肿瘤干细胞来源的DC-CIK对同源肿瘤细胞的杀伤作用
2014-07-05张腾月王长利
庞 冲 张腾月 王长利△
肿瘤干细胞来源的DC-CIK对同源肿瘤细胞的杀伤作用
庞 冲1张腾月2王长利1△
目的 观察干细胞负载树突细胞诱导的杀伤细胞(CSC-DC-CIK)作为效应细胞对同源肿瘤细胞的杀伤作用,探讨CSC抗原参与肿瘤杀伤作用的可行性。方法培养肾癌细胞株A498和肺癌细胞株A549,用流式分选术分离纯化CD133+细胞,分别作为肾癌干细胞(KSC)和肺癌干细胞(LSC),冻融法制备抗原。提取健康产妇脐带血的单个核细胞,体外扩增诱导生成DC和CIK细胞。分别用上述CSC抗原负载DC,与CIK共培养(CSC-DC-CIK),流式细胞术分析DC和CIK细胞免疫表型,ELISA法检测细胞因子分泌水平,用乳酸脱氢酶(LDH)释放法检测CSCDC-CIK对同源肿瘤细胞的杀伤效率。结果CSC-DC的DC免疫表型CD40+、CD80+、CD86+及HLA-DR+的表达均高于单纯DC的相应免疫表型的表达(P<0.01);DC、CSC-DC与CIK共培养后的DC免疫表型CD40+、CD80+、CD86+及HLA-DR+的表达均高于共培养前(P<0.01);CSC-DC与CIK共培养后的DC免疫表型CD40+、CD80+、CD86+及HLA-DR+的表达高于DC与CIK共培养后的相应免疫表型(P<0.01);DC、CSC-DC与CIK共培养后的CIK免疫表型CD3+、CD8+、CD56+的表达高于共培养前(P<0.01);CSC-DC与CIK共培养后的CIK免疫表型CD3+、CD8+、CD56+的表达高于DC与CIK共培养后的CIK相应免疫表型(P<0.01);DC、CSC-DC与CIK共培养后的IFN-γ、TNF-α和IL-2分泌水平高于共培养前(P<0.01);CSC-DC与CIK共培养后的IFN-γ、TNF-α和IL-2分泌水平高于DC与CIK共培养后的相应细胞因子表达(P<0.01);KSC-DC-CIK组和LSC-DC-CIK组对靶细胞的杀伤率为(50.21±4.24)%和(49.32±3.89)%,明显高于DC-CIK组的(30.25±3.11)%(F=89.157,P<0.01)。结论CSC抗原负载DC活化CIK (CSC-DC-CIK)对同源肿瘤细胞有更好的杀伤作用,对其作用机制和临床应用的可能性尚需深入研究。
肿瘤干细胞;树突细胞;免疫疗法;肾肿瘤;肺肿瘤;细胞因子诱导的杀伤细胞
