Isolation and Characterization of Fucoidans from Five Brown Algae and Evaluation of Their Antioxidant Activity
2014-04-20QUGuiyanLIUXuWANGDongfengYUANYiandHANLijun
QU Guiyan,LIU Xu,WANG DongfengYUAN Yi,and HAN Lijun,
1)Department of Food Science and Engineering, Ocean University of China, Qingdao 266003, P.R.China
2)Institute of Oceanology,Chinese Academy of Sciences, Qingdao 266071, P.R.China
3)College of Tea & Food Science and Technology, Anhui Agricultural University, Hefei 230036,P.R.China
© Ocean University of China,Science Press and Spring-Verlag Berlin Heidelberg 2014
1 Introduction
Fucoidans are complex sulfated polysaccharide found mainly in the cell-wall matrix of various brown algae(Aleet al.,2011).In recent years,development of new drugs has raised interests in fucoidans because of their antiviral,anticoagulant and anti-inflammatory function.Fucoidans of different structures have been isolated from various algal species,mainly the members of order Fucales and Laminariales with their biological activity elucidated in detail(Aleet al.,2011; Hanet al.,2008; Souzaet al.,2007).The bioactivity of fucoidans depends on their structural features such as sulfating degree,molecular weight and major sugar component type(Azofeifaet al.,2008; Jinet al.,2013; Wanget al.,2008; Chandía and Matsuhiro,2008; Barahonaet al.,2011).
Owing to the harsh condition in their habitats,many macroalgae have developed effective defense mechanisms,and as a result,are rich in bioactive compounds(Michelleet al.,2010).Substantial amounts ofMacrocystis(M.pyrifera)andLessonia(L.nigrescens and L.trabeculata)are traditionally harvested and exported from Chile and South Africa as the raw material of alginate(Westermeier,2006).Ascophyllum mackaii,a variant ofA.nodosum,is a dominant rocky intertidal brown alga in class Phaeophyceae,which is commonly used for industrial-scale preparation of alginate(Jianget al.,2010).Ecklonia maximais a dominant brown alga along the west coast of South Africa,forming large algal beds in the southern part of Benguela region(Bolton,2012).Laminaria japonicais the most important economic brown alga,which was cultured in China and other Asian countries such as Japan and Korea(Wanget al.,2010).Ecklonia maximaandLaminaria japonicawere also used as the raw material of alginate production.A number of studies have been carried out inLaminaria japonica.It is found that the fucoidans content of this species is determined by multi-factors such as geographic location and extraction method.This study was designed to determine the difference in the fucoidans content of selected algae and the chemical property and antioxidant activity of the fucoidans they contained.
2 Materials and Methods
2.1 Brown Algae
Fucoidans were extracted fromLaminaria japonica(China),Lessonia nigrescens(Chile),Lessonia trabeculata(Peru),Ascophyllum mackaii(South Africa) andEcklonia maxima(South Africa).The dry alga was milled in a disintegrator fitted with a sieve of 0.45 mm and sealed in glass bottles until use.
2.2 Fucoidans Extraction
Fucoidans were isolated with the method described early(Ponceet al.,2003)with some modifications.The milled alga was homogenized and fucoidans were extracted using selective solvents with a constant mechanical stirring.Fats,pigments and proteins were first extracted with EtOH-H2O(85:15)at room temperature(about 20℃,RT)for 24 h.The solvent was separated from residuals by vacuum filtration,and the alga was then dried at 40℃ for 6 h.The residuals(20 g)and distilled water(3×700 mL)were mixed in an ultrasonic washer set at 300 W for 11 min and then mechanically stirred at 100℃ for 2 h.The vessel was immediately cooled with running water and the suspension was filtrated through a nylon fiber(5 mm)to separate the residual alga.The supernatant was collected and concentrated by using a rotary evaporator under reduced pressure to an appropriate volume.Subsequently,1% CaCl2solution was added to the supernatant and the mixture was stored at 5℃ overnight to remove alginate.After centrifugation(2253 g,20 min),three-volume EtOH-H2O(95:5)was added to the resultant supernatant and then stored at 5℃ overnight to extract fucoidans.The precipitate formed was obtained after centrifuged at 2253 g for 20 min and then dissolved in water.The solution was dialyzed(cut off 8000–14000 Da)for 48 h for decreasing salinity and lyophilized to obtain crude fucoidans.
