基于苯并噻二唑和硅芴的一系列红光材料的载流子传输性质及荧光性质
2014-06-23邹陆一任爱民
李 岩 邹陆一 任爱民,*
(1吉林大学理论化学研究所,理论与计算化学重点实验室,长春130023;2吉林化工学院化学与制药工程学院物理化学系,吉林吉林132022)
1 Introduction
Conjugated organic polymers have attracted significant attention because of their wide potential applications in polymer light-emitting diodes(PLEDs),1-5organic field effect transistors(OFETs),6-10and organic solar cells(OSCs)11-15etc.Red emission can be achieved by introducing narrow band gap on the main chain.Recently,Cao's group16,17reported a new type of red or green polymers synthesized by incorporating 4,7-di(4-hexyl-2-thienyl)-2,1,3-benzothiadiazole(DHTBT)and 2,1,3-benzothiadiazole(BT),respectively,to poly(2,7-silafluorene)(PSiF),in which the 9-carbon in fluorene ring is replaced by silicon to improve the spectral stability at thermal annealing of polyfluorene and the electronic transmission performance.Inspired by the experimental work above,we designed a series of red-light-emitting oligomers.The chemical structures are shown in Fig.1.
In order to provide theoretical guidance for designing new polymer-based organic light-emitting diodes(OLEDs),the various properties of these oligomers,such as the highest occupied molecular orbitals(HOMOs),the lowest unoccupied molecular orbitals(LUMOs),HOMO-LUMO gaps(ΔEH-L,the energy difference between the HOMO and LUMO),ionization potentials(IPs),electron affinities(EAs),reorganization energies(λs),the lowest excitation energies(Eg),exciton binding energies(Eb),and fluorescence emission energies(EFlu)were calculated and discussed in detail in this work.All these parameters of the polymers were estimated through a linear extrapolation technique.18,19And the results were summarized by comparing the properties between polymer and oligomer as well as the properties of polymers with different proportions and coordination architectures of dithienyl-benzothiadiazole and silafluorene.We hope that it can predict how to change the hole/electron injection/transport abilities and the optical properties of polymers with the position of benzothiadiazole group on silafluorene group or the position of butyl group on thiophene group changes.
2 Computational methods and theory
2.1 Computational methods
The geometries of the ground,ionic,and the lowest excited singlet states of all the considered oligomers were fully optimized without any symmetry constraints using density functional theory(DFT)20withBecke′sthree-parameter functional and the Lee-Yang-Parr functional(B3LYP)21methods and the configuration interaction with single-excitation(CIS)22-24levels,respectively,with 6-31g*basis set for all atoms.On the basis of the optimized ground-and excited-state geometries,the absorption and fluorescence properties were investigated using time-dependent DFT(TD-DFT)calculations.25-27The polarizable continuum model(PCM)was used in treating the solvent(chloroform)effect on the absorbance and emission spectra.The properties of the polymers were obtained through the extrapolation method:18,19
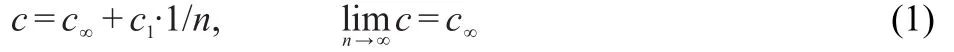
where c is energy,c∞is the energy of the polymers,c1is slope.
All calculations have been performed with the Gaussian 09 package.28
2.2 Reorganization energy and carrier transport
In OLEDs,holes and electrons must be injected from opposite electrodes,form excitons in an emission layer,and then the excitons decay to emit light.So the hole and electron injection/transport capabilities of the materials in a device must be taken into account for OLED fabrication.The intermolecular charge transport in disordered polymers can be regarded as a charge hopping process that can be represented as follows:M+/-+M=M+M+/-,where M+/-denotes the molecule in the cationic and hole(h)/electron(e)ionic states,M is the neutral molecule.According to theories of the Marcus and Hush,29-31the hole(or electron)transferrate(K)can be calculated by the following equation:
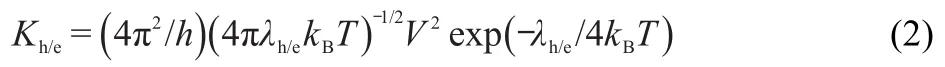
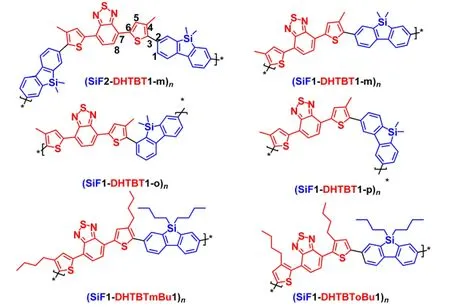
Fig.1 Sketch map of the structures of the polymers studied
whereTis the temperature,kBis the Boltzmann constant,his the Plank constant,λis the reorganization energy,andVis the electronic couplings between the two adjacent molecules in organic single crystal.Obviously,λandVare the two most important parameters for the charge-transfer rate.And meanwhile,Vin the amorphous materials can almost be regarded as a constant.32,33Thus we mainly investigate the internal reorganization energy for the studied polymers.The internal hole(or electron)reorganization energies can be obtained as follows:34

where HEP is hole extraction potential and EEP is electron extraction potential.
In view of equation(2),the reorganization energy can provide a useful link between the electronic structures and the transport properties.In OLEDs,low and close reorganization energies of the hole and the electron will be benefit to achieve highly efficient electroluminescence.The IPs and EAs can be either for vertical excitations(v,at the geometry of the neutral molecule)or adiabatic excitations(a,optimized structure for both the neutral and charged molecules).It is well known that the lower IP the hole transport layer(HTL),the easier it accepts a hole from anode.On the other hand,the higher EA the electron transport layer(ETL),the easier it snatches an electron from cathode.
2.3 Exciton binding energy
The exciton binding energy(Eb)is one of the key parameters for the electroluminescence quantum efficiency of OLEDs,35which can be determined by the following equation:EFlu.Excitons are created when the electrons and holes meet each other in the emission layer,then may decay radiatively and emit photons in the OLED devices.36,37SoEbis the energy of the Coulomb interactions between an electron and a hole.One electron is promoted from the HOMO into the LUMO and an exciton is formed.38Stable excitons will be formed only whenEb>>kBT(thermal energy).On the other hand,ifEbis less than or comparable tokBT(~25 meV,at room temperature),the bound excitons will decay into charged polaron pairs.39Generally,the process of electron-hole pair recombination in OLEDs is enhanced by the large exciton binding energy.40,41
3 Results and discussion
3.1 Geometric structures and frontier orbitals of singlet ground states
The optimized structures of the ground states of all the oligomers change slightly with increasing the repeated unitsn(n=1-4)(shown in Table 1).With different proportions of SiF and DHTBT,there is almost no change in the structures of the oligomers for the ground states.C2―C3 bond of(SiF1-DHTBT-mBu1)4(butyl group at themeta-position on thiophene group)is longer,its C6―C7 bond is shorter,and its dihedral angle C5=C6―C7=C8 is closer to 180°but its dihedral angle C1=C2―C3=C4 is further away from 180°than those of(SiF1-DHTBToBu1)4(butyl group at theortho-position on thiophene group).In(SiF1-DHTBT1-o)n,thienyl and benzothiadiazole are located almost in the same plane,but SiF is approximately perpendicular to DHTBT.And(SiF1-DHTBT1-o)nis less coplanar compared to(SiF1-DHTBT1-m)nand(SiF1-DHTBT1-p)n.It will affect the charge carrier transport and optical properties of the oligomersandpolymers.
