HRE和hTERT修饰条件复制型腺病毒携带Egr-1介导的Smac对人食管癌细胞Eca109周期和凋亡的影响
2014-05-06陈文庆金新天
陈文庆,金新天,闫 松,刘 刚,王 巍
(吉林省肿瘤医院,吉林长春130012)
HRE和hTERT修饰条件复制型腺病毒携带Egr-1介导的Smac对人食管癌细胞Eca109周期和凋亡的影响
陈文庆,金新天,闫 松,刘 刚,王 巍*
(吉林省肿瘤医院,吉林长春130012)
目的 探讨以HRE和hTERT修饰条件复制型腺病毒携带Egr-1介导的Smac(CARd.pE-Smac)对人食管癌细胞Eca109周期和凋亡的影响。方法 利用氯化钴进行化学乏氧,病毒感染滴度为5MOI[Multiplicity of infection(virus/cell)]感染人食管癌Eca109细胞24h,并进行2Gy照射,12h后分别利用Western blotting检测Smac蛋白的表达,PI染色和AnnexinⅤ-FITC双染,流式细胞术检测细胞周期和细胞凋亡变化。实验分为control、CARd.pESmac、hypoxia、2Gy、H+CARd.pE-Smac、CARd.pE-Smac+2Gy和H+CARd.pE-Smac+2Gy组。结果 CARd.pE-Smac感染常氧和乏氧的Eca109细胞后均未见Smac蛋白表达增加,而2Gy照射后,常氧、感染CARd.pE-Smac和乏氧再感染CARd.pE-Smac均能使Smac蛋白表达增加,尤其以三者联用后Smac表达增加最大;感染CARd.pESmac未对细胞周期有明显改变,而乏氧、2Gy和感染CARd.pE-Smac任二者(乏氧与2Gy除外)或者三者联用均能显著增加S期和G2/M期细胞百分比增加(P<0.05,P<0.01),尤其以三者联用作用更强;而且,对于细胞凋亡的诱导作用与周期进程变化的规律相类似。结论 HRE和hTERT修饰条件复制型腺病毒携带Egr-1介导的Smac在人食管癌细胞Eca109中显著过表达,且能够导致细胞发生S期延迟和G2/M期阻滞,并诱导细胞发生凋亡。
缺氧反应元件;人端粒酶逆转录酶;条件复制型腺病毒;人食管癌
(Chin J Lab Diagn,2014,18:1054)
食管癌是消化系统常见恶性肿瘤,我国是食管癌的高发地区,食管癌主要采用以手术为主,放化疗为辅的治疗方案,但由于个体差异导致患者产生放化疗耐受,从而影响效果。肿瘤基因-放射治疗提出将早期生长因子-1(early growth factor-1,Egr-1)启动子置于治疗基因的上游,从而增强辐射条件下治疗基因的表达,提高效果[1,2]。但是,肿瘤基因放射治疗中如何提高靶向性和选择高效的靶基因非常重要。第2个线粒体衍生的胱天蛋白酶激活剂(second mitochondria-derived activator of caspase,Smac)是2000年Wang等[3,4]从HeLa细胞中分离出并的一种新型线粒体蛋白质,具有显著的促凋亡活性,其过表达具有增强肿瘤细胞放射敏感性的功能,可以作为靶基因;另外,人端粒酶逆转录酶(human telomerase reverse transcriptase,hTERT)启动子具有肿瘤细胞内高表达的特点,而缺氧反应元件(Hypoxia response elements,HREs)具有乏氧诱导特性[5,6],将HRE和hTERT串联来修饰条件复制型腺病毒(conditionally replicative adenovirus,CRAd)对Smac的调控,有望实现靶基因在乏氧肿瘤细胞的高效表达,而且也充分结合Egr-1的辐射诱导特性,大大提高肿瘤基因-放射治疗效果。基于此,本研究利用HRE和hTERT修饰条件复制型腺病毒携带Egr-1介导的Smac表达,观察对食管癌细胞Eca109周期和凋亡的影响,以期为食管癌的基因-放射治疗提供新思路。
1 材料与方法
1.1 细胞系及培养
人食管癌细胞株Eca109购自中国科学院上海细胞生物学研究所,利用含10%胎牛血清、100U/ml青霉素和链霉素的PRMI1640培养基(均为Gibco产品,美国)培养,条件为37℃,5%CO2.