2.3 Chemical Composition Analysis
Total sugar and fucose content of fucoidans were analyzed with the phenol-sulfuric acid(DuBoiset al.,1956)and cysteine hydrochloride method(Gibbons,1955),respectively,using fucose(Sigma Chemical Co.,St.Louis,MO,USA)as standard.The sulfate group content was determined using the barium chloride-gelatin method(Dodgson and Price,1962).The concentration of soluble protein was determined using the Bradford method(Bradford,1976)with bovine serum albumin(BSA,Sigma)as standard.
2.4 Molecular Weight Determination
The molecular weight(MW)of fucoidans was determined by high-performance gel permeation chromatography(HPGPC)using dextrans(Sigma)as standard.The HPGPC system consisted of a TSK-gel G3000 PWxl column,a 0.05 mol L−1Na2SO4eluant used to irrigate the column at 1 mL min−1,and a refractive index detector(Shimadzu RID-10A,Kyoto,Japan).
2.5 Monosaccharide Composition Determination
The monosaccharide composition of fucoidans were analyzed by precolumn derivation high-performance liquid chromatography(HPLC)with 1-phenyl-3-methyl-5-pyrazolone(PMP).Acid hydrolysis of the sample was carried out with trifluoroacetic acid at 110℃ for 8 h,and then the sugars were converted into their PMP derivatives.A Thermo Hypersil ODS-2 HPLC column(250 mm × 4.6 mm)was used at ambient temperature of 30℃.The PMP derivatives elution was performed with a mixture of acetonitrile and phosphate buffer(0.1 mol L−1)at a flow rate of 1 mL min−1,and UV absorbance of effluent was monitored at 245 nm.
2.6 In vitro Antioxidant Activity Assaying
2.6.1 Reducing power assay
The reducing power of fucoidans was assayed according to the method developed by Yen and Chen(1995).Sample was dissolved in distilled water at different concentrations.Sample solution(1 mL)was mixed with sodium phosphate buffer(2.5 mL,0.2 mol L−1,pH 6.6)and potassium ferricyanide aqueous solution(K3Fe(CN)6,2.5 mL,1%).The mixture was incubated at 50℃ for 20 min,followed by addition of trichloroacetic acid solution(2.5 ml,10%)and ferric chloride aqueous(FeCl3,0.5 mL,1%).The absorbance of reaction mixture was measured at 700 nm on a UV-2000 spectrophotometer(Unico(Shanghai)Instrument Co.,Ltd.,China).The reducing power was proportional to the absorbance of reaction mixture.
2.6.2 Hydroxyl radical scavenging activity assaying
The hydroxyl radical scavenging activity(HRSA)of fucoidans was assayed by using the 1,10-phenanthroline-Fe2+oxidative method(Jinet al.,1996)with some modifications.The reaction mixture contained 1 mL of fucoidan sample solution,1.5 mL of 5 m mol L−11,10-phenanthroline,2.0 mL of 0.2 mol L−1sodium phosphate buffer solution(pH 7.4),1.0 mL of 7.5 mmol L−1FeSO4,1.0 mL of 0.1% H2O2,and 4.5 mL of H2O.After incubation at 37℃ for 1 h,the absorbance of reaction mixture was measured at 536 nm.HRSA was evaluated as the inhibition rate of 1,10-phenanthroline-Fe2+oxidation by hydroxyl radical.The activity was calculated with the equation
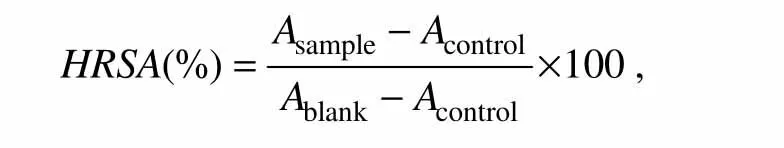
whereAis the absorbance of sample,control and blank reaction mixture at 536 nm.
