Cs取代对Ni-H3PW12O40/SiO2催化剂结构性质和催化性能的影响
2014-06-23孙晓丹吴义志孙素华杰朱慧红伊晓东方维平
金 浩 孙晓丹 董 澍 吴义志 孙素华 刘 杰朱慧红 杨 光 伊晓东 方维平
(1中国石油化工股份有限公司抚顺石油化工研究院,辽宁抚顺113001;2厦门大学化学化工学院,固体表面物理化学国家重点实验室,醇醚酯化工清洁生产国家工程实验室,福建厦门361005;3中国石油抚顺石化公司石油二厂,辽宁抚顺113004)
1 Introduction
Hydrocracking is a catalytic petroleum refining process that is commonly applied to convert the heavier petroleum fractions such as vacuum distillates into gasoline or middle distillates.1,2As the growing demand for high quality middle distillates and more stringent specifications,hydrocracking becomes a strategic process in a modern refinery.3
Hydrocracking catalysts are bifunctional,i.e.,the acid sites which provide the cracking function and metal sites with a hydrogenation-dehydrogenation function.4-7The typical acidic supports are amorphous oxides,mixtures of oxides,zeolites,and silicoaluminaphosphates.The metals most commonly used are Pt,Pd or bimetallic systems(i.e.,NiW,NiMo,and CoMo).The balance between the acidity of the support-concentration of acidic sites and their strength-hydro/dehydrogenation activity of the metal is of primary importance in determining the selectivity of hydroisomerization and distribution of cracking products.
Heteropolyacids(HPAs)with Keggin structure and their salts have been widely investigated as catalysts in many oxidation and acid-catalyzed reactions due to their strong acidity,high oxidation potential,and redox character.8-12The tungstophosphoric acid(H3PW12O40)(HPW)is among the most extensively studied,13-15since it possesses the highest Brønsted acidity.Nevertheless,the main drawback of such materials for catalytic application is their low specific surface area(<10 m2·g-1).Therefore,for many catalytic applications,they are usually impregnated on different porous materials with high surface area.Among these carriers,silica has been widely favored as the supporting material for HPA,since it interacts weakly with the Keggin anions and thus preserves their structure.16,17Yet in reactions that involve polar media,true heterogenization of H3PW12O40could not be achieved on silica,and the acid leached out into the reaction mixture.18,19
Heteropolyacid salts are prepared by exchanging part of the protons of HPA with cations with higher ionic radii,like Cs+and NH4+.20,21They have higher surface area(up to 150 m2·g-1compared to 10 m2·g-1of HPA)and improved thermal stability than their parent acids.In addition,they are known to be insoluble even in liquids as polar as water.Consequently,HPA salts should be better suited for practical applications that might involve polar reagents in harsh operating conditions.However,these salts tend to form colloidal suspensions in polar media,resulting in difficulties in the catalyst separation.22Moreover,their small particle size(unit inµm)limits their application for use as catalysts in commercial fixed bed or slurry type reactors.23
An obvious solution as often applied in industrial practice is to support these HPA salts on a larger particle size(unit in mm)carrier.Unfortunately,the insolubility of HPA salts with big cation makes conventional aqueous impregnation on different supports impossible.Consequently,the catalysts were prepared by sequential impregnation andin situreaction on different types of supports,as reported in previous literature.24-28
In our previous work,16we reported that non-sulfided supported Ni-H3PW12O40/SiO2catalysts exhibited high hydrocracking activity ofn-decane.But H3PW12O40has an excessive acidity and an overhigh cracking activity,which increases the probability to undergo secondary reactions.We also studied hydrogen spillover on Ni-CsxH3-xPW12O40(x=0,1,2)double-function hydrocracking catalysts by temperature programmed desorption and thermodynamics calculation.29The results show that the hydrogen adsorption amount on the two-component Ni-CsxH3-xPW12O40(x=0,1,2)catalysts is much greater than that on single-component catalysts,such as nickel,tungstophosphoric acid,and its cesium salts.Moreover,the Cs salts of H3PW12O40overcome these disadvantages,which have a more widely tunable acidity,a higher thermal stability,and much lower water solubility.21
Recently,we30also reported that non-sulfided supported Ni-CsxH3-xPW12O40/SiO2catalysts prepared by direct synthesis using tetraethyl orthosilicate as SiO2source.Taking into account the thermal stability of CsxH3-xPW12O40and the strong interaction between CsxH3-xPW12O40and support by direct synthesis,the calcination temperature of the catalyst could not be too high.The properties of support were restricted by calcination temperature and the pore size of the support was about 4 nm.