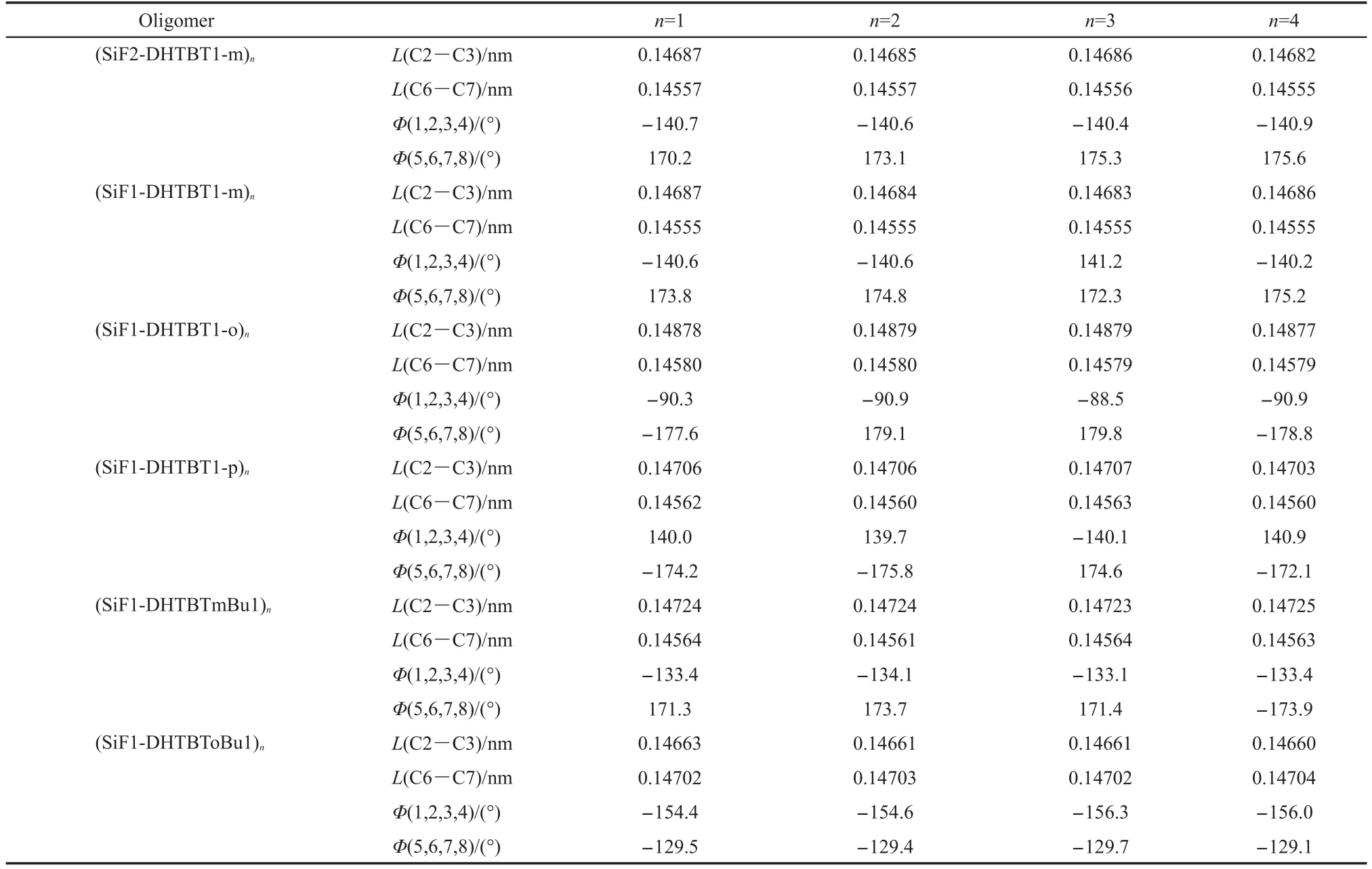
Table 1 Selected important bond lengths(L),and dihedral angles(Φ)of all the oligomers in the ground state with B3LYP/6-31g*
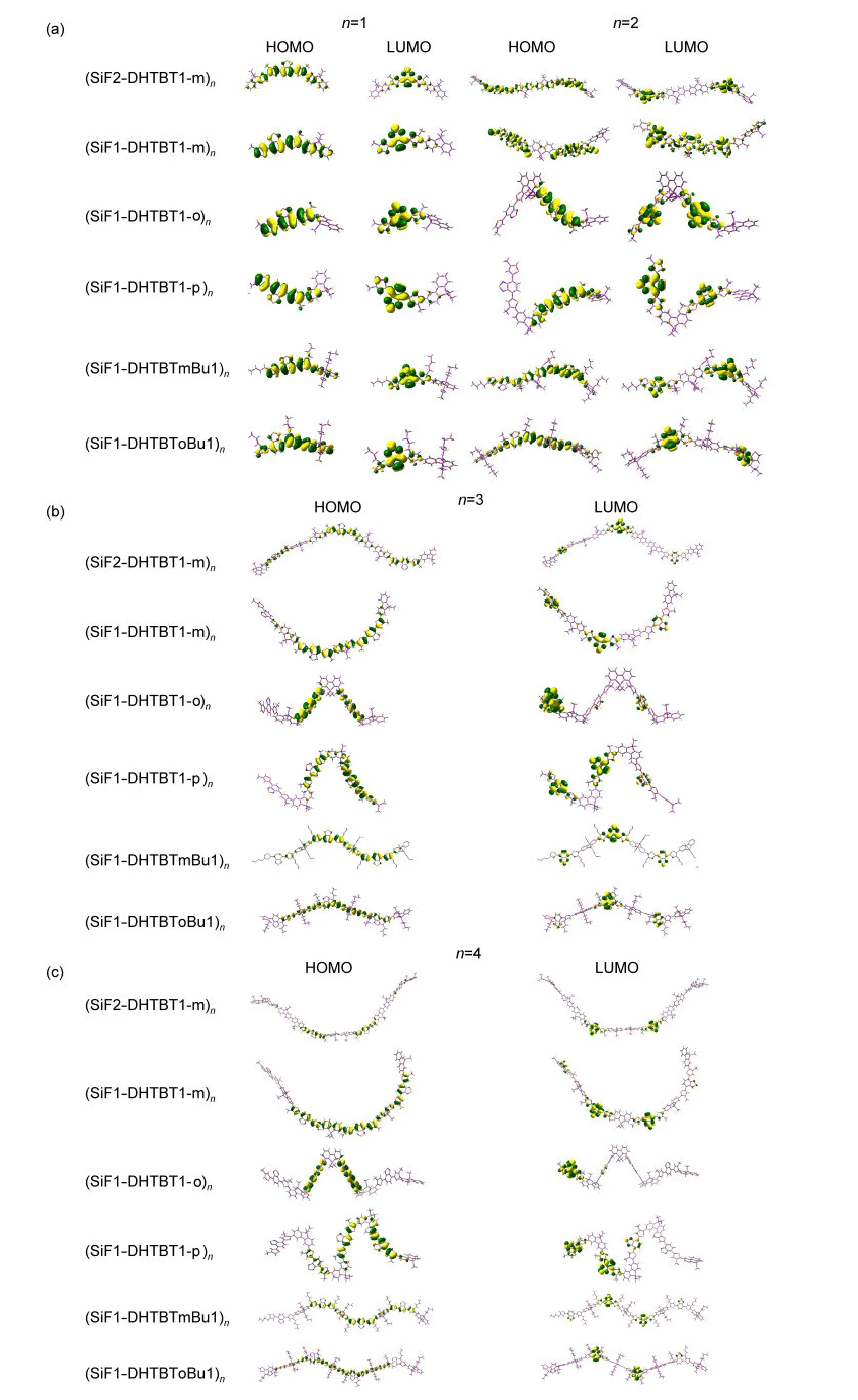
Fig.2 Plots of HOMO and LUMO of all the oligomers(n=1-4)by DFT//B3LYP/6-31g*
In order to characterize the optical and electronic properties,it is significant to examine the HOMO and LUMO energy levels of the oligomers and polymers,and their delocalized electron density and energy gaps.The contour plots of HOMO and LUMO for all the oligomers(n=1-4)are shown in Fig.2,and the frontier molecular orbital compositions of the fragments(arranged from left to right in the order of Fig.2)for the oligomers(n=4)are given in Table 2.It can be seen from Fig.2 that with the increase ofn,for(SiF1-DHTBT1-o)n,the HOMOs gradually localize on the core DHTBT groups,the LUMOs gradually localize on one side benzothiadiazole group of the oligomers.In(SiF1-DHTBT1-p)n,the HOMO gradually localizes on one side DHTBT groups,the LUMOs gradually localize on the other side DHTBT groups of the oligomers,both HOMO and LUMO are more delocalized compared with(SiF1-DHTBT1-o)n.In(SiF1-DHTBTmBu1)n,(SiF1-DHTBToBu1)n,and(SiF1-DHTBT1-m)n,the HOMOs gradually delocalize on the core DHTBT and SiF groups,the LUMOs gradually localize on the core DHTBT groups of the oligomers.While in(SiF2-DHTBT1-m)n,the HOMOs and LUMOs gradually localize on the core DHTBT groups.The frontier molecular orbital compositions of the oligomers(n=4)in the ground state(seen in Table 2)also confirm this fact.Interestingly,in(SiF2-DHTBT1-m)4,the HOMOs and LUMOs are both centrosymmetric.All the analyses given above indicate that electronic charge transfer may take place easily when an electron is excited from the ground state to the first excited state in(SiF1-DHTBT1-o)nand(SiF1-DHTBT1-p)n.
