New Version of GDP in EU and Its Enlightenment
2014-05-15ZHAOShuangchunCHENYuwenZHAOHongju
ZHAO Shuang-chun, CHEN Yu-wen ZHAO Hong-ju
(1.School of Business Administration, Shenyang Pharmaceutical University, Shenyang 110016, China; 2.Drug Certification Department, Center for Certification of Drug, Shenyang 110003, China)
New Version of GDP in EU and Its Enlightenment
ZHAO Shuang-chun1,2, CHEN Yu-wen1, ZHAO Hong-ju2
(1.School of Business Administration, Shenyang Pharmaceutical University, Shenyang 110016, China; 2.Drug Certification Department, Center for Certification of Drug, Shenyang 110003, China)
Objective To provide a brief introduction on the new version of GDP in EU and to analyze its enlightenment. Methods Documents analysis and comparative study were conducted. Results and Conclusion The amendment to the version of GDP in EU is conducive to drug quality assurance. Some contents such as self-inspection, quality risk management and validation can provide some enlightenment.
the new version of GDP; quality management; GSP
Medicine is a special commodity which is related to people’s live and health, drug circulation is an important industry in the society. In recent years, some drug hazards occurred in the circulation field in China. For instance, in November 2008, 6 patients in a hospital in Yun Nan province were found to have serious adverse drug reaction when they used the Acanthopanax Senticosus injection produced by Wan Dashan Pharmaceutical Company in Heilongjiang Province, it led to three deaths eventually[1]. The government investigated this event and found that some of the herbal injections were contaminated by bacteria when soaked in the rain.
In order to meet the requirements of drug safety for the national Five-year Plan, the new edition of Good Supply Practice (GSP) was issued on January 22nd, 2013, and it was carried out from June 1st, 2013[2]. This regulation substitutes the old version of GSP issued in 2000 and it is the principle for the quality management in drug circulation industry. It is a standard to guarantee the quality of drugs.
Similarly, The EU also has its regulation named GDP which stands for Good Distribution Practice. It can ensure the quality of medicinal products from production to consumers. The EU views GDP as an extension of Good Manufacturing Practice (GMP). Thus the regulation was issued together with GMP on the EU website[3].
1 Brief introduction on the new version of GDP in EU
The distribution of medicinal products is an important part in the integrated supply chain management. The quality of medicinal products can be affected by a lack of adequate control. Therefore, the EU Commission published Guidelines on Good Distribution Practice of Medicinal Products for Human Use (94/C 63/03) in 1994. The guidelines had 34 clauses that contained the general principle, personnel, documents, premises and facilities, sales, returns and self-inspection, etc.
However, with the development of economy, the distribution network for medicinal products is becoming complex and involves many business entities. The old rules can not satisfy the actual needs of business and government supervision. Thus the EU drug administration revised GDP and issued the draft on its website. The new version of GDP was issued on March 8th, 2013, and it was carried out from September 8th, 2013[3].
1.1 Main point of the new version of GDP
The new version of GDP contains the introduction and 10 chapters:
The introduction specifies that this regulation substitutes the version of 1994 and it applies not only to the drug distribution companies, but also to any individuals and entities involved in the distribution.
Chapter 1 is on the QUALITY MANAGEMENT which contains the principle, quality management, outsourcing management, quality review and monitoringand quality risk management.
Chapter 2 is on PERSONNEL which contains the principle, entrepreneurs, other personnel, training and hygiene.
Chapter 3 is on PREMISES and FACILITIES which contains the principle, premises and facilities.
Chapter 4 is on DOCUMENTS which contains the principle and general rules.
Chapter 5 is on OPERATION which contains the principle, qualification of suppliers and purchasers, receiving medicinal products, storage, destruction of obsolete goods, picking, supply and export to third countries.
Chapter 6 is on COMPLAINTS, RETURNS, FAKE or INFERIOR MEDICINAL PRODUCTS and MEDICINAL PRODUCT RECALLS which contain the principle, complaints, returned medicinal products, fake or inferior medicinal products and medicinal product recalls.