2.6.3 Superoxide anion radical scavenging activity assaying
The superoxide anion radical scavenging activity(SARSA)was assayed by measuring the inhibition of pyrogallol auto-oxidation using the method of Marklund and Marklund(1974)with minor modifications.Sample solution(4.2 mL)was added into 4.5 mL of 50 mmol L−1Tris-HCl buffer(pH 8.24)and incubated in a water bath at 25℃ for 20 min.The reaction was terminated by adding 0.3 mL of 3 mmol L−1pyrogallol(dissolved in 10 mmol L−1HCl).The inhibition rate of pyrogallol autooxidation was measured by determining the change in the absorbance of reaction mixture at 325 nm.Absorbance of each extract was recorded at a 30s interval for 4 min and the increment of absorbance was calculated by the difference(the absorbance at 10 min–the absorbance at the starting time).
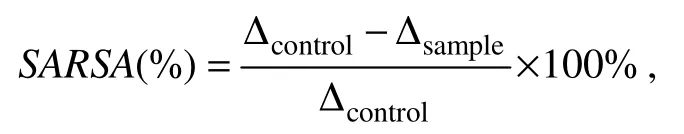
where Δ is the absorbance of sample and control reaction mixture at 325 nm.
2.7 Data Analysis
All values are expressed as means ± standard deviation(n=3).EC50is the efficient concentration defined as the concentration inhibiting 50% radical generation or scavenging 50% radical generated.The data were processed by Excel 2007 and GraphPad Prism 6.00 for Windows(GraphPad Software,Inc).
3 Results and Discussions
3.1 Chemical Composition
Fucoidans were isolated from five brown algae,Laminaria japonica,Lessonia nigrescens,Lessonia trabeculata,Ascophyllum mackaiiandEcklonia maxima,which were named as LJF,LNF,LTF,AMF and EMF,respectively.Their relative yield ranged from 1.39% to 4.95%(Table 1),being similar to those reported in the species in order Fucales and Laminariales(Riouxet al.,2007; Sakaiet al.,2002; Wanget al.,2008).Before fucoidans extraction,ethanol was used to extract fats,pigments and proteins,causing a sharp decrease in dry weight of alga(16%–20%)except forLaminaria japonica(0.8%)andEcklonia maxima(3.2%).These findings were similar to those obtained by Riouxet al.(2007),and demonstrated that this procedure may positively affect the yield and purity of fucoidans.
The results of chemical composition analyses demonstrated that all algae contained a high level of polysaccharides(57.46% –65.26%)and a low level of protein(< 3%).The major components of fucoidans were fucose(20.05%–29.23%)and sulfate(14.16%–22.36%).The molar ratio of sulfate to fucose of EMF was 0.97,20%– 30% lower than those of others(1.21–1.41)(Table 1).
The molecular weight(MW)of all fucoidans showed a high polydispersity.LNF and LTF had smaller MW than other fucoidans as was indicated by a precipitation phenomenon in the dialysis of fucoidan solution.A similar phenomenon was found inSaccharina longicruris(Riouxet al.,2007),which was related to the small proportion of insoluble fucoidans and the presence of high molecular weight fucoidans.