In the present paper,the Ni-CsxH3-xPW12O40/SiO2catalysts were prepared by two-step impregnation andin situreaction on the SiO2support.It avoided the restriction of support properties due to calcination temperature and the strong interaction between CsxH3-xPW12O40and support by direct synthesis.The catalysts were characterized by N2adsorption(BET),inductively coupled plasma atomic emission spectrometry(ICP),X-ray diffraction(XRD),Raman,in situXRD,NH3-temperature programmed desorption(NH3-TPD),H2-temperature programmed reduction(H2-TPR),H2-TPD,and Fourier transform infrared(FTIR)spectra of pyridine adsorption.The influence of Cs substitution on catalytic performance of the catalysts for hydrocracking ofn-decane was investigated.
2 Experimental
2.1 Preparation of catalysts
The catalysts were prepared by two-step impregnation andinsitureaction on the SiO2support.Typical procedures for the preparation of Ni-CsxH3-xPW12O40/SiO2catalysts are as follows:SiO2support(Qingdao Haiyang Chemical Co.,specific surface area(378 m2·g-1),40-60 mesh)was impregnated with a solution containing the desired quantities of Ni(NO3)2(Shanghai Hengxin Chemical Reagent Co.,analyzed grade)and Cs2CO3(Sinopharm Chemical Reagent Co.,3N).Impregnated samples were dried overnight at 110 °C and then calcined in air at 400 °C for 3 h.Then the samples were impregnated with a solution containing the desired quantities of H3PW12O40(Sinopharm Chemical Reagent Co.,analyzed grade).After impregnation,samples were dried overnight at 110°C without calcination.
Samples prepared with 8%amount of nickel and 50%amount of CsxH3-xPW12O40were labeled as 8%Ni-50%CsxH3-xPW/SiO2,wherein“x”stands for the molar of replaced by Cs inthe CsxH3-xPW12O40(x=0-3),while CsxH3-xPW stands for the CsxH3-xPW12O40.
2.2 Characterization
The chemical composition of the samples was determined using an IRIS Intrepid II XSP ICP atomic emission spectrometer(Thermo,USA).
The surface area(BET)and pore volume of the catalysts were determined by means of nitrogen adsorption at-196°C on an adsorption automatic instrument(Micromeritics Tristar 3020,USA).The samples were pretreated at 300°C for 3 h in a vacuum.
Powder X-ray diffraction(XRD)characterization was carried out on a Panalytical(NetherLands)X2 Pert PRO automatic powder diffractometer operated at 40 kV and 30 mA,using CuKα(λ=0.15406 nm)monochromatized radiation in all cases.Each step of 0.0167°was measured for 10 s from 10°to 90°(2θ).JCPDS file database was used for peak identification.
Raman spectra were recorded with a Renishaw(UK)inVia Raman System equipped with a charge-coupled device(CCD)detector at room temperature.The 532 nm of diode laser was used as the exciting source with a power of 22 mW.
In situXRD was performed under the 5%H2/Ar mixture atmosphere.The first spectrum was recorded at room temperature(25°C).The temperature was then raised up to 300,350,400,450,500,550,600,650,700,750,and 800°C,maintained at each value for 0.5 h before recording a new spectrum.
Acid properties were determined by ammonia temperatureprogrammed desorption in a Micromeritics AutoChem II 2920 analyzer(USA).0.2 g of catalyst sample was filled in a U-shaped quartz reactor tube and a thermocouple was placed onto the top of the sample.All samples were pretreated inAr(20 mL·min-1)at 400 °C for 2 h then in H2(20 mL·min-1)for 1 h.After cooling down to 100°C,10%NH3/Ar was passed over the samples for 30 min.Then,the samples were swept with Ar for 60 min and finally the desorption step was performed from 100 to 700 °C at a heating rate of 10 °C·min-1and 30 mL·min-1of Ar total flow.The desorbed products were monitored by thermal conductivity detector(TCD)and mass spectrometry(MS)equipment simultaneously.