1.2 条件复制型腺病毒
HRE和hTERT修饰的条件复制型腺病毒Ad.Egr-1-Smac-HRE-hTERT-E1A-E1Bp-E1B55K(CARd.pE-Smac)由吉林大学卫生部放射生物学重点实验室刘威武博士构建并惠赠[7],由HRE和hTERT串联修饰腺病毒载体的复制,而且还具有Egr-1介导Smac过表达的特性。
1.3 实验分组、乏氧与照射
实验分为control、CARd.pE-Smac、hypoxia(H)、2Gy、H+CARd.pE-Smac、CARd.pE-Smac+2Gy和H+CARd.pE-Smac+2Gy。人食管癌细胞Eca109采用化学乏氧剂氯化钴(CoCl2,Sigma产品,美国)模拟缺氧状态,终浓度为150μmol/L,乏氧时间为24h[7,8]。采用X射线深部治疗机进行照射(Philips),照射条件为200kV电压,10mA电流,滤板为0.5mm Cu和1.0mm Al,靶皮距离为50cm,剂量率为0.287Gy/min,总剂量为2Gy[7]。
1.4 Smac在人食管癌细胞Eca109中的表达
对数生长期的Eca109细胞按照1×106个/孔接种于6孔板,24h后待细胞稳定后给予终浓度为150μmol/L的CoCl2模拟乏氧状态,24h后将病毒感染滴度5MOI[Multiplicity of infection(virus/cell)]CARd.pE-Smac感染细胞,24h给予2Gy照射12h后,按照《分子克隆技术》步骤提取各组细胞蛋白,分装后冻存于-70℃冰箱中,备用。按照每条泳道50μg蛋白上样后,进行SDS-PAGE电泳进行分离,转膜后利用5%脱脂奶粉封闭1h,β-actin和Smac一抗4℃孵育过夜,TBST缓冲液洗2次,利用辣根过氧化物酶标记的二抗在37℃条件下继续孵育1h,发光,定影,拍照,分析。
1.5 细胞周期检测
细胞分组及处理方式同1.4,按密度为3×105个/孔接种24孔板,细胞收集后PBS洗2次后弃上清,每个离心管中加入RNAse A 50μl,PI 200μl,混匀,室温避光反应20min后上机检测,每个样品收取1.0×104个细胞。CellQuest软件收集并分析数据,结果以细胞百分比表示。
1.6 细胞凋亡检测
细胞分组及处理方式同1.5,细胞收取后采用Annexin V-FITC试剂盒检测细胞凋亡变化。细胞PBS洗2次后弃上清,每个离心管中加入500μl缓冲液重悬细胞沉淀,加入5μl Annexin V-FITC和5 μl PI,混匀,室温避光反应5-15min,2h内上机检测,每个样品收取1.0×104个细胞。CellQuest软件收集并分析数据,结果以细胞百分比表示。
1.7 统计学处理
实验数据以¯x±s表示,利用SPSS17.0统计软件one-way ANOVA检验进行分析,P<0.05或P<0.01表示具有统计学差异。
2 实验结果
2.1 Smac蛋白的表达
由图1可见,单纯感染CARd.pE-Smac和乏氧不能诱导Eca109中Smac蛋白表达增加,而2Gy照射后可以使Smac表达增加,乏氧后再感染CARd.pE-Smac后也未见Smac表达增加,而感染CARd.pE-Smac后在给予2Gy照射,Smac蛋白表达显著增加,特别是乏氧后感染CARd.pE-Smac后在给予2Gy照射,Smac表达增加最大。