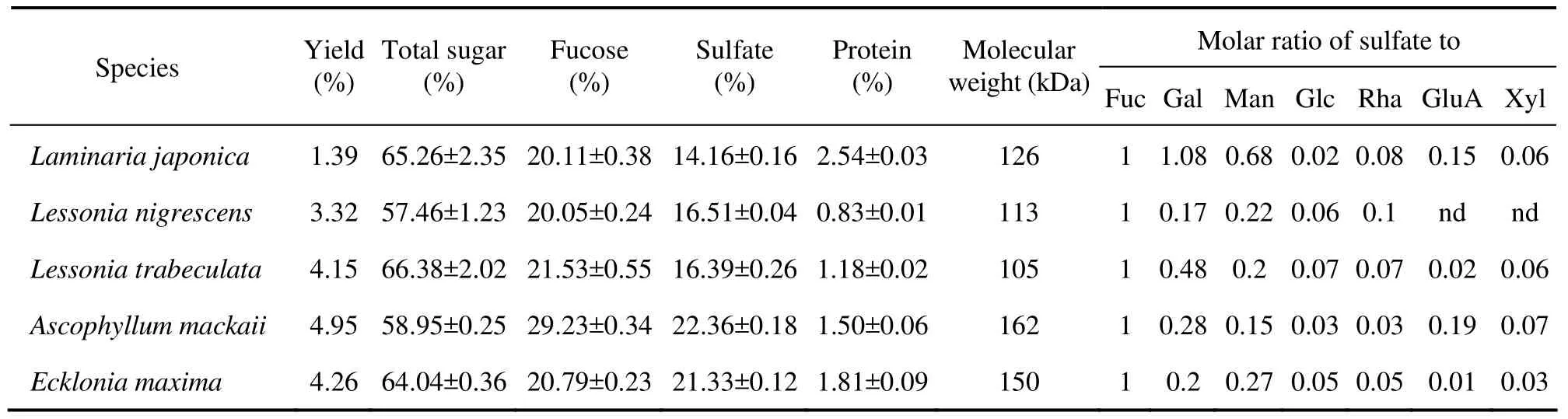
Table 1 Relative yield and chemical composition of fucoidans from five brown algae
The monosaccharide present in fucoidans was constituted of fucose as the major sugar.An exception was that LJF contained two major sugars,galactose and fucose(molar ratio 1:1).In addition to fucose and galactose,another five monosaccharides were detected in the isolated fucoidans,but glucuronic acid and xylose were absent in LNF.
According to the reported early,fucoidans extracted fromLaminaria japonicacontained approximately 28.3%–33.0% of sulfate,52.8%–62.1% of fucose,24.3%–27.4% of galactose and 6.1% of mannose with(or without)3.3% rhamnose and 1.9% xylose(Wanget al.,2008; Xueet al.,2001).In present study,the sulfate content was found to be extremely low inLaminaria.japonica(14.16%),which may be attributed to the presence of unsulfated fucoses in the linear part of polysaccharides(Riouxet al.,2007),as galactose and mannose content were high(Table 1).In 1990,a report on the chemical composition of a number of fucoidans from various sources stated thatFucus vesiculosusfucoidans contained 14%–39% sulfate(Foleyet al.,2011).This demonstrated that the fucoidans were quantitatively different among algae.Moreover,it has been demonstrated that extraction procedure may impact on the polysaccharide structure(Ponceet al.,2003),and that materials harvested at different places and in different seasons and methods may cause the difference in chemical composition(Riouxet al.,2007).Ascophyllum mackaiifucoidans contained approximately 19%–22.1% sulfate(Foleyet al.,2011; Riouxet al.,2007),being similar to the findings of present study.Nevertheless,the galactose and mannose content reported here were higher while the glucose and xylose content were lower than those obtained by Foleyet al. (2011).The content and composition ofLessonia nigrescens,Lessonia trabeculata,andEcklonia maximafucoidans have not been documented yet,thus our findings provided the basic information for further studied.
Overall,the fucoidans extracted from five alga have substantially different chemical properties.Such differences may have great influences on their antioxidant activity as analyzed in the following text.
3.2 Reducing Power
The reducing power of all fucoidans was concentration dependent.The reducing power of AMF and EMF was three times higher than that of other fucoidans(Fig.1).Except for AMF and EMF,the fucoidans had lower reducing power than ascorbic acid,α-tocopherol and BHA(0.80,0.89 and 0.92 at 1.0 mg mL−1,respectively)(Mauet al.,2004).
The reducing power of fucoidans significantly correlated with their sulfate content and molecular weight(Fig.1).However,Wanget al.(2008)and Costaet al.(2010)found that the relation between reducing power and sulfate content of fucoidans was not significant,and that the reducing power of fucoidans was less related to fucose content and not obviously related to the ratio of sulfate to fucose content.AMF and EMF with strong reducing power were highly able to donate hydrogen,by which the reduction can break free radical chain(Chunget al.,2002).Our data showed that the reducing capacity of fucoidans may serve as a significant indicator for its potential antioxidant activity.