近年来有关肿瘤生物免疫治疗领域的研究极为活跃,成为继手术、化疗、放疗之外不可忽视的治疗手段,包括细胞因子、单克隆抗体及各种免疫细胞等,并且取得了显著效果。其中树突状细胞(DC)联合细胞因子诱导的杀伤细胞(DC-CIK)过继免疫治疗具有很好的抗肿瘤作用,DC-CIK共同培养可明显提高CIK的增殖活性和细胞毒作用,提高患者自身免疫力,其安全性及疗效已经在转移性黑素瘤[1]、晚期非小细胞肺癌[2]、白血病[3]的治疗中得到了验证。肿瘤干细胞(cancer stem cell,CSC),具有无限自我更新及分化的潜能,是肿瘤发生发展、转移复发和放化疗抗性等产生的根源,成为恶性肿瘤研究的热点[4]。本研究从肾癌细胞A498和肺癌细胞A549分离肿瘤干细胞制备抗原负载DC,活化CIK,观察CSC-DC-CIK作为效应细胞对同源肿瘤细胞的杀伤作用,探讨CSC抗原参与肿瘤杀伤作用的可行性。
1 材料与方法
1.1 细胞株、试剂及仪器 肾癌细胞株A498和肺癌细胞株A549,本室保存。RPMI-1640培养基(赛默飞世尔生物化学制品有限公司);新生胎牛血清(FCS)、淋巴细胞分离液(天津血液研究所试剂公司);异硫氰酸荧光素(FITC)-CD40、APC-HLA-DR、PE-CD80、PE-CD86,叶绿素蛋白(Percp)-cy5.5-CD56、PE-CD8、FITC-CD3(美国BD Bioscience公司),重组人干细胞生长因子(rhSCF)、重组人FMS样酪氨酸激酶3(FLT3)配体、重组人粒细胞巨噬细胞刺激因子(rhGMCSF)、重组人白介素-4(rhIL-4)、重组肿瘤坏死因子-α(rhTNF-α)、重组人白介素-2(rhIL-2)、重组干扰素(rhINF)-γ(英国Peprotech公司);抗人CD3单抗(武汉生物制品研究所)。流式分析仪(美国BD公司),CO2孵箱(Thermo Forma公司)。
1.2 方法
1.2.1 肿瘤干细胞冻融抗原的制备 流式分析仪分别收集肾癌细胞A498和肺癌细胞A549的CD133+细胞,前者作为肾癌肿瘤干细胞(KSC),后者作为肺癌肿瘤干细胞(LSC),调整细胞浓度为1×107∕mL,加入500 μL生理盐水中,置液氮中5 min,迅速放入37℃水浴中,反复3次,离心、微孔滤膜过滤除菌,4℃保存备用。
1.2.2 细胞活率的测定 用Hanks液配制0.1%台盼蓝溶液;用0.5%胰蛋白酶∶0.2%EDTA=1∶1混合液来消化培养的贴壁细胞;加入适量Hanks液制成细胞悬液;每0.1 mL细胞悬液约加新鲜配制的染液一小滴,室温下染3~5 min;染色过的细胞材料,取一滴细胞悬液置玻片上,加盖玻片后放高倍镜下观察;计数1 000个细胞中的活细胞和死细胞数目;统计未染色细胞。细胞活率(%)=未染色的细胞数∕观察的细胞总数×100%。
1.2.3 单个核细胞的收集和DC体外诱导培养 经本人知情同意,采用健康产妇新鲜的脐带血,使用淋巴细胞分离液梯度离心法获取单个核细胞。将单个核细胞置于含10%新生牛血清的RPMI-1640完全培养基中,收集黏附细胞,加培养液调细胞浓度为1×106∕mL,加入rhGM-CSF和rhIL-4,培养至第5天时,用上述制备的冻融抗原以1∶5的比例进行冲击,即为CSC-DC(分别命名为KSC-DC或LSC-DC),继续培养2 d,与未冲击的DC均于第7天加入肿瘤坏死因子(TNF)-α。培养结束后用荧光倒置显微镜观察细胞形态,并流式细胞术进行CSC-DC和DC的表型检测。