The H2-temperature programmed reduction experiments were performed with a gas chromatography(GC)-TPR apparatus.The samples(50 mg)were treated in a flow of Ar(20 mL·min-1)at 300 °C for 30 min and then cooled to 50 °C.The samples were subsequently switched to a flow of 5%H2/Ar mixture(20 mL·min-1)and heated from 50 to 900 °C at a rate of 10 °C·min-1.The effluent gas mixture was passed through a cold trap at 0°C to remove water.Hydrogen consumption was monitored by an on-line gas chromatograph equipped with a TCD.
H2-TPD measurements were done in a Micromeritics Auto-Chem II 2920 analyzer.0.2 g of catalyst sample was filled in a U-shaped quartz reactor tube and a thermocouple was placed onto the top of the sample.All samples were pretreated in Ar(20 mL·min-1)at 400 °C for 2 h then in H2(20 mL·min-1)for 1 h.After cooling down to 50°C,the samples were swept with Ar for 60 min and finally the desorption step was performed from 50 to 700 °C at a heating rate of 10 °C·min-1and 30 mL·min-1ofAr total flow.
FTIR spectra of pyridine adsorption were recorded using a Thermo Nicolet Nexus spectrometer equipped with a liquidnitrogen-cooled mercury cadmium telluride(MCT)detector.The samples were pressed into self-supporting wafers and treated in H2at 400°C in an IR cell for 1 h followed by evacuation at 400°C for 5 min to remove the gas phase H2.After cooling to 100°C,the samples were exposed to pyridine vapor for 10 min.Then the spectra were recorded after evacuation at high temperatures.The IR spectra were recorded in the spectral range of 1700 to 1400 cm-1with 32 scans and at a resolution of 4 cm-1.
2.3 Catalytic studies
n-Decane used in the present study was purchased from Tianjin Kermel Chemical Reagent Co.(analyzed Grade)without further purification.
The catalytic performance of the catalysts was measured in a down flow fixed-bed quartz tube reactor cased in a stainless steel tube(inner diameter(id)=8 mm;50 cm in length)at 2.0 MPa,T=300°C,liquid hourly space velocity(LHSV)=2.92 h-1and H2/n-decane volume ratio of 1500.Prior to reaction,all the catalysts were reduced by a flow of H2at 400°C for 1 h.0.5 g of the catalyst was used in each experiment.n-Decane was introduced into the reactor using a micro pump(2ZB-1L10).The products were collected and identified when the reaction had begun for 4 h.The activity data were usually obtained after 10 h reaction.The products were directly analyzed on-line in a gas chromatograph with an OV-101 capillary column(30 m)and flame ionization detector(FID).
For comparison,an industrial hydrocracking catalyst FC-16(NiW/USY zeolite)was also measured for hydrocracking ofndecane under the same conditions.The industrial catalyst was obtained from FuShun Research Institute of petroleum and petrochemicals,SINOPEC.
3 Results and discussion
3.1 Catalysts characterization
The structural information,pore size distribution,and composition of the catalysts are presented in Table 1 and Table 2.The determined chemical composition from ICP is as expected,indicating that the results of chemical analysis of the catalysts are in good agreement with desired stoichiometries for Ni and CsxH3-xPW.It can be observed that the surface area and pore volume of SiO2after supporting Ni and CsxH3-xPW decrease remarkably,while the pore size increases slightly.This may be due to Ni and CsxH3-xPW blocking the micropores of the SiO2support,therefore,the surface area and pore volume decrease,the pore size increases slightly.However,the surface area and pore volume of the 8%Ni-50%CsxH3-xPW/SiO2catalysts increase with increasing the proportion of Cs in CsxH3-xPW.This may be due to the much higher surface area of CsxH3-xPW with increasing proportion of Cs in CsxH3-xPW,which makes the surface area of the catalysts become higher.
The XRD patterns of 8%Ni-50%CsxH3-xPW/SiO2and CsxH3-xPW catalysts are presented in Fig.1.It is clear that the intensity of the diffraction peaks of the 8%Ni-50%CsxH3-xPW/SiO2catalysts decreased compared to the CsxH3-xPW due to the interaction of CsxH3-xPW with Ni species and SiO2support.The 8%Ni-50%H3PW/SiO2catalyst shows the presence of characteristic peaks of NiO(37.0°,43.1°,and 62.6°)and Keggin structure of H3PW.27It is interesting to notice that the diffraction peaks of the Keggin structure of CsxH3-xPW(18.2°,23.7°,25.9°,29.9°,35.4°,43.2°,54.3°,and 62.2°)appeared and the intensities of these peaks increased with increasing the proportion of Cs in CsxH3-xPW on the catalysts.This could be attributed to the interaction of CsxH3-xPW with Ni species and SiO2support.