The HOMO and LUMO can be used to estimate the barrier height of injection hole and electron of polymers at ambient conditions.42,43The higher HOMO and LUMO energy levels,lead to the easier hole injection and the more difficult electron injection.The HOMO and LUMO levels as well as their energy gaps of all the oligomers(n=1-4)were computed and listed in Fig.3.As the repeat unitnincreasing,the separation energies between the frontier occupied or unoccupied molecular orbitals get gradually smaller,the HOMO energy levels increase,the LUMO energy levels decrease and thus their gaps get smaller(seen in Figs.3 and 4).For the isomers,the HOMO energy levels decrease from(SiF1-DHTBT1-m)nto(SiF1-DHTBT1-p)n,and then to(SiF1-DHTBT1-o)n;the LUMO energy levels decreasein the opposite trend,thus the energy gaps increase in the same order as the HOMO energy levels do(seen in Fig.5).That indicates that(SiF1-DHTBT1-m)nare favorable for accepting holes and electrons efficiently among the isomers,next are(SiF1-DHTBT1-p)n,and last are(SiF1-DHTBT1-o)n.Furthermore,their absorption wavelengths are gradually blue-shifted.The HOMO,LUMO energy levels and their energy gaps(ΔEH-L)of(SiF1-DHTBTmBu1)nare higher than those of(SiF1-DHTBT1-m)nby 0.06,0.03,and 0.09 eV,respectively,it indicates that butyl has only a slight effect on the hole and electron injection performance of polymers,while the position of butyl group on thiazole group significantly affects the HOMO and LUMO energy levels and their gaps in view of the results of(SiF1-DHTBTm-Bu1)nand(SiF1-DHTBToBu1)n.(SiF1-DHTBTmBu1)n(the dihedral angle between the SiF and DHTBTmBu is close to 180°)show better hole and electron injection performance than(SiF1-DHTBToBu1)n(the dihedral angle between the SiF and DHTBTmBu is about 130°)because the former have better conjugation between the electron accepter and donor groups.The large proportion of SiF group decreases the HOMO energy but increases the LUMO energy,and thus broadens the energy gap,which allows the hole and electron injection energy barriers to be increased and makes absorption wavelength blueshifted.It can be seen from Fig.6 that it is easy to inject hole from indium tin oxide(ITO)(~4.7 eV)(except(SiF1-DHTBT1-o)n)and inject electron from Ba(~2.7 eV)to the polymers.Therefore,in this sense,these polymers except for(SiF1-DHTBT1-o)nare strong candidates as good electroluminescent materials.
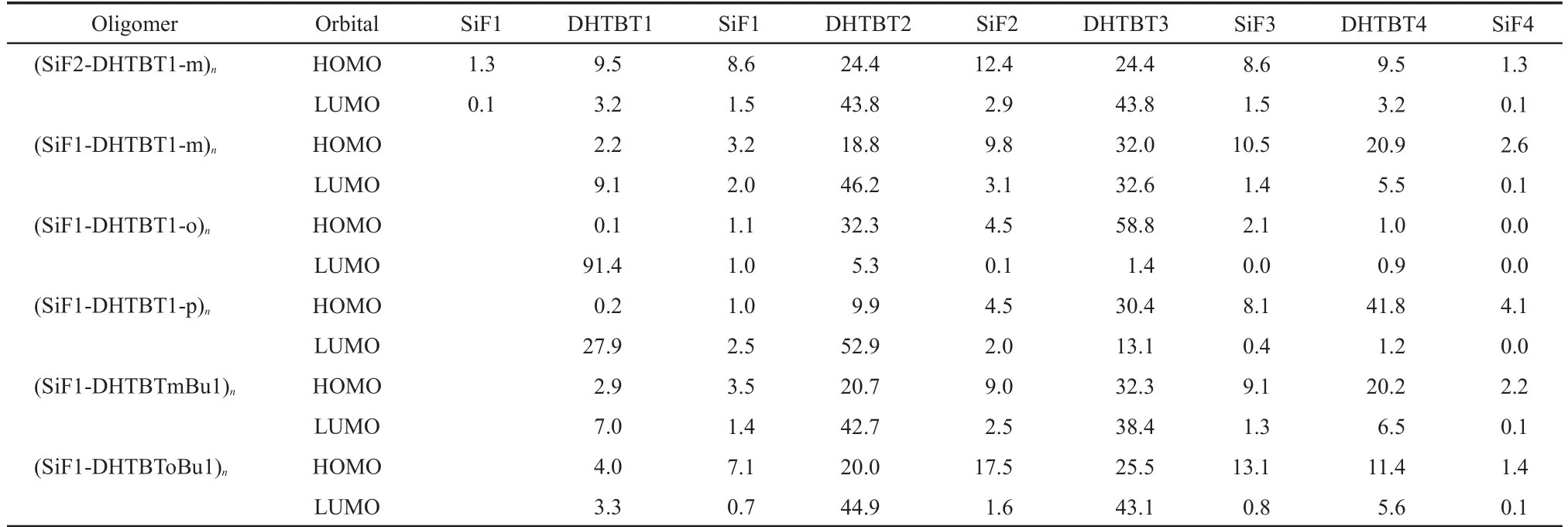
Table 2 Molecular orbital compositions(%)of the fragments(arranged from left to right in the order of Fig.2)in the S0states for the oligomers(n=4)by B3LYP/6-31g*
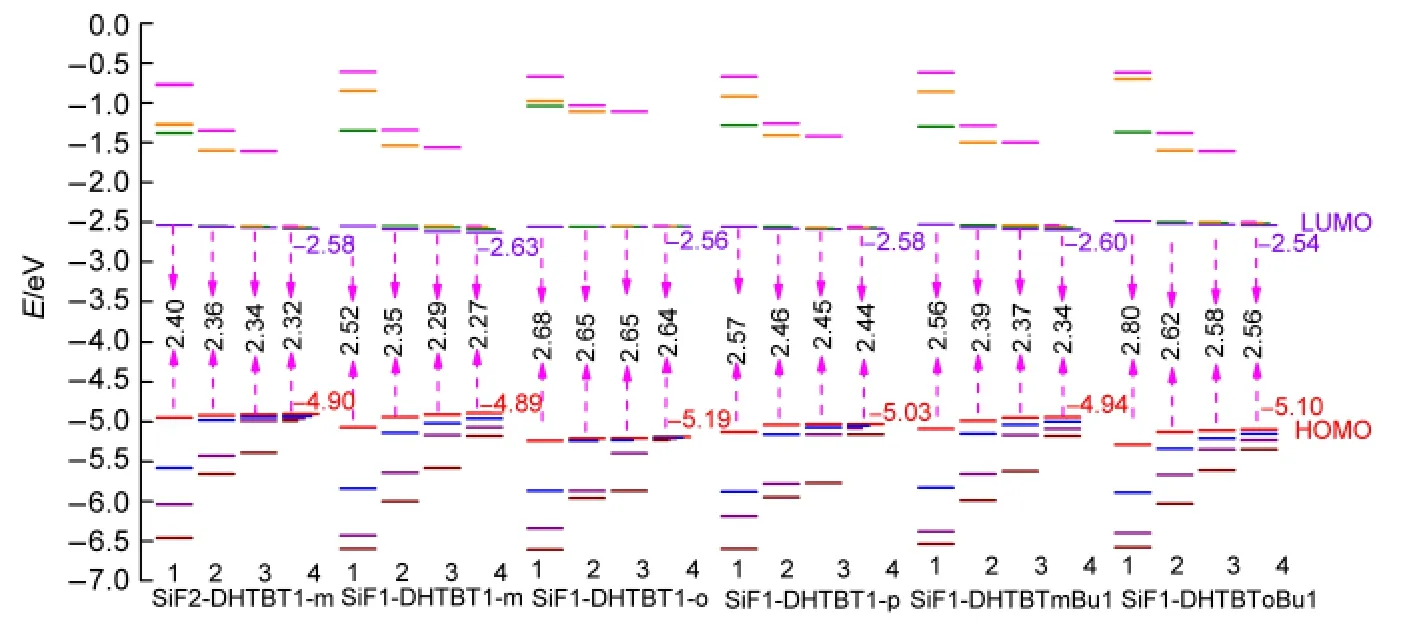
Fig.3 Diagram of the band gap region around the frontier molecular orbitals of all molecular monomers by B3LYP/6-31g*
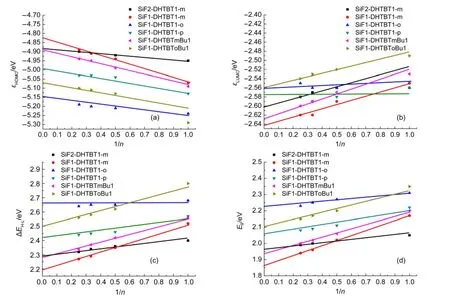
Fig.4 HOMO(a)and LUMO(b)energy levels(εHOMO,εLUMO),HOMO,LUMO energy gaps(ΔEH-L)(c)and the lowest singlet-singlet vertical transition energies(Eg)(d)of all the oligomers as a function of the reciprocal number of the repeated units
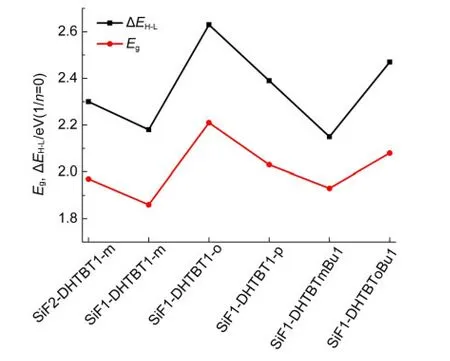
Fig.5 Variation of ΔEH-Land the lowest singlet-singlet vertical transition energies(Eg)of all polymers
3.2 Ionization potentials and electron affinities
According to Eq.(2),for efficient OLEDs,the hole and electron reorganization energies need to be small and close to each other to achieve rapid and balanced carrier transport in emitter,leading to higher fluorescence quantum efficiency of the OLED.