1.2.4 CIK细胞的分离及培养 收集单个核细胞中的非黏附细胞,用RPMI-1640完全培养基调细胞数为3×106∕mL,加入rhINF-γ,刺激24 h后,加入抗CD3单抗及rhIL-2;培养至第7天将CIK细胞分为4组,分别是冻融抗原冲击的DC 与CIK共培养组(KSC-DC-CIK组和LSC-DC-CIK组)、未冲击的DC与CIK共培养组(DC-CIK组)及CIK对照组,DC 与CIK细胞按照1∶10比例进行共培养。第10天后收集上述各组细胞,采用流式细胞术进行免疫表型检测;倒置显微镜观察细胞形态。
1.2.5 细胞因子的检测 取上述各组细胞上清液用酶联免疫吸附测定(ELISA)试剂盒进行干扰素(IFN)-γ、TNF-α和白细胞介素(IL)-2水平的检测,具体操作按试剂盒说明书进行。
1.2.6 对同源肿瘤细胞的杀伤作用 采用乳酸脱氢酶(LDH)释放法测定上述各组细胞对同源肿瘤细胞的杀伤作用,将CSC-DC-CIK作为效应细胞,并调整细胞浓度为1× 106∕mL,同源肿瘤细胞作为靶细胞,调细胞浓度为1×105∕mL。LDH释放法分别测定各实验组在效靶比20∶1的杀伤活性。设3个复孔,用酶标仪在490 nm波长下检测其光密度(OD)值。按以下公式计算杀伤率:杀伤率(%)=(实验组OD值-效应细胞自然释放OD值-靶细胞自然释放OD值)∕(靶细胞最大释放OD值-靶细胞自然释放OD值)×100%。
1.3 统计学方法 采用SPSS 18.0进行统计处理,计量数据用均数±标准差(±s)表示,组间比较采用方差分析,多重比较采用LSD-t法。组内共培养前后比较采用配对t检验,P<0.05为差异有统计学意义。
2 结果
2.1 肿瘤干细胞冻融抗原 倒置显微镜下KSC和LSC细胞形态一致,均为圆形,胞体较小,形似淋巴细胞,见图1。台盼蓝染色显示其细胞活率>95%。
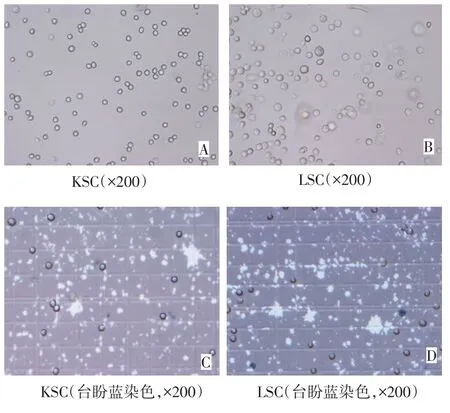
Fig.1 Microscopic morphologies of KSC and LSC图1 显微镜下KSC和LSC细胞形态
2.2 CSC-DC的培养与形态 新分离的脐带血单个核细胞呈球形散在分布,表面光滑;加入细胞因子组合培养2 d后,倒置显微镜下可见部分细胞悬浮并发生聚集样增生;培养第5天,出现细胞体积增大,并出现贴壁细胞,数量增多,多形性细胞增多。第5天用冻融抗原进行冲击后大部分细胞聚集成团,胞体较大,培养7 d后具有树突状突起的细胞增多且突起更为明显,见图2。