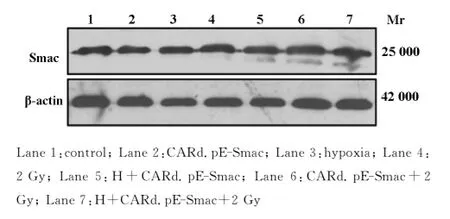
Fig.1 Change of Smac protein expression in each groups by Western blotting
2.2 细胞周期的变化
由表1可见,单纯感染CARd.pE-Smac后对Eca109细胞周期分布未见显著改变,而乏氧,2Gy照射和乏氧后感染CARd.pE-Smac均导致S期和G2/M期细胞百分比较control组显著增加(P<0.05),感染CARd.pE-Smac后给予2Gy照射,S期和G2/M期细胞百分比较control组显著增加(P<0.05,P<0.01),且G2/M期细胞百分比较2Gy组也显著增加(P<0.05),乏氧后感染CARd.pESmac后给予2Gy照射,则S期和G2/M期细胞百分比较control和2Gy组均显著增加(P<0.05,P<0.01)。
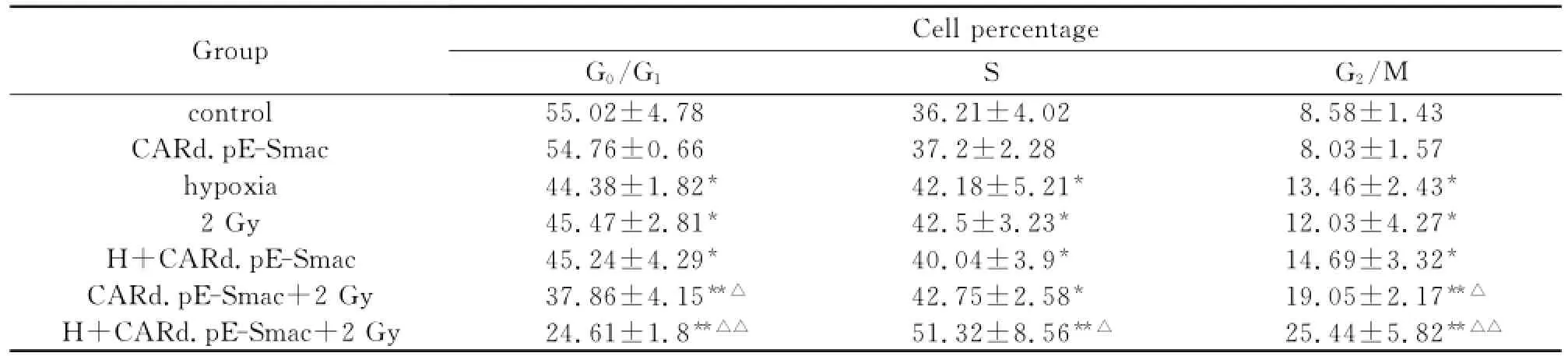
Tab.1 Cell cycle of each phage in Eca109 cells(n=3,¯x±s,%)
2.3 细胞凋亡的变化
流式细胞术(图2)结果显示,乏氧、2Gy照射和乏氧后感染CARd.pE-Smac都能诱导Eca109细胞凋亡较control组显著增加(P<0.05),只是2Gy诱导的程度更大,其他二者基本一致;感染CARd. pE-Smac且2Gy照射和乏氧后感染CARd.pESmac并2Gy照射均能导致细胞凋亡较control组和2Gy组显著增加(P<0.05,P<0.05),尤其以后者诱导程度更大(表2)。
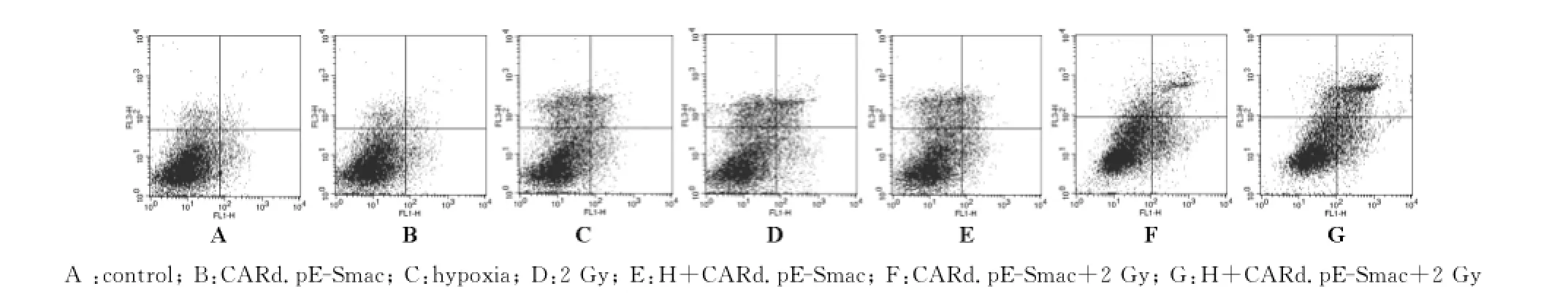
Fig.2 The representative FCM picture of apoptosis with AnnexinⅤ-FITC/PI staining

Tab.2 Cell apoptotic percentage detected by FCM with AnnexinⅤ-FITC/PI staining[n=4,¯x±s,%]
3 讨论
条件复制型腺病毒是比较理想的肿瘤基因治疗中靶基因的表达载体,通常在肿瘤细胞中产生级联的扩大效应[9,10]。而对其进行结构上的改造尤为关键,通常改变策略很多,针对肿瘤的乏氧环境这一特征,研究者考虑将HRE和hTERT改造到条件复制型腺病毒载体上,实现乏氧肿瘤细胞中病毒感染和复制的高效。另外,相关文献证实[11,12],电离辐射能够介导Egr-1启动下游基因的表达,但是Egr-1启动子虽然具有诱导下游基因表达增加的特性,但是因为乏氧这一特性,导致效率较低。研究者考虑将乏氧和辐射双重靶向应用到一个目标中,实现双重效应。在本实验中,笔者发现Smac蛋白表达结果显示了HRE和hTERT修饰的条件复制型腺病毒携带Egr-1介导的Smac在人食管癌细胞Eca109表达增加,且以乏氧后感染该病毒并进行照射表达最大,实现了本实验的初始目标,为下一步印证相关效应提供必要的基础。