Fig.1 XRD patterns of 8%Ni-50%CsxH3-xPW/SiO2catalysts
The Raman spectra of the 8%Ni-50%CsxH3-xPW/SiO2catalysts are presented in Fig.2.Raman scattering spectroscopy is an effective method to study the structure of the supported CsxH3-xPW because it is extremely sensitive to the Keggin unit,and the support has no significant interference on the Raman signals originating from the Keggin unit.All the 8%Ni-50%CsxH3-xPW/SiO2catalysts display similar Raman spectra.The sharp and intense peak at 1009 cm-1can be assigned to stretching vibrations of P―O bond of P―O4,whereas peaks at lower wavenumbers can be assigned to W=O(990 cm-1)and W―O―W(905 cm-1)stretching vibrations.31,32The strong interaction between H3PW and SiO2support reduces the symmetry of Keggin unit.Furthermore,it can be speculated that the intro-duction of Cs also will weaken the interaction between CsxH3-xPW and the SiO2support becomes weaker,reducing the influence on Keggin unit′s symmetry.Therefore,the intensity of these peaks increases with increasing the proportion of Cs in CsxH3-xPW on the catalysts.The Raman results are consistent with XRD characterization.

Table 1 Chemical composition and textural information of the catalysts

Table 2 Pore size distribution of the catalysts
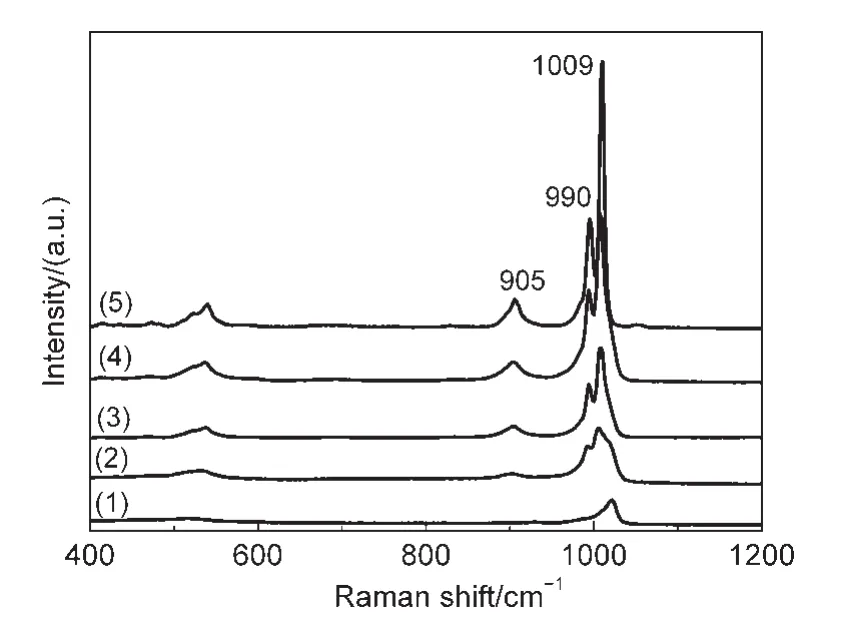
Fig.2 Raman spectra of 8%Ni-50%CsxH3-xPW/SiO2catalysts
The thermal stabilities of 8%Ni-50%Cs1.5H1.5PW/SiO2and 8%Ni-50%H3PW/SiO2catalysts under the hydrogen atmosphere were studied byin situXRD and the patterns are shown in Fig.3.When the temperature is lower than 500°C,the XRD patterns of the 8%Ni-50%Cs1.5H1.5PW/SiO2catalyst only present the characteristic peaks of the Keggin structure.Compared with the XRD pattern of the catalyst at 25°C,the characteristic diffraction peaks of the catalyst calcined at higher temperature have no change.In the XRD patterns of the catalyst calcined at temperature higher than 500°C,the new intense diffraction peaks of H0.5WO3(23.5°,34°)and Cs0.3WO3(44°)are observed.While for the 8%Ni-50%H3PW/SiO2catalyst calcined at 500°C,the new intense diffraction peak of H0.5WO3(23.5°)is observed.The 8%Ni-50%Cs1.5H1.5PW/SiO2catalyst has improved thermal stability than 8%Ni-50%H3PW/SiO2catalyst.These results indicate that Cs1.5H1.5PW decomposes when the calcination temperature exceeds 500°C.