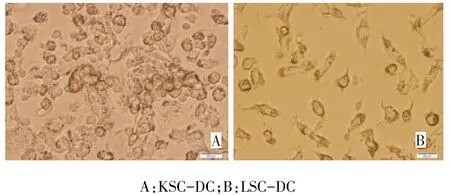
Fig.2 Microscopic morphology of dendritic cells induced from mononuclear cells isolated from cord blood after been pulsed with KSC and LSC frozen-thawed antigen(×400)图2 冻融抗原从脐带血单个核细胞诱导出的树突状细胞(×400)
2.3 CIK细胞的培养与鉴定 CIK细胞培养第4天开始数量倍增,体积开始增大,细胞核大而圆,部分细胞聚集成簇。培养10 d后CIK成熟表型检测结果为CD3+细胞占(68.321±3.891)%,CD3+CD8+细胞占(36.792±2.822)%,CD3+CD56+细胞占(25.334± 2.652)%,提示细胞达到成熟程度,见图3。
2.4 免疫表型表达的测定结果 KSC-DC组和LSC-DC组CD40+、CD80+、CD86+及HLA-DR+的表达均高于DC的相应免疫表型的表达(P<0.01);DC、 CSC-DC与CIK共培养后的DC免疫表型CD40+、CD80+、CD86+及HLA-DR+的表达均高于共培养前(P<0.01);KSC-DC和LSC-DC与CIK共培养后的DC免疫表型CD40+、CD80+、CD86+及HLA-DR+的表达高于DC与CIK共培养后的相应免疫表型(P<0.01),见表1。DC-CIK、KSC-DC-CIK和LSC-DCCIK组的CIK免疫表型CD3+、CD3+CD8+、CD3+CD56+的表达高于CIK组(P<0.01);KSC-DC-CIK和LSC-DC-CIK组的CIK免疫表型CD3+、CD8+、CD56+的表达高于DC-CIK组(P<0.01);KSC-DC-CIK组和LSC-DC-CIK组间差异无统计学意义(P>0.05),见表2。
2.5 细胞因子分泌水平的测定结果 DC-CIK、KSC-DC-CIK和LSC-DC-CIK组的IFN-γ、TNF-α 和IL-2表达高于CIK组(P<0.01);KSC-DC-CIK 和LSC-DC-CIK组的IFN-γ、TNF-α和IL-2表达高于DC-CIK组(P<0.01);KSC-DC-CIK组和LSCDC-CIK组TNF-α和IL-2表达的差异无统计学意义(P>0.05),见表3。
2.6 CSC-DC-CIK对同源肿瘤细胞的杀伤作用 KSC-DC-CIK组和LSC-DC-CIK组对靶细胞的杀伤率为(50.21±4.24)%和(49.32±3.89)%,明显高于DC-CIK组的(30.25±3.11)%(F=89.157,P<0.01),KSC-DC-CIK组和LSC-DC-CIK组对肾癌和肺癌细胞的杀伤作用差异无统计学意义(P>0.05)。