The IPs,EAs,HEPs,EEPs,and reorganization energies(λs)of the studied oligomers calculated by B3LYP/6-31g*are list-ed in Table 3.Through the extrapolation method,those of the polymers were obtained and depicted in Table 3.The order of the negative values of HOMO,LUMO energy levels,IP(a)and EA(a)for the polymers are shown in Fig.7.Even though the HOMO underestimates the ability of losing an electron but the LUMO overestimates the ability of capturing an electron for the polymers,the qualitative law is consistent with the IP and EA(Fig.7).It can be seen from Table 3 that polymers are favorable to injecting hole and electron from the opposite electrodes because of their lower IP and higher EA levels comparing to oligomers.For(SiF2-DHTBT1-m)n,(SiF1-DHTBT1-m)n,and(SiF1-DHTBTmBu1)n,they show relatively better hole and electron injection abilities than others in view of their lower IP and higher EA values.Increasing the proportion of SiF unit only changes the IP value but does not change the EA value,therefore,enhances the hole injection ability but has no effect on the electron injection ability.Compared to(SiF1-DHTBT1-m)n,the poorer hole and electron injection abilities of(SiF1-DHTBTmBu1)ncan be attributed to the steric congestion of butyl group.For(SiF1-DHTBToBu1)n,there is larger steric hindrance comparing to(SiF1-DHTBTmBu1)n,leading to its poorer hole and electron injection abilities.(SiF1-DHTBT1-o)nhave the highest IP and lowest EA values,implying its poorest hole and electron injection abilities,and followed by(SiF1-DHTBT1-p)n.
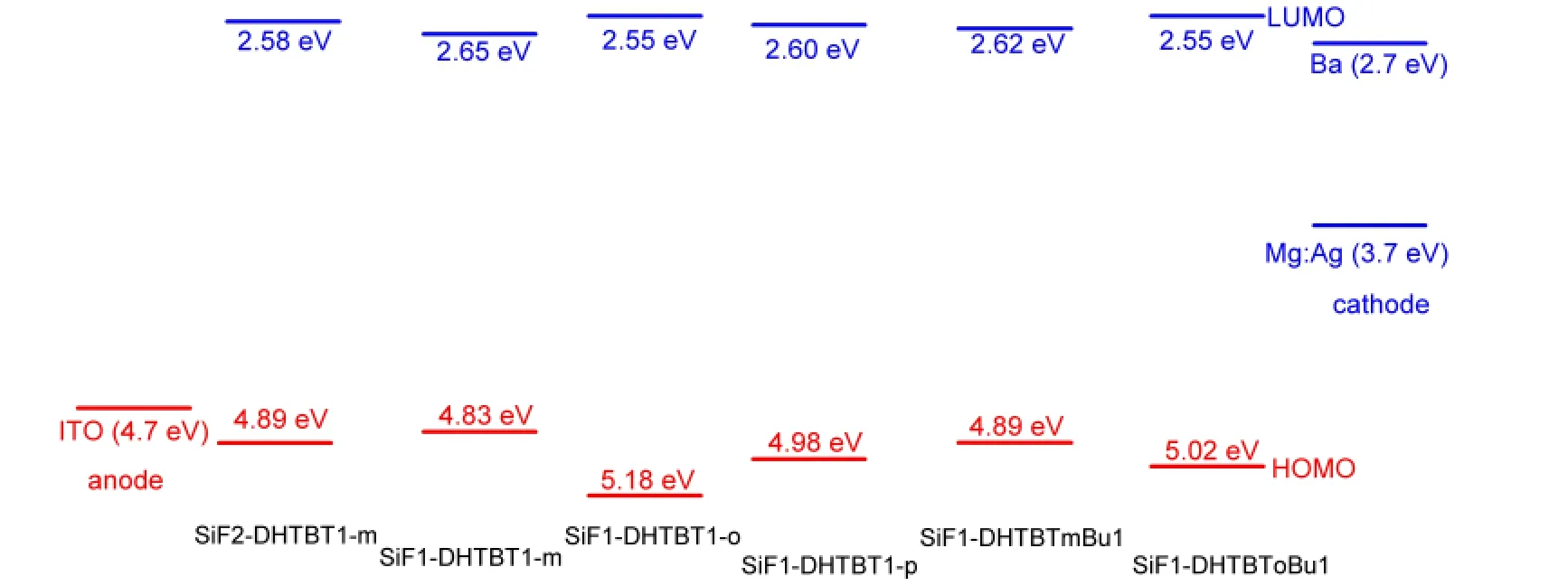
Fig.6 HOMO and LUMO energy levels of the polymers and the electrode potentials of the anode and cathode