Fig.3 In situ XRD patterns of the catalysts calcined at different temperatures under the 5%H2/Ar mixture atmosphere
The acidity of the 8%Ni-50%CsxH3-xPW/SiO2catalysts was characterized by NH3-TPD and the FTIR of pyridine adsorption.The NH3-TPD profiles of the 8%Ni-50%CsxH3-xPW/SiO2catalysts are shown in Fig.4.All the catalysts show two desorption peaks of ammonia near 170 and 540°C,respectively.It is evident from Fig.4 that the amount of NH3desorbed of the catalysts decreases with increasing the proportion of Cs in CsxH3-xPW.
In other words,the acid amount of the catalysts decreases with decreasing the H+content in CsxH3-xPW on the catalysts.The 8%Ni-50%CsxH3-xPW/SiO2catalysts show relatively higher acidity compared to the industrial catalyst.
The FTIR spectra of pyridine adsorbed on reduced 8%Ni-50%CsxH3-xPW/SiO2catalysts are shown in Fig.5.The use of IR spectroscopy to detect the adsorbed pyridine enables to distinguish different acid sites.The band at 1446 cm-1is due to the pyridine adsorbed on the Lewis acid sites.On the other hand,the band at 1538 cm-1is due to the pyridine adsorbed on the Brønsted acid sites.The band at 1488 cm-1is due to the contributions of Lewis and Brønsted acid sites.33,34The characteristic absorption bands of pyridine adsorbed on Lewis acid sites and Brønsted acid sites were observed for 8%Ni-50%CsxH3-xPW/SiO2catalysts.The IR results show that the intensities of absorption bands for Brønsted acid sites(1538 cm-1)and Lewis acid sites(1446 cm-1)decrease with increasing the proportion of Cs in CsxH3-xPW,and relative amount of Lewis acid sites is higher than that of Brønsted acid sites.The results are consistent with NH3-TPD characterization.

Fig.4 NH3-TPD profiles of reduced catalysts

Fig.5 FTIR spectra of pyridine adsorbed and desorbed on reduced catalysts
The H2-TPR profiles of the 8%Ni-50%CsxH3-xPW/SiO2catalysts are shown in Fig.6.The 8%Ni-50%H3PW/SiO2catalyst displays three reduction peaks.The first reduction peak near 400°C corresponds to the reduction of NiO species,which had weak interaction with the H3PW.Thein situXRD results showed that the CsxH3-xPW begins to decompose when the calcinations temperature exceeds 500°C.The second reduction peak near 580°C is mainly attributed to the reduction of NiO species,which had strong interaction with the W species of the catalysts.The third reduction peak near 700°C corresponds to the reduction of W species.However,the 8%Ni-50%CsxH3-xPW/SiO2(x=0.5,1,1.5,2)catalysts show two reduction peaks.The first reduction peak near 400°C corresponds to the reduction of NiO species and the peak area becomes larger.The second reduction peak near 690°C corresponds to the reduction of W species.This may be due to the interaction between NiO and CsxH3-xPW gradually weakened with increasing the proportion of Cs in CsxH3-xPW.The reduction peak(580°C)shifts to lower temperature(400 °C)and the peak area(around 400 °C)becomes larger.The phenomenon is consistent with the result of Raman characterization.
The H2-TPD profiles of the 8%Ni-50%CsxH3-xPW/SiO2catalysts are shown in Fig.7.All the catalysts present two H2-desorbed peaks near 160 and 450°C,respectively.The amount of H2desorbed decreases with increasing the proportion of Cs in CsxH3-xPW.In other words,the amount of H2desorbed increases with increasing content of H+in the CsxH3-xPW on the catalysts.