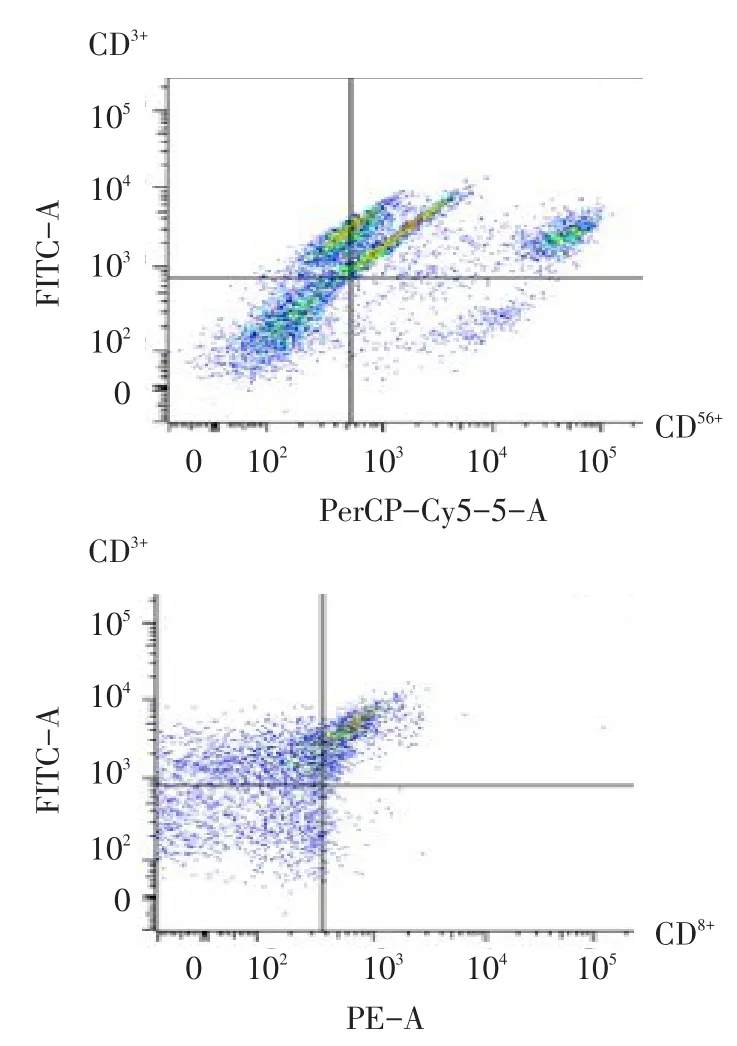
Fig.3 Phenotypes of CIK analyzed by flow cytometry图3 CIK细胞表型的流式细胞术检测分析图
Tab.1 Phenotype analysis of CIK before and after co-culture表1 与CIK共培养前后DC表型分析 (n=10,%,±s)
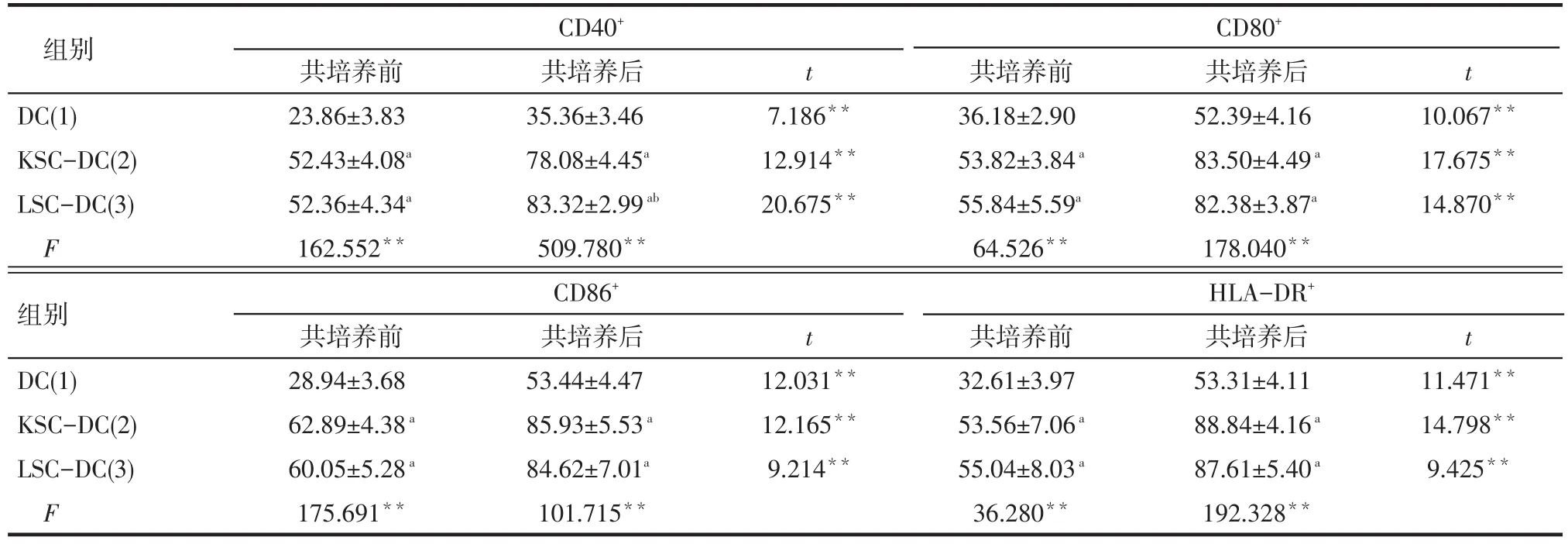
Tab.1 Phenotype analysis of CIK before and after co-culture表1 与CIK共培养前后DC表型分析 (n=10,%,±s)
**P<0.01;a与(1)组比较,b与(2)组比较,P<0.01;表2同
组别DC(1) KSC-DC(2) LSC-DC(3) F组别DC(1) KSC-DC(2) LSC-DC(3) F CD40+共培养前23.86±3.83 52.43±4.08a52.36±4.34a162.552**CD86+共培养前28.94±3.68 62.89±4.38a60.05±5.28a175.691**共培养后35.36±3.46 78.08±4.45a83.32±2.99ab509.780**t t 7.186**12.914**20.675**共培养后52.39±4.16 83.50±4.49a82.38±3.87a178.040**10.067**17.675**14.870**共培养后53.44±4.47 85.93±5.53a84.62±7.01a101.715**t t 12.031**12.165**9.214**CD80+共培养前36.18±2.90 53.82±3.84a55.84±5.59a64.526**HLA-DR+共培养前32.61±3.97 53.56±7.06a55.04±8.03a36.280**共培养后53.31±4.11 88.84±4.16a87.61±5.40a192.328**11.471**14.798**9.425**
Tab.2 Phenotype analysis of CIK before and after been co-cultured with DC表2 与DC共培养结束后CIK表型分析(n=10,%,±s)
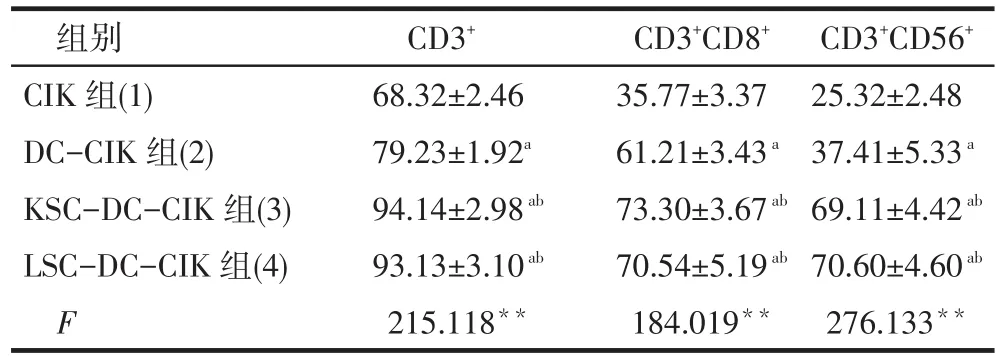
Tab.2 Phenotype analysis of CIK before and after been co-cultured with DC表2 与DC共培养结束后CIK表型分析(n=10,%,±s)
?