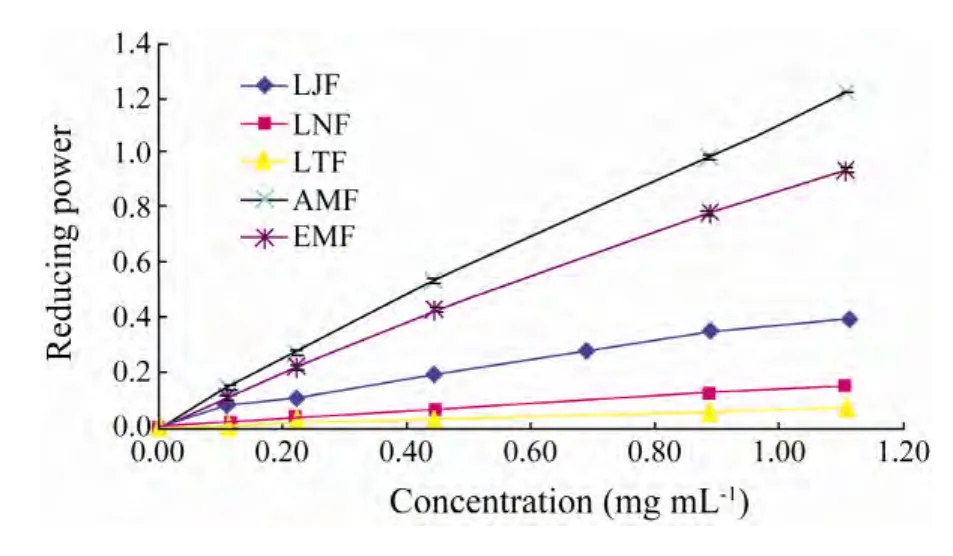
Fig.1 Reducing power of fucoidans extracted from five alga as a function of concentration.Data are mean ± SD(n =3).
3.3 Hydroxyl Radical Scavenging Activity
The HRSA of fucoidans extracted from five alga was dose dependent(Fig.2).LJF had the EC50value of 2.17,whereas EMF had the EC50value of 1.29 mg mL−1and other three had the EC50value < 1.0 mg mL−1.These findings indicated that the fucoidans(except for LJF)had stronger HRSA than vitamin C(EC50= 1.537 mg mL−1)(Xinget al.,2005).
The factors that affected the HRSA of fucoidans were similar to those that influenced the reducing power.However,as compared with the reducing power,LTF and LNF exhibited high inhibitory effect on hydroxyl radicals at low concentrations.Previous works indicated that sulfated galactan fromSchizymenia binderican quench hydroxyl radicals,whereas fucoidans from the brown algaLes vadosa(Barahonaet al.,2011)and polysaccharides from 11 species of tropical seaweeds are less effective as hydroxyl radical scavengers(Costaet al.,2010).Thus,a combination of multi-factors may have influenced the antioxidant activity of fucoidans,as was mentioned by Wanget al.(2008).
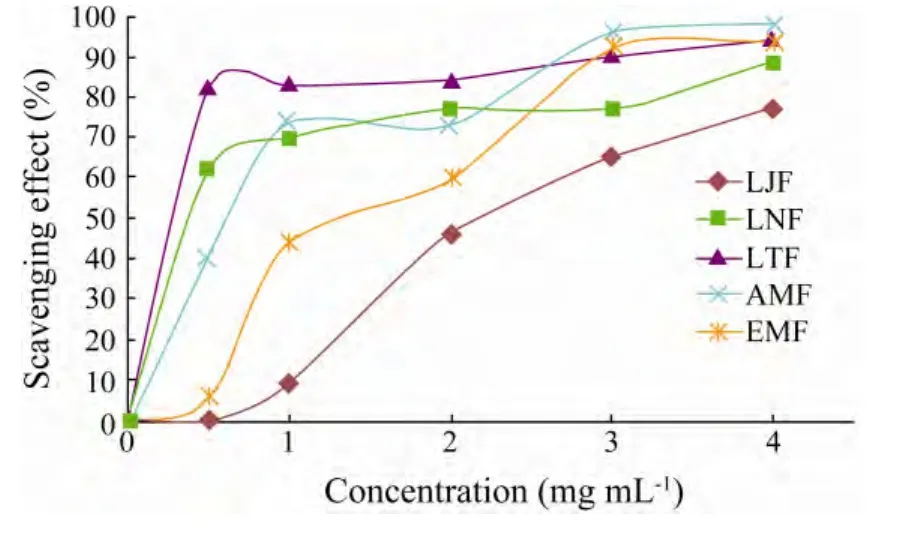
Fig.2 Scavenging effect of fucoidans from different alga on hydroxyl radicals.Data are mean ± SD(n=3).