Chapter 7 is on OUTSOURCING MANAGEMENT which contains the principle and two parties of the contract.
Chapter 8 is on SELF-INSPECTIONS which contains the principle and self-inspections.
Chapter 9 is on TRANSPORTATION which contains the principle, transportation, containers, packaging and labeling and products packaging requiring special conditions.
Chapter 10 is on SPECIFIC PROVISIONS for BROKERS which contains the principle, quality system, personnel and documents.
The annex contains 12 new terms.
1.2 Comparison between the new version of GDP in EU and the old one
The new version of GDP added more contents compared with the old one, which includes:
The introduction of a quality system which specified responsibilities, processes and risk management principles;
Appropriate documentation which is used to avoid misunderstanding caused by spoken communication;
Sufficient and competent personnel to carry out all the tasks;
Adequate premises, installations and equipment so as to ensure proper storage and distribution of medicinal products;
Appropriate management of complaints, returns, fake or inferior medicinal products and recalls;
Outsourcing activities should be defined clearly to avoid misunderstandings;
It emphasized on the transportation management to avoid breakage, adulteration and theft, and to ensure that the temperature conditions are good in the process of transportation;
Specific rules for brokers (personnel involved in activities in relation to the sales or purchase of medicinal products).
The biggest change in this new version of GDP is that it fully absorbs the quality management system in GMP; the pharmaceutical trading enterprises should establish a stable quality management system which includes organizational structure, procedure, recourses and any activity maintaining drug quality in the process of storage and transportation. This quality system should be well recorded and documented; any deviation from the established procedures should be investigated. The quality system requires the active participation of the enterprises manager. All of these can effectively connect GDP with GMP, and any activity in GDP can be adapted to the quality management system. It will help to maintain the quality in the process of distribution.
In addition, the new version of GDP requires that the enterprises should have necessary facilities and risk assessment should be used to determine the quality of these facilities and equipments. The enterprises should monitor the temperature in the process of transportation and establish emergency measures to guarantee the quality of drugs.