Tab.3 Cytokines levels in cell supernatant after co-culture表3 共培养后4组细胞上清中细胞因子的分泌水平(n=10,ng∕L,±s)

Tab.3 Cytokines levels in cell supernatant after co-culture表3 共培养后4组细胞上清中细胞因子的分泌水平(n=10,ng∕L,±s)
**P<0.01;a与(1)组比较,b与(2)组比较,c与(3)组比较,P<0.01
组别CIK组(1) DC-CIK组(2) KSC-DC-CIK组(3) LSC-DC-CIK组(4) F IFN-γ 871.05±82.46 1 532.51±60.23a3 238.14±246.50ab3 511.28±354.44abc337.212**TNF-α 326.67±55.89 498.31±43.07a986.67±73.00ab1 034.53±77.51ab305.320**IL-2 108.20±17.97 214.18±24.76a439.33±37.39ab418.90±31.84ab309.024**
3 讨论
DC是功能最强的抗原递呈细胞,能激活初始T细胞,激发特异性免疫应答和促进非特异性细胞毒杀伤,是抗肿瘤免疫的重要载体。DC诱导培养CIK可以使CIK细胞中的免疫抑制T细胞降低,达到增强CIK细胞的杀伤作用[5],而且体外扩增速度快,杀瘤谱广,对耐药肿瘤细胞敏感[6],是一种比较理想的具有细胞毒作用的免疫活性细胞。DC-CIK共同培养可明显提高CIK的增殖活性和细胞毒作用,体内外研究均表明DC-CIK过继免疫治疗具有很好的抗乳腺癌[7-8]、结肠癌[9]、肝癌作用[10]。谭洁等[11]报道DC-CIK细胞体外能有效杀伤肾癌细胞786-0和慢性粒细胞白血病细胞K562,输注后可提升患者的免疫水平,可作为晚期肿瘤患者的辅助治疗。王赛男等[12]也发现DC-CIK免疫治疗能改善肾癌患者的免疫抑制状态,提高机体的抗肿瘤免疫效应,有较好的近期疗效。陈诗萍等[13-14]等发现树突细胞诱导的CIK细胞能够有效抑制肺癌移植瘤生长,有望成为非小细胞肺癌有效的过继免疫治疗方法。本文未进行DC-CIK对比CIK的体外杀伤肿瘤实验,但通过对比DC与CIK共同培养前后DC免疫表型、CIK免疫表型及细胞因子分泌水平,可以推测出DC-CIK共同培养可以明显提高抗肿瘤效果,和前述文献结果一致。
CSC是影响肿瘤治疗效果和转归的重要因素之一,鉴于其对放化疗的抗性,故免疫治疗具有独特的优势[15]。理论上推测,CSC应具有一般肿瘤细胞抗原和CSC特有的抗原,以CSC抗原负载DC激活CIK,有望达到对一般肿瘤细胞和CSC的特异性杀伤作用。唐家平等[16]用负载胃癌干细胞抗原的DCCIK对胃癌细胞的杀伤作用明显高于未负载组,在效靶比为20∶1时对胃癌细胞的杀伤率为80.6%,而未负载组为49.7%(P<0.05)。本研究以CD133+作为筛选肾癌和肺癌CSC的表面标志[4],冻融法获取CSC抗原,制备CSC-DC-CIK细胞,确实能促进免疫细胞的成熟和细胞因子的分泌,当效靶比为40∶1时对同源肿瘤细胞的杀伤率达50%左右。本研究结果初步证实了DC-CIK负载KSC及LSC能够有效提高对同源肿瘤细胞的杀伤率,不同CSC抗原参与的同源肿瘤杀伤作用方面虽然LSC-DC与CIK共培养后的DC免疫表型CD40+与KSC-DC组有差异,KSC-DC-CIK组和LSC-DC-CIK组的IFN-γ表达有差异,但是在CD80+等其他表型及TNF-α和IL-2表达方面没有明显差异,因此笔者认为在杀伤其同源肿瘤细胞方面还不能说两种CSC抗原哪种更有特异性。对于是否存在某种CSC抗原能够较KSC和LSC抗原更有效地杀伤其同源肿瘤细胞仍需日后增加肿瘤干细胞种类以进一步深入探索。
[1]Bernatchez C,Radvanyi LG,Hwu P.Advances in the treatment of metastatic melanoma:Adoptive T-cell therapy[J].Semin Oncol, 2012,(2):215-226.doi:10.1053∕j.seminoncol.2012.01.006.
[2]Shi SB,Ma TH,Li CH,et al.Effect of maintenance therapy with dendritic cells:cytokine-induced killer cells in patients with advanced non-small cell lung cancer[J].Tumori,2012,98(3):314-319.doi:10.1700∕1125.12398.
[3]Pan Y,Tao Q,Wang H,et al.Dendritic cells decreased the concomitant expanded Tregs and Tregs related IL-35 in cytokine-induced killer cells and increased their cytotoxicity against leukemia cells [J]. PLoS One, 2014,9(4):e93591. doi: 10.1371∕journal. pone.0093591.eCollection 2014.