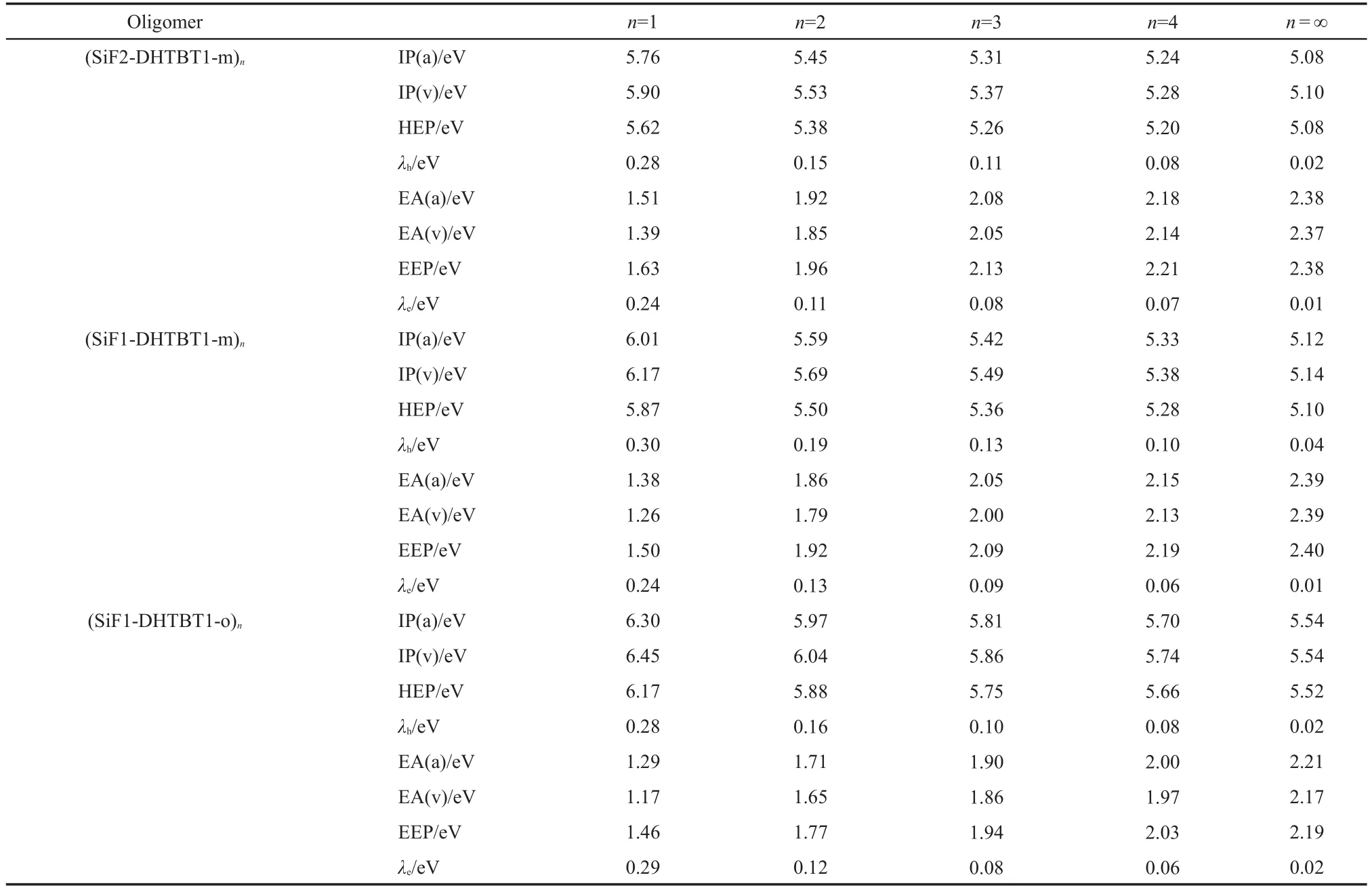
Table 3 Ionization potentials,electron affinities,hole extraction potentials,electron extraction potentials,and reorganization energies for all the oligomers(n=1-4)and polymers(n=∞)
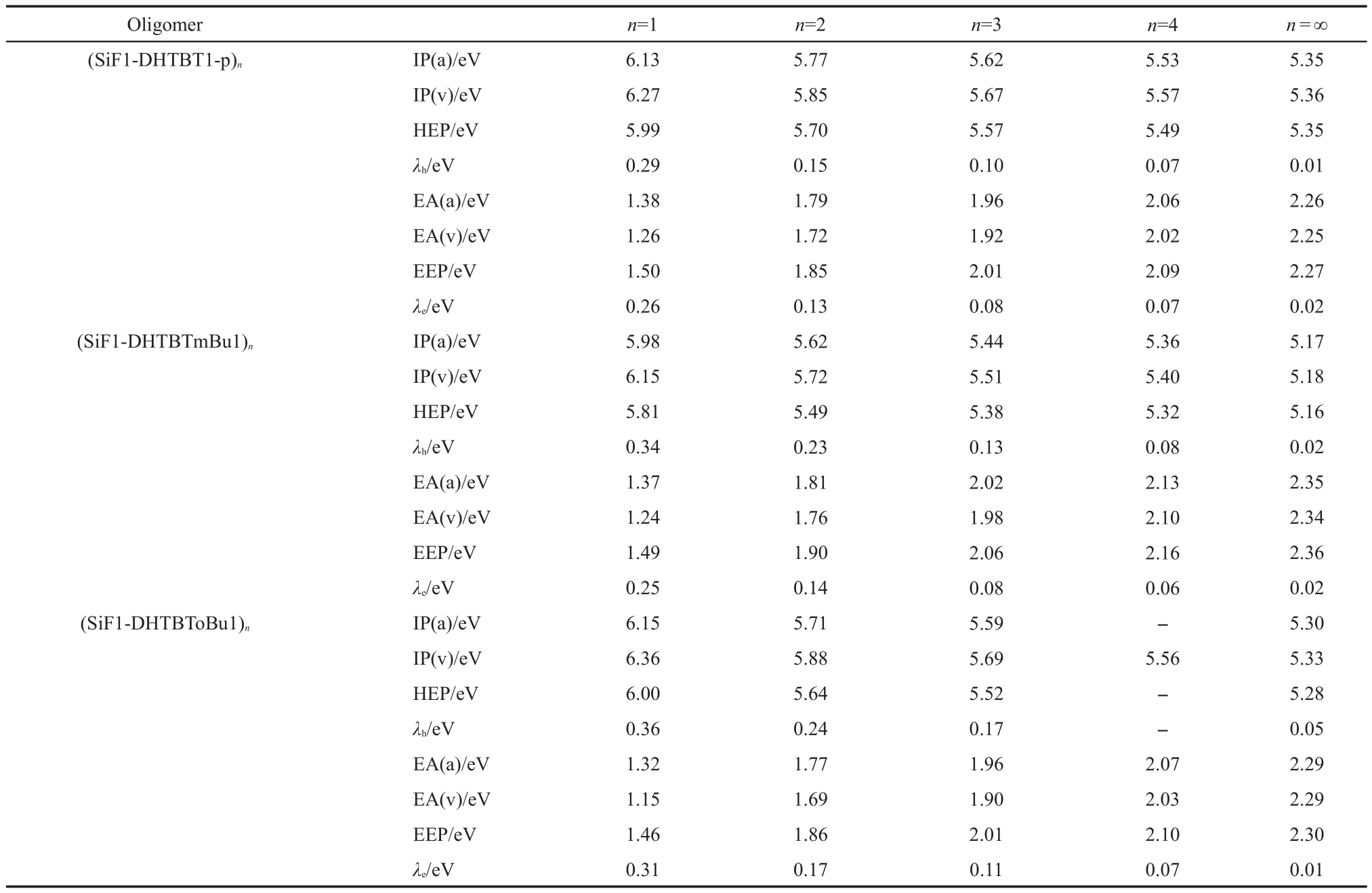
continued Table 3
Compared to PF,the most studied polymers in this work show favorable to injecting a hole and an electron in view of their smaller IP(v)and bigger EA(v)than PF(5.37 eV for IP(v)and 1.20 eV for EA(v)44),except for(SiF1-DHTBT1-o)n(5.54 eV for IP(v)).
According to equation(2),the hole and electron transfer rates increase with decreasing the reorganization energy.As increasing repeat unitn,reorganization energy gets small(see Ta-ble 3),thus the polymers have good hole and electron transport properties and would be good emitting material with high efficiency due to their hole and electron reorganization energies not only extremely small but almost the same,except for(SiF1-DHTBT1-m)nand(SiF1-DHTBToBu1)n.Introducing SiF group into the backbone allows the electron-hole balance.
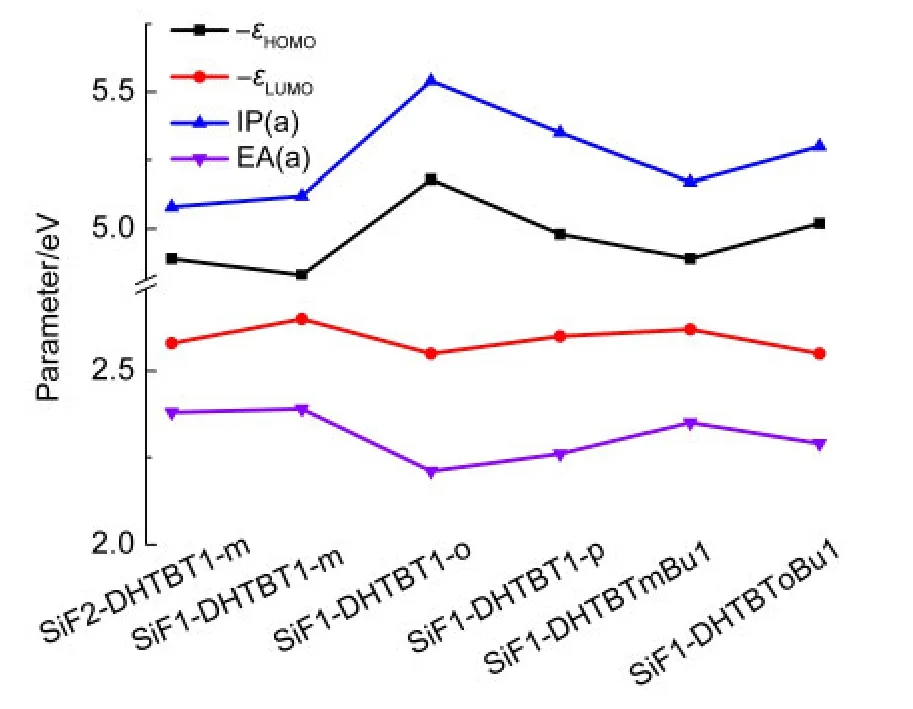
Fig.7 Calculated negative value of HOMO,LUMO energy levels,IP(a),and EA(a)of the polymers
3.3 Absorption spectra and fluorescent emission spectra
The energy gaps(ΔEH-L)are not equal to the optical band gap(Eg)because of the neglect of inter electronic interaction upon the single one electron excitation,45-49and the lowest singlet-singlet transition is not only the electron promotion from HOMO to LUMO,but a combined transition of HOMO-xto LUMO+y(x,y=1,2,3,…),which will subsequently be disscussed(see Fig.5).