3.4 Superoxide Radical Scavenging Activity
The SARSA of fucoidans isolated in this study concentration-dependently varied(Fig.3).Of the five fucoidans,LNF and LTF showed the highest SARSA(90% at 4.0 mg mL−1)which was higher than that of vitamin C(68.19% at 2.0 mg mL−1)(Xinget al.,2005).
The inhibitory effect on the superoxide radical scavenging activity of LNF and LTF was high at low concentrations,being similar to that observed in HRSA(Fig.2).However,in this study,low MW fucoidans generally had high SARSA,being opposite to the trend observed in reducing power(Fig.1).This result was in contrast to the finding of Wanget al.(2008)who found that the correlation between sulfate content and SARSA was positive among three fractions fromLaminaria japonica.
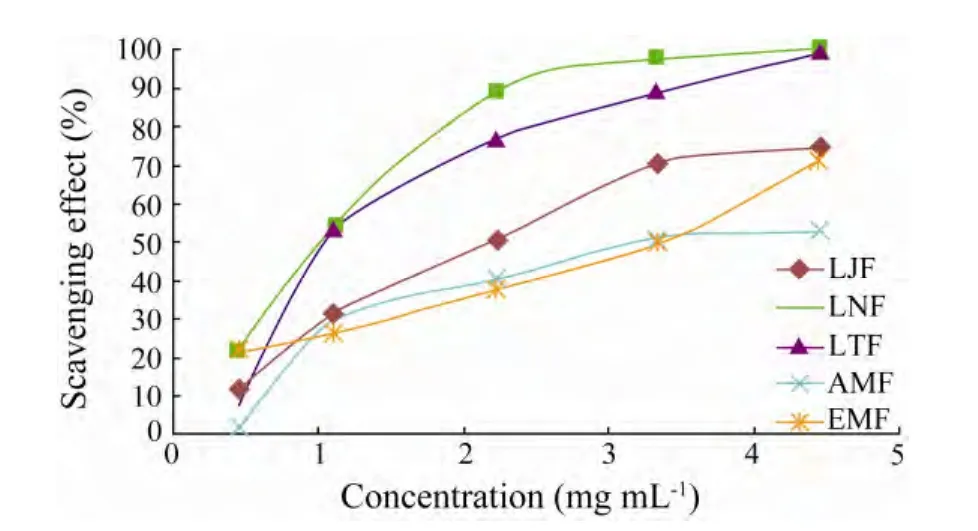
Fig.3 Scavenging effect of fucoidans from different alga on superoxide radicals.Data are mean ± SD(n=3).
4 Conclusions
The fucoidans extracted from five brown algae varied in chemical composition and antioxidant ability.The sulfate content and MW of fucoidans were indicators of their reducing power.Low MW fucoidans may have high SARSA.Further work is necessary in order to characterize the structure of fucoidans of these algal species.
Acknowledgements
This study was supported by the Ocean Public Welfare Scientific Research Project,State Ocean Administration of China(No.201105029-9).The authors wish to thank Dr.Bo Jiang for her help in manuscript preparation and Drs.Chao Guo,Shuju Guo and Dayong Shi(Institute of Oceanology,Chinese Academy of Sciences)for their assistance in experiment.
Ale,M.T.,Maruyama,H.,Tamauchi,H.,Mikkelsen,J.D.,and Meyer,A.S.,2011.Fucoidan fromSargassumsp.andFucus vesiculosusreduces cell viability of lung carcinoma and melanoma cellsin vitroand activates natural killer cells in micein vivo.International Journal of Biological Macromolecules, 49(3):331-336.