[4]Carsten R,Christian S,Adrian F,et al.From''magic bullet''s to specific cancerimmunotherapy[J].Swiss Medical Weekly,2013,143: w13734.doi:10.4414∕smw.2013.13734.
[5]Schmidt J,Eisold S,Buchler MW,et al.Dendritic cells reduce number and function of CD4+CD25+in cytokine-induced killer cells derived from patients with pancreatic carcinoma[J].Cancer Immunology immunotherapy,2004,53(11):1018-1026.doi:10.1007∕s00262-004-0554-4.
[6]Niu Q,Wang W,Li Y,et al.Cord blood-derived cytokine-induced killer cells biotherapy combined with second-line chemotherapy in the treatment of advanced solid malignancies[J].Int Immunopharmacol,2011,(04):449-456.doi:10.1016∕j.intimp.2010.12.014.
[7]Yue LL,Zhang LL,Chai Y,et al.Effect of cytokine-induced killer cells co-cultured with dendritic cells pulsed with antigens on cytotoxicity against multidrug resistant breast cancer cells[J].Chin J Cancer Biother,2012,19(4):437-441.[岳玲玲,张连生,柴晔,等.负载抗原的DC与CIK共培养对耐药乳腺癌细胞的杀伤作用[J].中国肿瘤生物治疗杂志,2012,19(4):437-441.]doi:10.3872∕j. issn.1007-385X.2012.04.017.
[8]Lyu Y,Pang H,Pang CM,et al.Study on the Kill Activity of the Whole Tumor Cell Antigen Pulsed DC Co-Culture with CIK for Breast Cancer Circulating Tumor Cells[J].Tianjin Med J,2013,41 (12):1173-1176.[吕艳,庞华,庞春淼,等.负载抗原DC-CIK共培养对富集培养乳腺癌CTCs杀伤作用研究[J].天津医药,2013,41 (12):1173-1176.]doi:10.3969∕j.issn.0253-9896.2013.12.010.
[9]Wang B,Liu YX,He CY,et al.Compare study of killing activity of DC and CIK on colorectal cancer SW1116[J].Chin J Lab Diagn, 2013,17(9):1571-1573.[王斌,刘玉侠,何春莹,等.抗原负载的DC及CIK对结肠癌细胞SW1116杀伤活性的比较研究[J].中国实验诊断学,2013,17(9):1571-1573.]doi:10.3969∕j.issn.1007-4287.2013.09.007.
[10]Zhou B,Zhang DC.Killing activity of antigen-pulsed dendritic cells combined with cytokine induced killer cells against hepatocarcinoma cells[J].Journal of Chongqing Medical University,2012,37 (8):703-706.[周兵,张德重.抗原致敏的树突状细胞联合细胞因子诱导的杀伤细胞对肝癌细胞杀伤活性的研究[J].重庆医科大学 学 报,2012,37(8):703-706.]doi: 10.3969∕j.issn.0253-3626.2012.08.012.
[11]Tan J,Wang B,He ZJ,et al.Curative effect of autologous dendritic cell stimulated by cytokine-induced killer cells on metastatic renal carcinoma[J].Chin J Cancer Biother,2013,20(6):711-716.[谭洁,王彬,何志洁,等.自体DC-CIK细胞治疗晚期肾细胞癌的疗效[J].中国肿瘤生物治疗杂志,2013,20(6):711-716.]doi:10.3872∕j. issn.1007-385X.2013.06.014.
[12]Wang SN,Gao HY,Pan X,et al.Impact of DC-CIK treatmeat on lymphocyte subsets of patients with renal cell carcinoma[J].Chin J Cancer Biother,2012,19(3):309-312.[王赛男,高海燕,潘欣,等. DC-CIK治疗对肾癌患者淋巴细胞亚群的影响[J].中国肿瘤生物治 疗 杂 志,2012,19(3):309-312.]doi: 10.3872∕j.issn.1007-385X.2012.03.016.
[13]Chen SP,Mai SJ,Yu XJ,et al.Inhibitory effect of cytokine-induced killer(CIK)cells induced by dendritic cells on lung cancer xenograft in nude mice[J].Chin Med Biotechnol,2012,7(2):125-129.[陈诗萍,买世娟,佘杏娟,等.树突状细胞诱导的CIK细胞对肺癌生长的抑制作用[J].中国医药生物技术,2012,7(2):125-129.]doi: 10.3969∕cmba.j.issn.1673-713X.2012.02.008.