电离辐射后细胞如果处于G2/M期阻滞,这样能够保证DNA损伤得以修复,细胞得以存活,不至于死掉。细胞处于不同周期时,对辐射的敏感性不同,通常G2/M期是敏感期。本研究发现,感染病毒并未导致细胞出现明显变化,药物乏氧即可导致G2/M期细胞显著增加(P<0.05),可能与乏氧药物的作用能够有关,并不是乏氧环境导致的。而2Gy照射后也能导致G2/M期细胞显著增加(P<0.05),这与临床放疗的分割照射方案相似,可以使细胞集中在该期,有利于后续照射效果的提高。感染病毒并给予2Gy照射后G2/M期细胞显著增加(P<0.05),这说明Egr-1介导Smac蛋白发挥了作用。而乏氧的细胞施以相同的处理,则诱导G2/M期阻滞的程度更大。这对于基于此的食管癌放疗方案是一个比较大的发现。
另外,Smac是凋亡诱导基因,电离辐射也能直接诱导细胞凋亡。以Smac为基础的肿瘤基因-放射治疗可以实现二者的相互协同作用。而且,辐射诱导细胞周期检查点的阻滞,这是临床肿瘤放射治疗的基础。本实验结果表明,感染病毒未见凋亡诱导作用,乏氧和辐射都能诱导Eca109细胞凋亡增加,而且二者的共同作用效果更强,乏氧所发挥的作用也是和所用化学试剂模拟乏氧相关。对于常氧的细胞,感染病毒并2Gy照射则显著增强凋亡诱导作用,且对乏氧的细胞施以相同处理,诱导能力更强。这说明本实验的HRE和和hTERT修饰的条件复制型腺病毒介导辐射诱导Smac过表达对Eca109细胞具有乏氧状态下辐射杀伤增强的作用,这对于食管癌的临床放疗具有重要意义。
综上,本研究以HRE和hTERT来增加腺病毒在肿瘤细胞乏氧状态下的高效复制,以Egr-1启动子实现辐射时Smac的过表达增强,这样的复合型表达载体能高效诱导Smac过表达;同时,该条件复制型腺病毒载体使细胞阻滞于G2/M期,并诱导细胞凋亡,实现对食管癌细胞的靶向杀伤增强,具有一定的科研价值和临床治疗参考意义。
[1]Hu Y,Ouyang W,Wu F,et al.Enhanced radiosensitivity of SW480cells via TRAIL up-regulation mediated by Egr-1promoter[J].Oncol Rep,2009,22(4):765.
[2]Wu JH,Wang HF,Wang ZC,et al.Conditionally replicating adenovirus combined with gene-targeted radiotherapy induces apoptosis via TRAIL death receptors in MDA-MB-231cells[J].Mol Med Rep,2013,8(1):299.
[3]Du C,Fan GM,Li Y,et al.Smac,a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition[J].Cell,2000,102(1):33.
[4]Qin Q,Zuo Y,Yang X,et al.Smac mimetic compound LCL161 sensitizes esophageal carcinoma cells to radiotherapy by inhibiting the expression of inhibitor of apoptosis protein[J].Tumour Biol,2014,35(3):2565.