Ale,M.T.,Mikkelsen,J.D.,and Meyer,A.S.,2011.Important determinants for fucoidan bioactivity:A critical review of structure-function relations and extraction methods for fucosecontaining sulfated polysaccharides from brown seaweeds.Marine Drugs, 9(10):2106-2130.
Azofeifa,K.,Angulo,Y.,and Lomonte,B.,2008.Ability of fucoidan to prevent muscle necrosis induced by snake venom myotoxins:Comparison of high- and low-molecular weight fractions.Toxicon,51(3):373-380.
Barahona,T.,Chandía,N.P.,Encinas,M.V.,Matsuhiro,B.,and Zúñiga,E.A.,2011.Antioxidant capacity of sulfated polysaccharides from seaweeds.A kinetic approach.Food Hydrocolloids,25(3):529-535.
Bolton,J.J.,Anderson,R.J.,Smit,A.J.,and Rothman,M.D.,2012.South African kelp moving eastwards:The discovery ofEcklonia maxima(Osbeck)Papenfuss at De Hoop Nature Reserve on the south coast of South Africa.African Journal of Marine Science, 34(1):147-151.
Bradford,M.M.,1976.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Analytical Biochemistry, 72(1–2):248-254.
Chandía,N.P.,and Matsuhiro,B.,2008.Characterization of a fucoidan fromLessonia vadosa(Phaeophyta)and its anticoagulant and elicitor properties.International Journal of Biological Macromolecules,42(3):235-240.
Chung,Y.-C.,Chang,C.-T.,Chao,W.-W.,Lin,C.-F.,and Chou,S.-T.,2002.Antioxidative activity and safety of the 50 ethanolic extract from red bean fermented byBacillus subtilisIMR-NK1.Journal of Agricultural and Food Chemistry, 50(8):2454-2458.
Costa,L.S.,Fidelis,G.P.,Cordeiro,S.L.,Oliveira,R.M.,Sabry,D.A.,Câmara,R.B.G.,Nobre,L.T.D.B.,Costa,M.S.S.P.,Almeida-Lima,J.,Farias,E.H.C.,Leite,E.L.,and Rocha,H.A.O.,2010.Biological activities of sulfated polysaccharides from tropical seaweeds.Biomedicine & Pharmacotherapy,64:21-28.
Dodgson,K.S.,and Price,R.G..,1962.A note on the determination of the ester sulphate content of sulphated polysaccharides.Biochemical Journal,84(1):106-110.
DuBois,M.,Gilles,K.A.,Hamilton,J.K.,Rebers,P.A.,and Smith,F.,1956.Colorimetric method for determination of sugars and related substances.Analytical Chemistry,28(3):350-356.
Ermakova,S.,Sokolova,R.,Kim,S.-M.,Um,B.-H.,Isakov,V.,and Zvyagintseva,T.,2011.Fucoidans from brown seaweedsSargassum hornery,Eclonia cava,Costaria costata:Structural characteristics and anticancer activity.Applied Biochemistry and Biotechnology, 164(6):841-850.
Foley,S.A.,Szegezdi,E.,Mulloy,B.,Samali,A.,and Tuohy,M.G.,2011.An unfractionated fucoidan fromAscophyllum nodosum:Extraction,characterization,and apoptotic effectsin vitro.Journal of Natural Products, 74(9):1851-1861.
Gibbons,M.N.,1955.The determination of methylpentoses.Analyst, 80:268-276.
Han,J.G.,Syed,A.Q.,Kwon,M.,Ha,J.H.,and Lee,H.Y.,2008.Antioxident,immunomodulatory and anticancer activity of fucoidan isolated fromFucus vesiculosus.Journal of Biotechnology, 136, Supplement(0):S571.
Jiang,Z.,Okimura,T.,Yokose,T.,Yamasaki,Y.,Yamaguchi,K.,and Oda,T.,2010.Effects of sulfated fucan,ascophyllan,from the brown algaAscophyllum nodosumon various cell lines:A comparative study on ascophyllan and fucoidan.Journal of Bioscience and Bioengineering,110(1):113-117.
Jin,M.,Cai,Y.X.,Li,J.R.,and Zhao,H.,1996.1,10-Phenanthroline-Fe2+oxidative assay of hydroxyl radical produced by H2O2/Fe2+.Progress of Biochemistry and Biophysics, 23(6):553-555.