[14]Cai JX,Tan J,Wang B,et al.Short-term curative efficacy of DCCIK cell-therapy on patients with advanced non-small cell lung cancer[J].Modern Oncology,2014,22(1):67-70.[蔡俊霞,谭洁,王彬,等.DC-CIK治疗43例晚期非小细胞肺癌的近期疗效观察[J].现代肿瘤杂志,2014,22(1):67-70.]doi:10.3969∕j.issn.1672-4992.2014.01.20.
[15]Yang L,Ren B,Li H,et al.Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small cell lung cancer patients[J].Cancer Immunol Immunother,2013,62:65-73.doi:10.1007∕s00262-012-1311-8.
[16]Tang JP,Li JY.A study of the immune function of DC-CIK loaded with gastric cancer stem cell antigen against the activity of gastric cancer[J].China Medical Engineering,2013,21(3)1-5.[唐家平,李锦毅.负载胃癌干细胞抗原的树突细胞对胃癌细胞免疫作用的研究[J].中国医学工程,2013,21(3):1-5.]
(2014-09-11收稿 2014-09-18修回)
(本文编辑 魏杰)
Effect of Tumor Stem Cell Derived CSC-DC-CIK on Destructing Homologous Tumor Cells
PANG Chong1,ZHANG Tengyue2,WANG Changli1△
1 Tianjin Medical University Cancer Institute and Hospital,National Clinical Research Center for Cancer,Key Laboratory of Cancer Prevention and Therapy,Tianjin,Tianjin 300060,China;2 Tianjin Eye Hospital△
E-mail:E-mail:wangchangli@medmail.com.cn
ObjectiveTo investigate the destructive effect of CSC-DC-CIK who were induced by cytokine induced killer(CIK)cells co-cultured with dendritic cells(DCs)on homologous tumor cells and to explore the possibility of CSC antigen involving in killing tumor.MethodsKidney cancer stem cells(KSCs)and lung cancer stem cells(LSCs)were isolated through FACS using CD133+as a selection marker from cultured kidney cancer cell line A498 and lung cancer cell line A549 respectively.Freeze-thaw method was used to obtain the cancer stem cells(CSCs)antigens.DC cells and CIK cells were collected by in vitro expansion and inducted from the mononuclear cells isolated from human cord blood.The CIK cells were co-cultured with the DCs which were pulsed with the CSCs antigens(CSC-DC-CIK)mentioned above.Immunophenotypes of DC and CIK were analyzed by flow cytometry;cytokines levels were detected by ELISA kits and the destructive effects of two kinds of CSC-DC-CIKs were tested by lactate dehydrogenase(LDH)release assay.ResultsThe expression of phenotypes CD40+,CD80+,CD86+and HLA-DR+were higher in CSC-DC than in CD(P<0.01);the expression of phenotypes CD40+,CD80+,CD86+and HLA-DR+of DC and CSC-DC were higher after co-culture than those before co-culture(P<0.01);the expression of phenotypes CD40+,CD80+,CD86+and HLA-DR+of CSC-DC after been co-cultured with CIK were higher than those of DC after been co-cultured with CIK(P<0.01).The CIK phenotypes:CD3+,CD8+,CD56+were increased in CIK co-cultured with both CSC-DC and DC than those before co-culture(P<0.01);the expression of phenotypes CD3+,CD8+,CD56+were higher in CSC-DC co-cultured with CIK than in DC co-cultured with CIK.DC-CIK and CSC-DC-CIK groups were more capable to express IFN-γ,TNF-α,IL-2 than they were before co-cultured with CIK(P<0.01).CSC-DC-CIK group can secrete more above cytokines than DC-CIK group does(P<0.01).The destructive rates of KSC-DC-CIK and LSC-DC-CIK on target cells were(50.21±4.24)%and(49.32±3.89)%respectively which were muchhigher than that in DC-CIK(30.25±3.11)%(F=89.157,P<0.01).ConclusionCSC-DC-CIKs have destructive effects on homologous tumor cells.More researches are needed to explore the mechanism and to evaluate the clinical applications.
neoplastic stem cells;dendritic cells;immunotherapy;kidney neoplasms;lung neoplasms;cytokine induced killer
R730.3
A
10.3969∕j.issn.0253-9896.2014.10.004
1天津医科大学肿瘤医院,国家肿瘤临床医学研究中心,天津市“肿瘤防治”重点实验室(邮编300060);2天津市眼科医院
△通讯作者 E-mail:wangchangli@medmail.com.cn