These changes greatly influenced the whole industry. The enterprises should adopt risk assessment in the quality management and control key points in every step to ensure the quality of drug.
Thus the new edition of GDP is an effective supplement to the 1994 edition of GDP.
1.3 Comparison between the new version of GDP in EU and the new GSP in China
The new version of GSP was carried out on June 1st, 2013. This new regulation contains general rules, quality management of drug wholesalers, quality management of drug retailers and supplementary provisions. Moreover, China Food and Drug Administration (CFDA) issued five appendices which should be used as supportive documents on November 23rd, 2013[4]. These appendices are Cold and Frozen Product’s Storage and Transportation, Computer System in Drug Distributors, Temperature and Humidity Automatic Monitoring, Acceptance and Verification Management.
Table 1 shows the comparison:
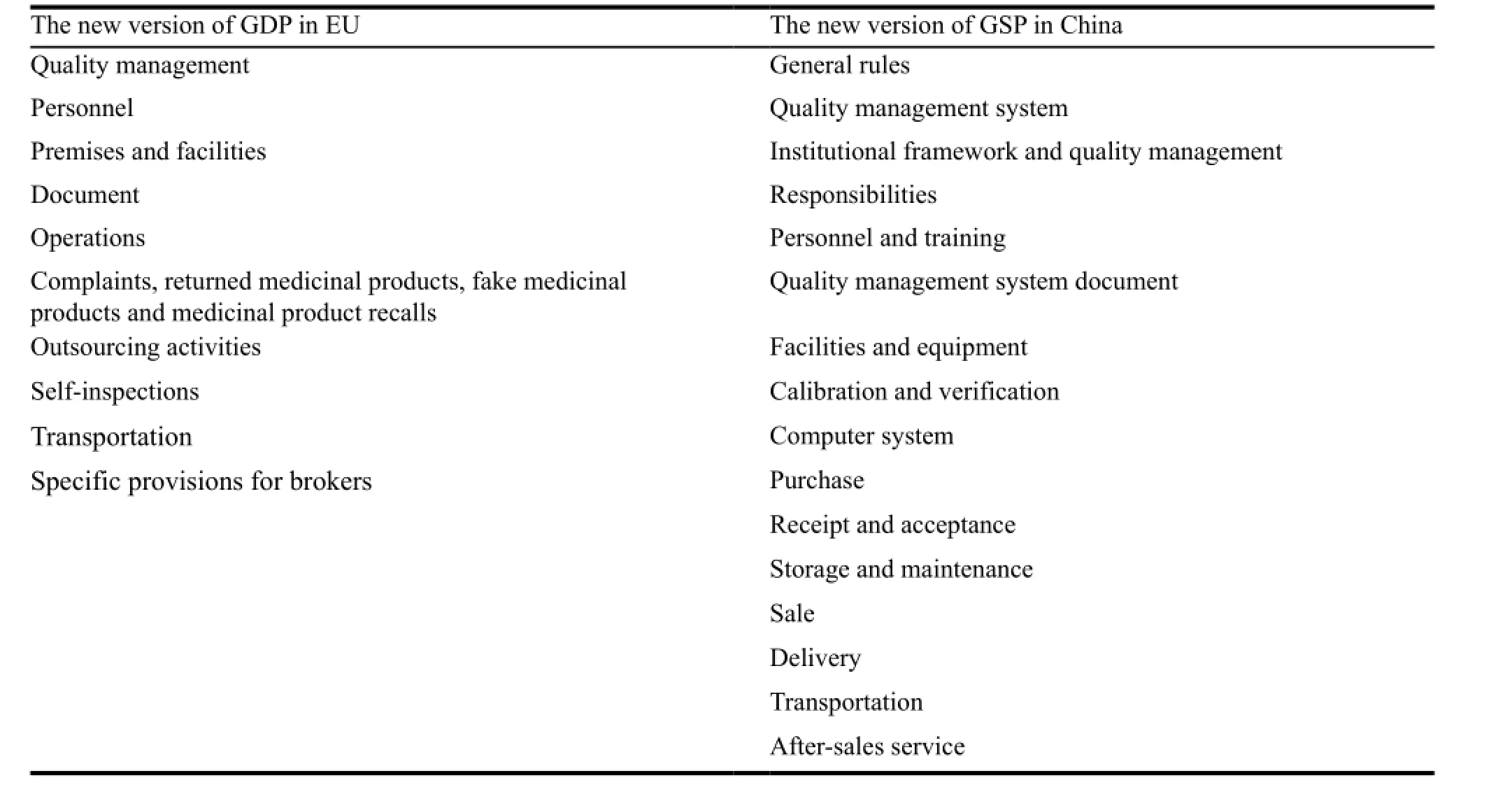
Table 1 The comparison between two regulations
The comparison clearly shows the new version of GSP in China is more specifically classified. It strengths the invoice management, cold chain management and transportation management. It also puts more emphasis on the channel management and temperature and humidity control. CFDA issued five supportive documents and required the distributors should implement them strictly. Thus the new version of GSP in China is more concrete than the EU’s GDP.
Our GSP is complicated as it reflects our national conditions. Now we have 13 thousand drug wholesalers and 420 thousand drug retailers which lead to some problems, and the quality of drugs cannot be guaranteed in the process of distribution. So we need a more strict regulation to deal with these problems. Our new version of GSP is to meet the requirement of the Twelfth Five-Year Plan, and it is well specified, for example, the head of quality control should hold a bachelor’s degree, a licensed pharmacist certification and at least 3 years working experiences. On the contrary, the EU’s GDP is much more ambiguous and inclusive, for example, it regulates that the head of quality control should have competence and experience, and he or she needs some training to learn GDP. The reason is that the number of drug trading enterprises in EU is fewer than that in China, and the personnel quality is high. So there is no need to specify its requirement for personnel.
Therefore, we can see the overall development and its general level in a certain area or a country from the regulation.
2 Enlightenment on the new version of GDP in EU
Although the length of the new version of GDP is less than GSP, some parts in this regulation are worth learning.