The absorption and emission oscillator strengths of the oligomers increase when the repeat unit increases(see Tables 4,5 and Fig.8).The wavelengths of the maximum absorptions are 627.1,663.3,559.0,611.1,638.1,and 592.5 nm for(SiF2-DHTBT1-m)n,(SiF1-DHTBT1-m)n,(SiF1-DHTBT1-o)n,(SiF1-DHTBT1-p)n,(SiF1-DHTBTmBu1)n,and(SiF1-DHTBToBu1)n(see Table 4)respectively,which confirms the prediction from the energy gaps discussed above.The maximum absorption peaks of all monomers arise fromS0toS1,which are dominated by HOMO→LUMO.With increasing the conjugated length,the lowest optically allowed electronic transition is not mainly the promotion of the electron from HOMO to LUMO becauseof the nearly degenerated HOMOs and LUMOs,except for(SiF1-DHTBToBu1)n.
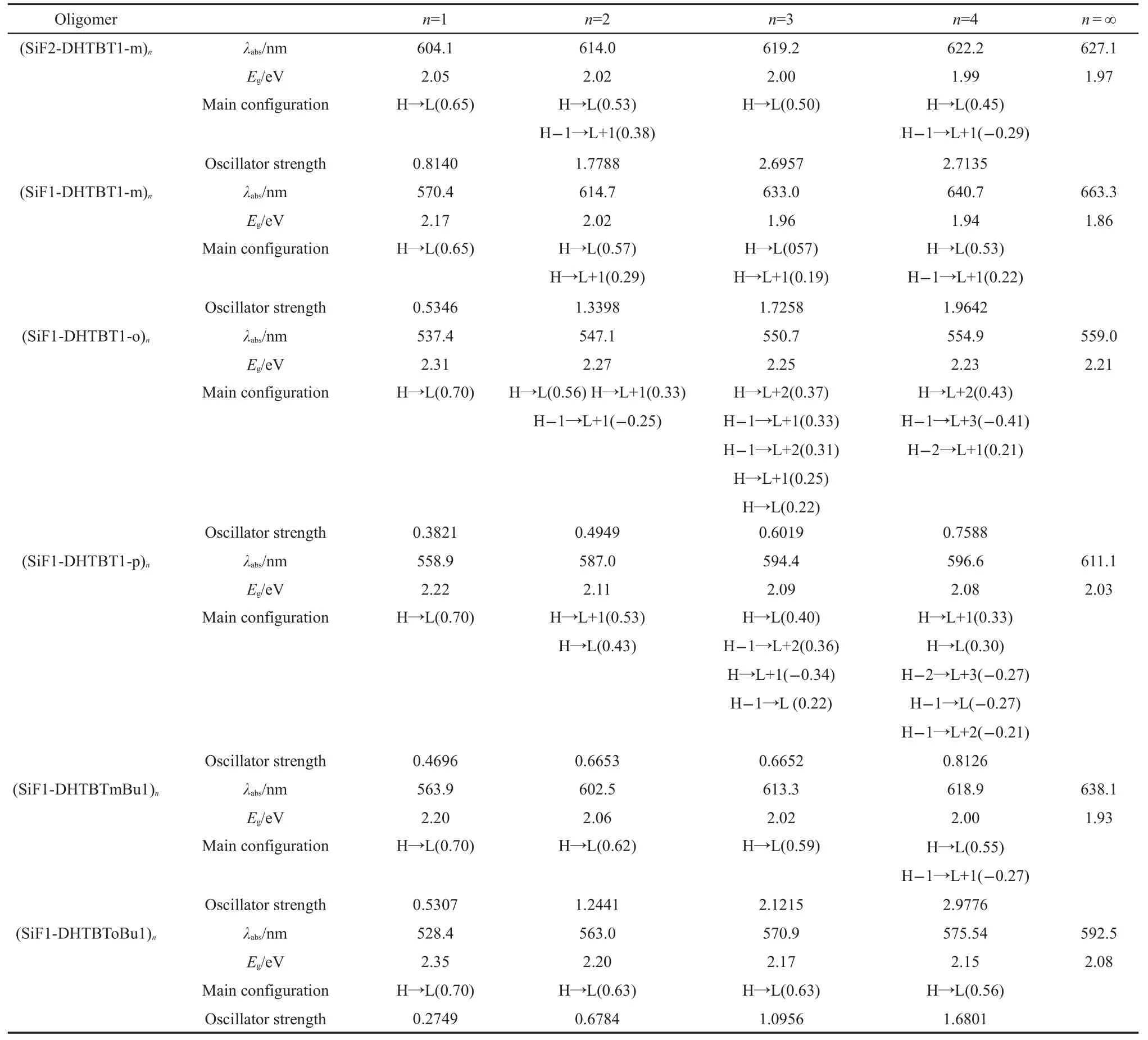
Table 4 Calculated absorption spectral data of all the oligomers(n=1-4)and polymers(n=∞)
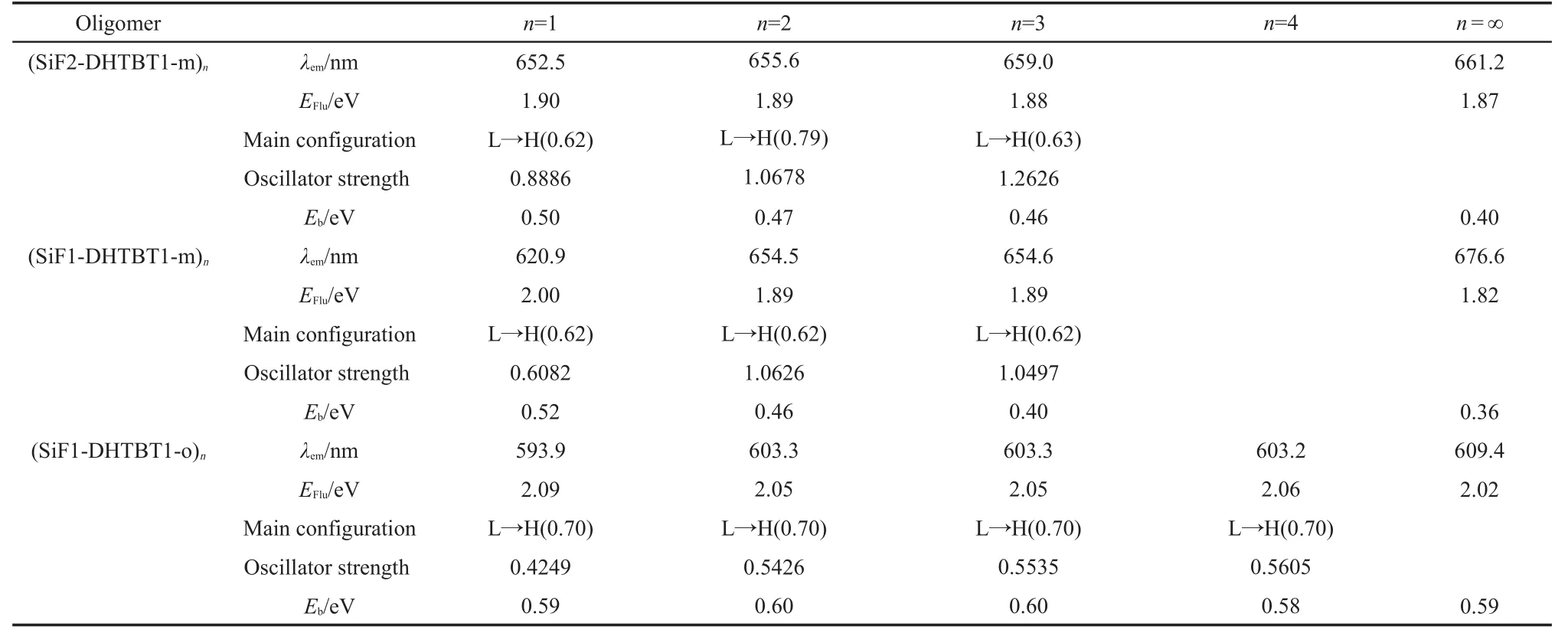
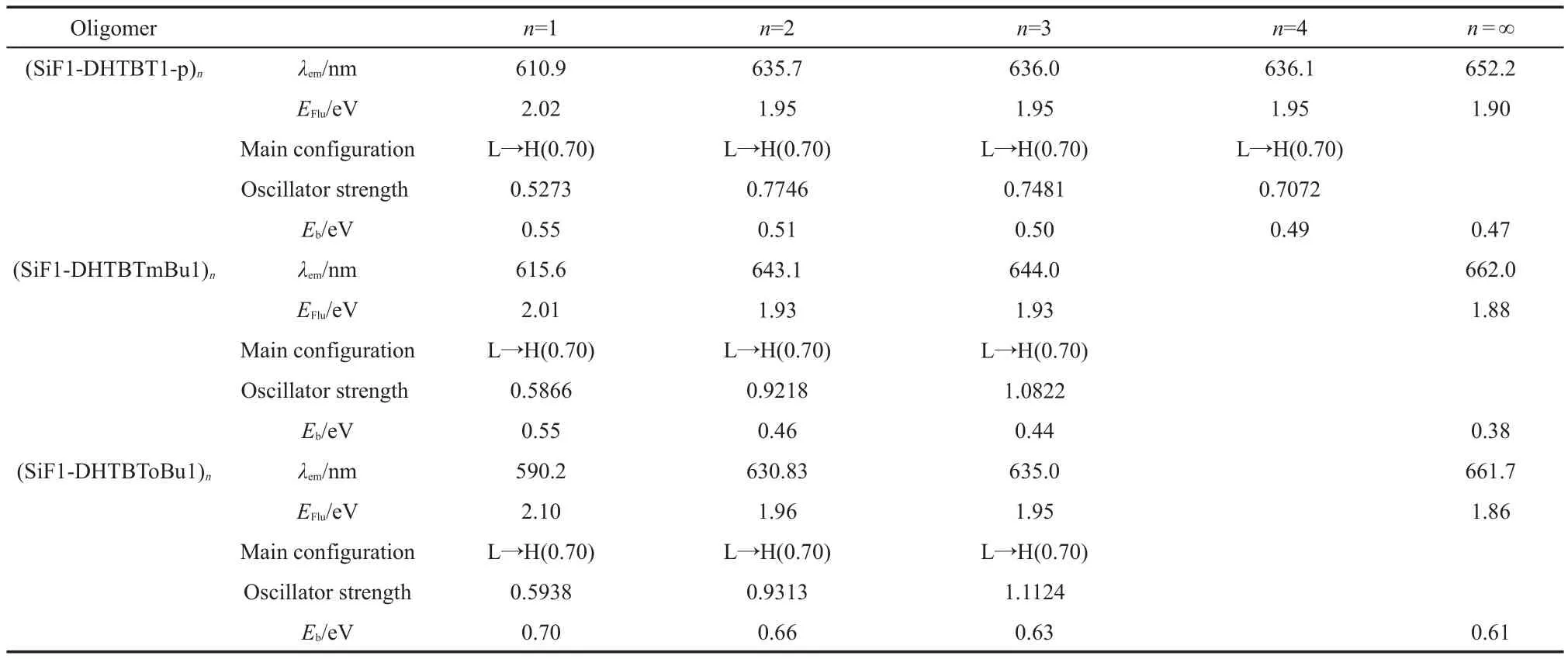
Table 5 Fluorescent emissions of all the oligomers(n=1-3(4))and polymers(n=∞)
continued Table 5
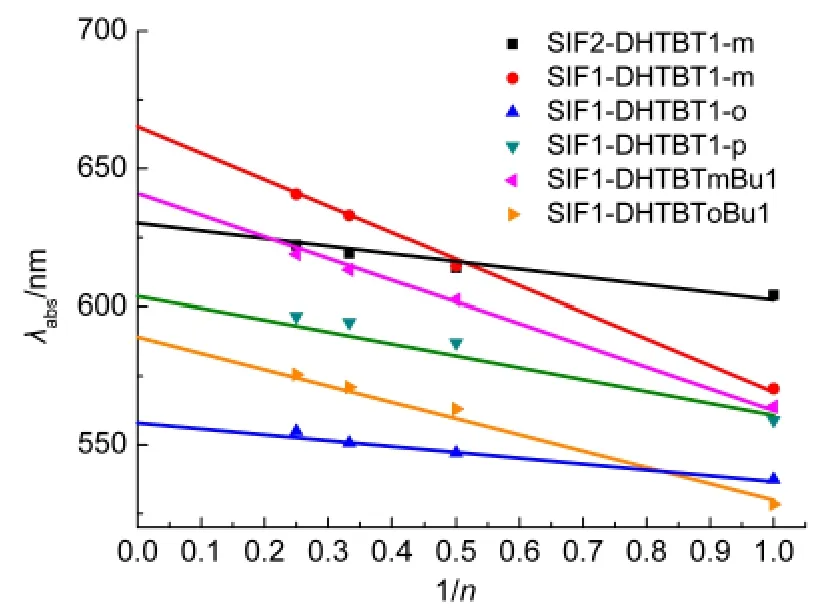
Fig.8 Absorption of all the oligomers as a function of the reciprocal number of the repeated units
The predicted emission spectra are located in near infrared light region(760 nm>λem>620 nm)(Table 5).The maximum emission peaks are all assigned toπ→π*electronic transitions,arising fromS1toS0,which are due to the electron transitions from LUMO to HOMO.
Moreover,the exciton binding energies of all studied polymers are at least 14 times larger than 25 meV,which implies their room-temperature stimulated emission.The largerEbvalues(>600 meV)of(SiF1-DHTBToBu1)nand(SiF1-DHTBT1-o)nindicate that the OLEDs with them may have higher fluorescent quantum efficiency because they are easier to form an exciton in the OLEDs.
4 Conclusions