This phenomenon may be explained by the hydrogen spillover,which has been found in our past work.16,17,27,35The dissociated hydrogen molecule on the metal Ni can spill to the acid sites(CsxH3-xPW)and combine with the H+.Both the dissociated molecule hydrogen and the H+of the CsxH3-xPW are highly reactive hydrogen species,which will produce the relatively stable species Hn+.It seems that there is a balance on the surface of the CsxH3-xPW,that is


Fig.6 H2-TPR profiles of the catalysts

Fig.7 H2-TPD profiles of the catalysts
As the concentration of Hn+species on the surface of CsxH3-xPW increases,the reactive hydrogen species H·can reversely spill over back to the Ni0sites.This process can form a reactive hydrogen species layer covering the catalyst′s surface.The CsxH3-xPW can not only act as the acid sites but also act as the hydro-dehydrogenation sites.This is a non-classical bifunctional mechanism which was reported in some papers.16,27,35,36
3.2 Catalytic activity
The activity of the 8%Ni-50%CsxH3-xPW/SiO2catalysts for hydrocracking ofn-decane is shown in Table 3,wherein the conversion ofn-decane and the C+5selectivity were taken to express the activity of the catalyst.The catalytic performance of the prepared catalysts was compared with that of a typical NiW/zeolite industrial catalyst.It is evident from Table 3 that the 8%Ni-50%CsxH3-xPW/SiO2catalysts and the industrial catalyst all exhibit high activity for the hydrocracking ofn-decane.Among the catalysts tested,8%Ni-50%H3PW/SiO2catalyst shows highest conversion ofn-decane and lower C5+selectivity.Moreover,after the introduction of Cs species,the conversion ofndecane decreases while the C5+selectivity is improved for 8%Ni-50%CsxH3-xPW/SiO2catalysts.Furthermore,the 8%Ni-50%CsxH3-xPW/SiO2catalysts show higher activity compared to the industrial catalyst.
With increasing the proportion of Cs in CsxH3-xPW,the conversion ofn-decane decreases from 99.2%to 89.5%for 8%Ni-50%CsxH3-xPW/SiO2catalysts.The reduced 8%Ni-50%H3PW/SiO2catalyst shows the highest activity,superior to the 8%Ni-50%CsxH3-xPW/SiO2(x≠0)catalyst and the industrial catalyst.Combined with the results of H2-TPD and H2-TPR characterization,it can be inferred that the hydrogenation ability of the catalysts is gradually weakened with increasing the proportion of Cs in CsxH3-xPW.
With increasing the proportion of Cs in CsxH3-xPW,the C5+selectivity of the catalysts increases from 74.1%to 85.2%.The reduced 8%Ni-50%Cs2HPW/SiO2shows the highest C5+selectivity,superior to the 8%Ni-50%H3PW/SiO2catalyst and the industrial catalyst.Based on the results of NH3-TPD and FTIR spectra of pyridine adsorption,it can be concluded that the acidity of the 8%Ni-50%CsxH3-xPW/SiO2catalysts is in line with their C5+selectivity,which is ascribed to the gradually lower cracking activity of the catalysts with increasing the proportion of Cs in CsxH3-xPW owing to their weaker acidity.According to bifunctional reaction scheme,37the hydroisomerization and hydrocracking go through the formation of carbonium ions,the lower the acid strength of the acid sites,the lower will be the average lifetime of the carbonium ions on the acid sites.This will decrease the probability to undergo secondary reactions.In addition,the pore size of the 8%Ni-50%CsxH3-xPW/SiO2catalysts increases slightly with increasing the proportion of Cs in CsxH3-xPW.Indeed,it is well-known that the micropore of zeolite is beneficial for secondary reactions.Therefore,it can be expected that large pore size of the 8%Ni-50%CsxH3-xPW/SiO2catalysts would favor the diffusion of liquid products while decreasing the probability of secondary reactions.Moreover,the 8%Ni-50%Cs1.5H1.5PW/SiO2catalyst shows the highest C5+yield of 80.3%in the 8%Ni-50%CsxH3-xPW/SiO2catalysts,which is much higher than the yield of 73.5%on the 8%Ni-50%H3PW/SiO2catalyst and the yield of 62.1%on the industrial catalyst.

