2013).
[23]H.Yu,C.L.Mazzanti,T.W.Whitf i eld,R.E.Koeppe, O.S.Andersen,and B.Roux,J.Am.Chem.Soc.132, 10847(2010).
[24]Y.Fang,S.Shigeto,N.Seong,and D.D.Dlott,J.Phys. Chem.A 113,75(2009).
[25]T.Takekiyo,Y.Yoshimura,Y.Ikeji,N.Hatano,and T. Koizumi,J.Phys.Chem.B 112,13355(2008).
[26]R.Zhang and W.Wu,J.Mol.Liq.162,20.(2011).
[27]X.Gao,Y.Liu,H.Li,J.Bian,Y.Zhao,Y.Cao,Y. Mao,X.Li,Y.Xu,Y.Ozaki,and J.Wu,J.Mol.Struct. 1040,122(2013).
[28]R.Zhang,H.Li,Y.Lei,and S.Han,J.Phys.Chem.B 108,12596(2004).
[29]P.Barczy´nski,Z.Dega-Szafran,A.Katrusiak,and M. Szafran,J.Mol.Struct.1000,127(2011).
[30]F.Chen,L.Selvam,and F.Wang,Chem.Phys.Lett. 493,358(2010).
∗Author to whom correspondence should be addressed.E-mail:zhangr-zju@hotmail.com