2.1 It pays more attention to self-inspection
Chapter 8 focuses more on self-inspection in distributor’s quality management. The self-inspection includes the quality system objective, assessment on the quality system such as complaints, deviation, corrective measures and risk assessment, etc. The results of self-inspection should be recorded immediately and communicated effectively.
However, the new version of GSP in China adopts the concept of internal audit to substitute self-inspection. But the content of internal audit is insufficient, article 8 and article 9 regulate that companies should carry out internal audit at regular intervals or when the key elements of quality management system changes. But it does not emphasize the scope of the internal audit and the effective communication between each department. We suggest the governmentdepartments increase the content of internal audit and the distributors must implement it strictly.
2.2 It focuses on the quality risk management
The new version of GDP in EU requires enterprises should focus more on the quality risk management. The quality risk management is a systematic course that occurs in the process of the assessment, control, communication and audit. Enterprises should adopt perspective or retrospective way to implement risk management. It puts forward that the risk assessment should be based on the scientific guidance, course control and the protection of patients. It suggests enterprises should implement the quality risk management according to ICH Q9. ICH Q9 provides some useful tools for enterprises such as HACCP, FMEA, FAT, etc[5]. It provides the theoretical basis for enterprises’ risk management.
However, the new edition of GSP in China only illustrates that enterprises should conduct quality risk management; it does not tell enterprises how to carry out this work. It cannot provide specific method on the technical level for enterprises, and the supervisors cannot guide enterprises to implement quality risk management effectively. We suggest the government should issue quality risk management as a supportive document to facilitate the effective implementation and supervision.
2.3 It focuses on the qualification and verification
The new version of GDP emphasizes that enterprises should identify their internal key equipment and key course to ensure the effective operation. Enterprises should conduct qualification to its key equipment and verification to its key course. It specifies that the scope of qualification and verification is on the basis of written risk assessment. Thus we can see clearly this new version of GDP emphasizes that enterprises should take initiative to carry out the risk assessment, qualification and verification.
Article 53 in the new edition of GSP regulates that enterprises should verify their refrigeration house, temperature and humidity monitoring system and cold chain transportation, and the use of related facilities according to the results of verification. From the regulation we can draw the conclusion that enterprises should verify their cold chain system according to GSP, however, it does not define the qualification and verification scope on the results of risk assessment. We suggest that the government should take enterprises as executors to carry out the risk management.
3 Conclusions
This paper briefly introduces the main content of the new version of GDP in EU, and compares it with the new version of GSP in China. Then we analyze the enlightenment of GDP to us. We want to provide some reference for our government department to make our regulation more effective in the future.
[1] ZHANG Ying-qiu. Quality Risk Management in Drug Circulation Section [M]. Shandong: Shandong University, 2012: 9.
[2] Good Sales Practice (Number 90 by MOH) [EB/OL]. http://www. sfda.gov.cn/WS01/CL0053/78464.html, 2013-01-22.
[3] Good Distribution Practice [EB/OL]. http://ec.europa.eu/health/ human-use/good_distribution_practice/index_en.htm, 2013-03-08.
[4] The Announcement about Issuing 5 Appendixes Relating to GSP [EB/OL]. http://www.sfda.gov.cn/WS01/CL0087/93757.html, 2013-10-30.
[5] State Food and Drug Administration Certificate Center. EU Guidelines of GMP [M]. Beijing: Chinese Medical Technology Press, 2008: 230.
Author’s information: CHEN Yu-wen, Professor. Major research area: GSP. Tel: 024-23986552, E-mail: cywwyc@163.com
杂志排行
亚洲社会药学杂志的其它文章
- Marine Pharmaceutical Industry Development in Developed Countries and Its Enlightenment
- Study on the Establishment of Efficiency Evaluation System of Drug Supervision and Sampling Test
- FDA Practices: GMP Quality System Inspection and Evaluation
- Family Economic Risk Caused by Five Major Chronic Diseases among Urban Residents: A Comparative Study Based on the Data from 9 Cities in China
- Three Therapeutic Regimens in Treatment of Community-acquired Pneumonia: A Cost-effectiveness Analysis
- Pharmacovigilance System for Pharmaceutical Enterprises in EU: Regulation and Its Enlightenment