Table 3 Catalytic performance of the catalysts for n-decane hydrocracking
4 Conclusions
The results obtained in the present work indicate that the 8%Ni-50%CsxH3-xPW/SiO2catalystsʹacidityviaNH3-TPD and FTIR spectra of pyridine adsorption,and hydrogenation-dehydrogenation functionviaH2-TPR and H2-TPD decrease with Cs gradual substituting in CsxH3-xPW.The conversion ofn-decane decreases slightly and the C5+selectivity of the catalysts increases with increasing the proportion of Cs in CsxH3-xPW.The best result was obtained on the 8%Ni-50%Cs1.5H1.5PW/SiO2catalyst with the C5+selectivity of 83.8%at then-decane conversion of 95.8%,which is much higher than that of the industrial catalyst.
(1) Morawski,I.;Mosio-Mosiewski,J.Fuel Process.Technol.2006,87,659.doi:10.1016/j.fuproc.2006.01.006
(2) Ancheyta,J.;Sánchez,S.;Rodríguez,M.A.Catal.Today2005,109,76.doi:10.1016/j.cattod.2005.08.015
(3) Roussel,M.;Lemberton,J.L.;Guisnet,M.;Cseri,T.;Benazzi,E.J.Catal.2003,218,427.doi:10.1016/S0021-9517(03)00164-7
(4) Calemma,V.;Peratello,S.;Perego,C.Appl.Catal.A:Gen.2000,190,207.doi:10.1016/S0926-860X(99)00292-6
(5)Ren,X.T.;Li,N.;Cao,J.Q.;Wang,Z.Y.;Liu,S.Y.;Xiang,S.H.Appl.Catal.A:Gen.2006,298,144.doi:10.1016/j.apcata.2005.09.031
(6) Zeng,S.Q.;Blanchard,J.;Breysse,M.;Shi,Y.H.;Su,X.T.;Nie,H.Appl.Catal.A:Gen.2005,294,59.doi:10.1016/j.apcata.2005.07.015
(7) Roussel,M.;Norsic,S.;Lemberton,J.L.;Guisnet,M.;Cseri,T.;Benazzi,E.Appl.Catal.A:Gen.2005,279,53.doi:10.1016/j.apcata.2004.10.011
(8) Timofeeva,M.N.Appl.Catal.A:Gen.2003,256,19.doi:10.1016/S0926-860X(03)00386-7
(9)Zhang,Q.D.;Tan,Y.S.;Yang,C.H.;Han,Y.Z.J.Mol.Catal.A:Chem.2007,263,149.doi:10.1016/j.molcata.2006.08.044
(10) Zhang,P.;Huang,M.;Chu,W.;Luo,S.Z.;Li,T.Acta Phys.-Chim.Sin.2013,29,770.[张 坡,黄 明,储 伟,罗仕忠,李 通.物理化学学报,2013,29,770.]doi:10.3866/PKU.WHXB201301152
(11) Gu,L.Y.;Gao,B.J.;Fang,X.L.Acta Phys.-Chim.Sin.2013,29,191.[顾来沅,高保娇,房晓琳.物理化学学报,2013,29,191.]doi:10.3866/PKU.WHXB201210266
(12) Yuan,C.Y.;Chen,J.Chin.J.Catal.2011,32,1191.doi:10.1016/S1872-2067(10)60236-7
(13) Kumar,G.S.;Vishnuvarthan,M.;Palanichamy,M.;Murugesan,V.J.Mol.Catal.A:Chem.2006,260,49.doi:10.1016/j.molcata.2006.07.050
(14) Yang,X.K.;Chen,L.F.;Wang,J.A.;Noreña,L.E.;Novaro,O.Catal.Today2009,148,160.doi:10.1016/j.cattod.2009.03.022
(15) Wang,J.A.;Chen,L.F.;Noreña,L.E.;Navarrete,J.Appl.Catal.A:Gen.2009,357,223.doi:10.1016/j.apcata.2009.01.023
(16)Qiu,B.;Yi,X.D.;Lin,L.;Fang,W.P.;Wan,H.L.Catal.Today2008,131,464.doi:10.1016/j.cattod.2007.10.095