The electronic structures and photophysical properties of a series of red-emitting polymers based on benzothiadiazole and silafluorene were investigated in this work.The results show that(SiF1-DHTBT1-m)nare favorable for accepting a hole and an electron among the isomers,next are(SiF1-DHTBT1-p)n,and last are(SiF1-DHTBT1-o)n.The position of butyl group on thiazole group significantly affects the hole and electron injection abilities as well as the absorption and emission wavelengths.
Overall,(SiF2-DHTBT1-m)nand(SiF1-DHTBTmBu1)ndemonstrate good hole and electron injection abilities as well as fast and balanced electron/hole transport performance.Meanwhile,theirEbvalues are at least 14 times larger thankBT(~25 meV,at room temperature).So they can be used as materials for high efficient OLEDs.(SiF1-DHTBT1-o)nare most unfavorable for being used as the hole and electron injection materials because their largest IP and smallest EA,but they may be good emitter layer materials because they are easier to form an exciton in OLEDs.
The predicted emission spectra of polymers are located in red visible light region(652.2-676.6 nm)except for(SiF1-DHTBT1-o)n(609.4 nm).
(1) Garcia,A.;Bakus,R.C.,II;Zalar,P.;Hoven,C.V.;Brzezinski,J.Z.;Nguyen,T.Q.J.Am.Chem.Soc.2011,133(8),2492.doi:10.1021/ja106268w
(2) Pei,Q.;Yu,G.;Zhang,C.;Yang,Y.;Heeger,A.J.Science1995,269(5227),1086.doi:10.1126/science.269.5227.1086
(3)Burn,P.L.;Holmes,A.B.;Kraft,A.;Bradley,D.D.C.;Brown,A.R.;Friend,R.H.;Gymer,R.W.Nature1992,356(6364),47.doi:10.1038/356047a0
(4)Xu,X.;Han,B.;Chen,J.;Peng,J.;Wu,H.;Cao,Y.Macromolecules2011,44(11),4204.doi:10.1021/ma200191p
(5) Park,M.J.;Lee,J.;Jung,I.H.;Park,J.H.;Wang,D.H.;Shim,H.K.Macromolecules2008,41(24),9643.doi:10.1021/ma802043q
(6)Allard,S.;Forster,M.;Souharce,B.;Thiem,H.;Scherf,U.Angew.Chem.Int.Edit.2008,47(22),4070.