Jin,W.,Zhang,Q.,Wang,J.,and Zhang,W.,2013.A comparative study of the anticoagulant activities of eleven fucoidans.Carbohydrate Polymers, 91(1):1-6.
Marklund,S.,and Marklund,G.,1974.Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase.European Journal of Biochemistry,47(3):469-474.
Mau,J.-L.,Chang,C.-N.,Huang,S.-J.,and Chen,C.-C.,2004.Antioxidant properties of methanolic extracts fromGrifola frondosa,Morchella esculentaandTermitomyces albuminosusmycelia.Food Chemistry, 87(1):111-118.
Ponce,N.M.A.,Pujol,C.A.,Damonte,E.B.,Flores,M.L.,and Stortz,C.A.,2003.Fucoidans from the brown seaweedAdenocystis utricularis:Extraction methods,antiviral activity and structural studies.Carbohydrate Research, 338(2):153-165.
Rioux,L.E.,Turgeon,S.L.,and Beaulieu,M.,2007.Characterization of polysaccharides extracted from brown seaweeds.Carbohydrate Polymers, 69(3):530-537.
Rocha de Souza,M.C.,Marques,C.T.,Guerra Dore,C.M.,Ferreira da Silva,F.R.,Oliveira Rocha,H.A.,and Leite,E.L.,2007.Antioxidant activities of sulfated polysaccharides from brown and red seaweeds.Journal of Applied Phycology,19(2):153-160.
Sakai,T.,Kimura,H.,and Kato,I.,2002.A marine strain of flavobacteriaceae utilizes brown seaweed fucoidan.Marine Biotechnology, 4(4):399-405.
Tierney Michelle,S.,Croft Anna,K.,and Hayes,M.,2010.A review of antihypertensive and antioxidant activities in macroalgae.Botanica Marina,53(5):387-408.
Wang,J.,Zhang,Q.,Zhang,Z.,and Li,Z.,2008.Antioxidant activity of sulfated polysaccharide fractions extracted fromLaminaria japonica.International Journal of Biological Macromolecules,42(2):127-132.
Wang,J.,Zhang,Q.,Zhang,Z.,Song,H.,and Li,P.,2010.Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted fromLaminaria japonica.International Journal of Biological Macromolecules, 46(1):6-12.
Westermeier,R.,Patiño,D.,Piel,M.I.,Maier,I.,and Mueller,D.G.,2006.A new approach to kelp mariculture in Chile:Production of free-floating sporophyte seedlings from gametophyte cultures ofLessonia trabeculataandMacrocystis pyrifera.Aquaculture Research, 37:164-171.
Xing,R.,Yu,H.,Liu,S.,Zhang,W.,Zhang,Q.,Li,Z.,and Li,P., 2005.Antioxidant activity of differently regioselective chitosan sulfatesin vitro.Bioorganic and Medicinal Chemistry,13(4):1387-1392.
Xue,C.H.,Fang,Y.,Lin,H.,Chen,L.,Li,Z.J.,Deng,D.,and Lu,C.X.,2001.Chemical characters and antioxidative properties of sulfated polysaccharides fromLaminariajaponica.Journal of Applied Phycology,13(1):67-70.
Yen,G.C.,and Chen,H.Y.,1995.Antioxidant activity of various tea extracts in relation to their antimutagenicity.Journal of Agricultural and Food Chemistry, 43(1):27-32.
杂志排行
Journal of Ocean University of China的其它文章
- Experimental Study on the Flow Around Two Tandem Cylinders with Unequal Diameters
- Identification of Fucans from Four Species of Sea Cucumber by High Temperature1H NMR
- Decomposition of the Unsteady Wave Patterns for Bessho form Translating-Pulsating Source Green Function
- Sedimentary Evolution of the Holocene Subaqueous Clinoform off the Southern Shandong Peninsula in the Western South Yellow Sea
- Revision of P-wave Velocity and Thickness of Hydrate Layer in Shenhu Area, South China Sea
- Impact of Convective Momentum Transport by Deep Convection on Simulation of Tropical Intraseasonal Oscillation