(17) Qiu,B.;Yi,X.D.;Lin,L.;Fang,W.P.;Wan,H.L.Catal.Commun.2009,10,1296.doi:10.1016/j.catcom.2009.02.007
(18) Vazquez,P.;Pizzio,L.;Romanelli,G.;Autino,J.;Caceres,C.;Blanco,M.Appl.Catal.A:Gen.2002,235,233,doi:10.1016/S0926-860X(02)00266-1
(19) Haber,J.;Pamin,K.;Matachowski,L.;Mucha,D.Appl.Catal.A:Gen.2003,256,141.doi:10.1016/S0926-860X(03)00395-8
(20)Narasimharao,K.;Brown,D.R.;Lee,A.F.;Newman,A.D.;Siril,P.F.;Tavener,S.J.;Wilson,K.J.Catal.2007,248,226.doi:10.1016/j.jcat.2007.02.016
(21)Luzgin,M.V.;Kazantsev,M.S.;Volkova,G.G.;Wang,W.;Stepanov,A.G.J.Catal.2011,277,72.doi:10.1016/j.jcat.2010.10.015
(22) Okuhara,T.;Kimura,M.;Kawai,T.;Xu,Z.;Nakato,T.Catal.Today1998,45,73.doi:10.1016/S0920-5861(98)00251-X
(23) Choi,S.;Wang,Y.;Nie,Z.;Liu,J.;Peden,C.H.F.Catal.Today2000,55,117.doi:10.1016/S0920-5861(99)00231-X
(24) Soled,S.;Miseo,S.;McVicker,G.;Gates,W.E.;Gutierrez,A.;Paes,J.Catal.Today1997,36,441.doi:10.1016/S0920-5861(96)00235-0
(25) Yang,W.;Billy,J.;Taârit,Y.B.;Védrine,J.C.;Essayem,N.Catal.Today2002,73,153.doi:10.1016/S0920-5861(01)00508-9
(26)Gao,R.H.;Chen,H.;Le,Y.Y.;Dai,W.L.;Fan,K.N.Appl.Catal.A:Gen.2009,352,61.doi:10.1016/j.apcata.2008.09.031
(27) Jin,H.;Yi,X.D.;Sun,S.H.;Liu,J.;Yang,G.;Zhu,H.H.;Fang,W.P.Fuel Process.Technol.2012,97,52.doi:10.1016/j.fuproc.2012.01.011
(28) Popa,A.;Sasca,V.;Holclajtner-Antunovi,I.Microporous Mesoporous Mat.2012,156,127.doi:10.1016/j.micromeso.2012.02.030
(29)Yuan,S.H.;Ji,N.H.;Xia,W.S.;Yi,X.D.;Fang,W.P.React.Kinet.Mech.Catal.2012,106,475.doi:10.1007/s11144-012-0448-y
(30) Jin,H.;Guo,D.Y.;Sun,X.D.;Sun,S.H.;Liu,J.;Zhu,H.H.;Yang,G.;Yi,X.D.;Fang,W.P.Fuel2013,112,134.doi:10.1016/j.fuel.2013.05.007
(31) Rocchiccioli-Deltcheff,C.;Fournier,M.;Franck,R.;Thouvenot,R.Inorg.Chem.1983,22,207.doi:10.1021/ic00144a006
(32)Qu,X.S.;Guo,Y.H.;Hu,C.W.J.Mol.Catal.A:Chem.2007,262,128.doi:10.1016/j.molcata.2006.08.026
(33) Chen,L.F.;Noreña,L.E.;Wang,J.A.;Zhou,X.L.;Navarrete,J.;Hernández,I.;Montoya,A.;Pérez Romo,P.;Salas,P.;Castella Pergher,S.Catal.Today2008,133-135,331.
(34) Varisli,D.;Dogu,T.;Dogu,G.Ind.Eng.Chem.Res.2008,47,4071.doi:10.1021/ie800192t
(35) Jin,H.;Yi,X.D.;Sun,X.D.;Qiu,B.;Fang,W.P.;Weng,W.Z.;Wan,H.L.Fuel2010,89,1953.doi:10.1016/j.fuel.2009.11.031
(36) Kuba,S.;Lukinskas,P.;Grasselli,R.K.;Gates,B.C.;Knözinger,H.J.Catal.2003,216,353.doi:10.1016/S0021-9517(02)00125-2
(37) Corma,A.;Martinez,A.;Pergher,S.;Peratello,S.;Perego,C.;Bellusi,G.Appl.Catal.A:Gen.1997,152,107.doi:10.1016/S0926-860X(96)00338-9