(7) Dimitrakopoulos,C.D.;Malenfant,P.R.L.Adv.Mater.2002,14,99.
(8) Bao,Z.;Lovinger,A.J.;Brown,J.J.Am.Chem.Soc.1998,120(1),207.doi:10.1021/ja9727629
(9) Forrest,S.R.Chem.Rev.1997,97,1973.
(10) Sirringhaus,H.Proc.IEEE2009,97(9),1570.doi:10.1109/JPROC.2009.2021680
(11)Ho,C.L.;Wang,Q.;Lam,C.S.;Wong,W.Y.;Ma,D.;Wang,L.;Gao,Z.Q.;Chen,C.H.;Cheah,K.W.;Lin,Z.Chem.Asian J.2009,4(1),89.doi:10.1002/asia.v4:1
(12) Cerezo,A.;Clifton,P.H.;Galtrey,M.J.;Humphreys,C.J.;Kelly,T.F.;Larson,D.J.;Lozano-Perez,S.;Marquis,E.A.;Oliver,R.A.;Sha,G.;Thompson,K.;Zandbergen,M.;Alvis,R.L.Mater.Today2007,10(12),36.doi:10.1016/S1369-7021(07)70306-1
(13) Roncali,J.;Leriche,P.;Cravino,A.Adv.Mater.2007,19(16),2045.
(14) Roncali,J.Accounts Chem.Res.2009,42(11),1719.doi:10.1021/ar900041b
(15) Jorgensen,M.;Krebs,F.C.J.Org.Chem.2005,70(15),6004.doi:10.1021/jo0506783
(16)Wang,E.;Li,C.;Zhuang,W.;Peng,J.;Cao,Y.J.Mater.Chem.2008,18(7),797.doi:10.1039/b716607a
(17)Wang,E.;Wang,L.;Lan,L.;Luo,C.;Zhuang,W.;Peng,J.;Cao,Y.Appl.Phys.Lett.2008,92(3),033307.doi:10.1063/1.2836266
(18) Kuhn,H.J.Chem.Phys.1949,17(12),1198.doi:10.1063/1.1747143
(19) Lahti,P.M.;Obrzut,J.;Karasz,F.E.Macromolecules1987,20(8),2023.doi:10.1021/ma00174a056
(20) Hohenberg,P.Phys.Rev.B1964,136(3B),864.
(21) Becke,A.D.J.Chem.Phys.1993,98(7),5648.doi:10.1063/1.464913
(22) Stanton,J.F.;Gauss,J.R.;Ishikawa,N.;Head-Gordon,M.J.Chem.Phys.1995,103(10),4160.doi:10.1063/1.469601
(23) Foresman,J.B.;Head-Gordon,M.;Pople,J.A.;Frisch,M.J.J.Phys.Chem.1992,96(1),135.doi:10.1021/j100180a030
(24)Walters,V.A.;Hadad,C.M.;Thiel,Y.;Colson,S.D.;Wiberg,K.B.;Johnson,P.M.;Foresman,J.B.J.Am.Chem.Soc.1991,113(13),4782.doi:10.1021/ja00013a011
(25) Casida,M.E.;Jamorski,C.;Casida,K.C.;Salahub,D.R.J.Chem.Phys.1998,108(11),4439.doi:10.1063/1.475855
(26) Stratmann,R.E.;Scuseria,G.E.;Frisch,M.J.J.Chem.Phys.1998,109(19),8218.doi:10.1063/1.477483
(27) Matsuzawa,N.N.;Ishitani,A.;Dixon,D.A.;Uda,T.J.Phys.Chem.A2001,105(20),4953.doi:10.1021/jp003937v
(28) Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 09,Revision B.01;Gaussian Inc.:Wallingford,CT,2009.
(29) Marcus,R.Rev.Mod.Phys.1993,65(3),599.doi:10.1103/RevModPhys.65.599
(30) Hush,N.S.J.Chem.Phys.1958,28(5),962.doi:10.1063/1.1744305
(31) Marcus,R.A.J.Chem.Phys.1956,24(5),966.doi:10.1063/1.1742723
(32) Nelsen,S.F.;Blomgren,F.J.Org.Chem.2001,66(20),6551.doi:10.1021/jo001705m
(33) Nelsen,S.F.;Trieber,D.A.;Ismagilov,R.F.;Teki,Y.J.Am.Chem.Soc.2001,123(24),5684.doi:10.1021/ja003436n
(34) Hutchison,G.R.;Ratner,M.A.;Marks,T.J.J.Am.Chem.Soc.2005,127(7),2339.doi:10.1021/ja0461421
(35) Heeger,A.J.;Cao,Y.;Parker,I.D.;Yu,G.;Zhang,C.Nature1999,397(6718),414.doi:10.1038/17087
(36) Karabunarliev,S.;Bittner,E.Phys.Rev.Lett.2003,90(5),057402.doi:10.1103/PhysRevLett.90.057402
(37) Chen,L.;Zhu,L.;Shuai,Z.J.Phys.Chem.A2006,110(50),13349.doi:10.1021/jp0652998
(38) Baldo,M.;Segal,M.Phys.Status Solidi A-Appl.Res.2004,201(6),1205.
(39) Heeger,A.J.Nature of the Primary Photoexcitations in Poly(arylenevinylenes):Bound Neutral Excitons or Charged Polaron Pairs,in Primary Photoexcitations in Conjugated Polymers:Molecular Excitation Versus Semiconductor Band Model.Singapore and River Edge:NJ,1997;pp 20-47.
(40) Seo,J.H.;Jin,Y.;Brzezinski,J.Z.;Walker,B.;Nguyen,T.Q.ChemPhysChem2009,10(7),1023.
(41)Li,Y.;Zou,L.Y.;Ren,A.M.;Feng,J.K.Comput.Theor.Chem.2012,981,14.doi:10.1016/j.comptc.2011.11.021
(42)Xu,X.;Zhu,E.;Bian,L.;Wang,Z.;Wang,J.;Zhuo,Z.;Wang,J.;Zhang,F.;Tang,W.Chin.Sci.Bull.2012,57(9),970.doi:10.1007/s11434-011-4964-3
(43) Tang,W.;Hai,J.;Dai,Y.;Huang,Z.;Lu,B.;Yuan,F.;Tang,J.;Zhang,F.Sol.Energy Mater.Sol.Cells2010,94(12),1963.doi:10.1016/j.solmat.2010.07.003
(44)Wang,J.F.;Feng,J.K.;Ren,A.M.;Liu,X.D.;Ma,Y.G.;Lu,P.;Zhang,H.X.Macromolecules2004,37(9),3451.doi:10.1021/ma035725c
(45) Li,Q.;Yu,J.S.;Li,L.;Jiang,Y.D.;Suo,F.;Zhan,X.W.Acta Phys.-Chim.Sin.2008,24,133.[李 青,于军胜,李 璐,蒋亚东,锁 钒,占肖卫,物理化学学报,2008,24,133.]doi:10.3866/PKU.WHXB20080123
(46) Hay,P.J.J.Phys.Chem.A2002,106(8),1634.doi:10.1021/jp013949w
(47) Curioni,A.;Andreoni,W.;Treusch,R.;Himpsel,F.J.;Haskal,E.;Seidler,P.;Heske,C.;Kakar,S.;van Buuren,T.;Terminello,L.J.Appl.Phys.Lett.1998,72(13),1575.doi:10.1063/1.121119
(48)Hong,S.Y.;Kim,D.Y.;Kim,C.Y.;Hoffmann,R.Macromolecules2001,34(18),6474.doi:10.1021/ma010254k
(49)Liu,X.J.;Lin,T.;Gao,S.W.;Ma,R.;Zhang,J.Y.;Cai,X.C.;Yang,L.;Teng,F.Acta Phys.-Chim.Sin.2012,28,1337.[刘小君,林 涛,高少伟,马 睿,张晋悦,蔡新晨,杨 磊,滕 枫.物理化学学报,2012,28,1337.]doi:10.3866/PKU.WHXB201204092