[5]Okino ST,Chichester CH,Whitlock JP Jr.Hypoxia-inducible mammalian gene expression analyzed in vivo at a TATA-driven promoter and at an initiator-driven pro-moter[J].J Biol Chem,2004,273(37):23837.
[6]Kwon OJ,Kim PH,Huyn S,et al.A hypoxia-and{alpha}-fetoprotein-dependent oncolytic adenovirus exhibits specific killing of hepatocellular carcinomas[J].Clin Cancer Res,2010,16(24):6071.
[7]Liu WW,Liu Y,Liang S,et al.Hypoxia-and radiation-induced overexpression of Smac by an adenoviral vector and its effects on cell cycle and apoptosis in MDA-MB-231human breast cancer cells[J].Exp Ther Med,2013,6(6):1560.
[8]Dai M,Cui P,Yu M,et al.Melatonin modulates the expression of VEGF and HIF-1alpha induced by CoCl2in cultured cancer cells[J].2008,44(2):121.
[9]Yang SW,Chanda D,Coody JJ,et al.Conditionally replicating adenovirus expressing TIMP2increases survival in a mouse model of disseminated ovarian cancer[J].PLoS One,2011,6(10):e25131.
[10]Zheng FQ,Xu Y,Yang RJ,et al.Combination effect of oncolytic adenovirus therapy and herpes simplex virus thymidine kinase/ganciclovir in hepatic carcinoma animal models[J].Acta Pharmacol Sin,2009,30(5):617.
[11]Liu LL,Smith MJ,Sun BS,et al.Combined IFN-gamma-endostatin gene therapy and radiotherapy attenuates primary breast tumor growth and lung metastases via enhanced CTL and NK cell activation and attenuated tumor angiogenesis in a murine model[J].Ann Surg Oncol,2009,16(5):1403.
[12]Li Y,Guo C,Wang Z,et al.Enhanced effectes of TRAIL-endostatin-based double-gene-radiotherapy on suppressing growth,promoting apoptosis and inducing cell cycle arrest in vascular endothelial cells[J].J Huazhong Univ Sci Technolog Med Sci,2012,32(2):153.
Effects on cell cycle and apoptosis of HRE and hTERT-modified replicative adenovirus carrying Smac mediated by Egr-1in human esophageal cancer Eca109 cells
CHEN Wen-qing,JIN Xin-tian,YAN Song,et al.
(Jilin Province Cancer Hospital,Changchun130012,China)
Objective To explored the effects on cell cycle and apoptosis of HRE and hTERT-modified replicative adenovirus carrying Smac mediated by Egr-1(CARd.pE-Smac)in human esophageal cancer Eca109cells.Methods Chemical hypoxia was achieved by CoCl2,human esophageal cancer Eca109cells were infected with CARd.pE-Smac by infection titer of 5MOI,after 24hcells were irradiated by 2Gy,then after 12h,cells were collected.Smac protein was measured by Western blotting,cell cycle change and apoptosis were measured by flow cytometry with PI staining and AnnexinⅤ-FITC double staining,respectively.All groups included control,CARd.pE-Smac,hypoxia,2Gy,H+CARd.pE-Smac,CARd.pE-Smac+2Gy and H+CARd.pE-Smac+2Gy.Results After hypoxic and normoxic cells were infected with CARd.pE-Smac,there were not obvious Smac expression increasing,but after 2Gy irradiation,Smac expression increased in cells of normaxia,CARd.pE-Smac infection and hypoxia+CARd.pE-Smac infection,especially,Smac expression reached to maximum under three combination.Cell cycles had not obvious changes after CARd.pESmac infecting cells,but hypoxia,2Gy and CARd.pE-Smac infection,as the two(except hypoxia and 2Gy)or any three party,significantly increased S and G2/M cell percentages(P<0.05,P<0.01),especially,its role was most strong in combination with the three;in addition,apoptotic induction regularity was similar with cell cycle changes.Conclusion HRE and hTERT-modified replicative adenovirus carrying Smac mediated by Egr-1caused Smac obvious overexpression in human esophageal cancer Eca109cells,and leaded S phage delay and G2/M arrest,and also induced apoptosis.
Hypoxia response elements;hTERT;conditionally replicative adenovirus;Human esophageal carcinoma
Q78
A
2013-10-14)
1007-4287(2014)07-1054-04
吉林省自然科学基金资助(201115215)
*通讯作者
