Antifouling Potential of Bacteria Isolated from a Marine Biofilm
2014-04-20GAOMinWANGKeSURongguoLIXuzhaoandLUWei
GAO Min, WANG Ke, SU Rongguo,, LI Xuzhao, and LU Wei
1) Key Laboratory of Marine Chemistry Theory and Technology of Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
2) Marine Chemical Research Institute, Qingdao 266071, P. R. China
Antifouling Potential of Bacteria Isolated from a Marine Biofilm
GAO Min1), WANG Ke2), SU Rongguo1),*, LI Xuzhao2), and LU Wei2)
1) Key Laboratory of Marine Chemistry Theory and Technology of Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
2) Marine Chemical Research Institute, Qingdao 266071, P. R. China
Marine microorganisms are a new source of natural antifouling compounds. In this study, two bacterial strains, Kytococcus sedentarius QDG-B506 and Bacillus cereus QDG-B509, were isolated from a marine biofilm and identified. The bacteria fermentation broth could exert inhibitory effects on the growth of Skeletonema costatum and barnacle larvae. A procedure was employed to extract and identify the antifouling compounds. Firstly, a toxicity test was conducted by graduated pH and liquid-liquid extraction to determine the optimal extraction conditions. The best extraction conditions were found to be pH 2 and 100% petroleum ether. The EC50value of the crude extract of K. sedentarius against the test microalgae was 236.7 ± 14.08 μg mL-1, and that of B. cereus was 290.6 ± 27.11 μg mL-1. Secondly, HLB SPE columns were used to purify the two crude extracts. After purification, the antifouling activities of the two extracts significantly increased: the EC50of the K. sedentarius extract against the test microalgae was 86.4 ± 3.71 μg mL-1, and that of B. cereus was 92.6 ± 1.47 μg mL-1. These results suggest that the metabolites produced by the two bacterial strains are with high antifouling activities and they should be fatty acid compounds. Lastly, GC-MS was used for the structural elucidation of the compounds. The results show that the antifouling compounds produced by the two bacterial strains are myristic, palmitic and octadecanoic acids.
marine biofilm; bacteria; antifouling activity; fatty acids
1 Introduction
Biofouling is known as the colonization of natural or artificial surfaces in the marine environment by a number of microorganisms, including bacteria, fungi, diatoms, cyanobacteria, and microalgae as well as by macroalgae and invertebrates (Little and Wagner, 1997; Callow and Callow, 2002; Buma et al., 2009). Every year, biofouling causes serious operational problems and huge economic losses for marine industries and the navies around the world (Yebra et al., 2004; Silkina et al., 2012). To control the effects of foulers, antifouling coatings have been widely applied over the last several decades. However, these coatings, which include organotin and copper compounds, are highly toxic and can cause irreversible environmental deformations (Alzieu, 1998; Iyapparaj et al., 2013). With the global ban on the application of organotin-based marine coatings by the International Maritime Organization in 2008, the development of alternative, environmentally friendly, low-toxic and nontoxic antifouling compounds for marine industries has become an urgent need (Dahlstrom et al., 2002).
In recent years, extensive studies on novel antifouling compounds against biofouling have been conducted (Holmstroem and Kjelleberg, 1999; Kitano et al., 2011). Marine natural products have been considered as promising sources of antifouling compounds. Thus far, a number of potent antifouling compounds have been isolated and identified from extracts of marine microorganisms (bacteria and fungi) and macroorganisms (sponges, corals, seaweeds, and nudibranchs). Diketopiperazines were identified from a deep-sea bacterium, Streptomyces fungicidicus, showing promising antifouling activity (Li et al., 2006). 10b-Formamidokalihinol-A and kalihinol A, which are isolated and purified from the marine sponge Acanthella cavernosa, inhibit bacterial growth, suppress larval settlement, and exhibit antifouling properties (Yang et al., 2006). Floridoside, produced by the red alga Grateloupia turuturu, show potent antibarnacle activity (Hellio et al., 2004). Most of the natural antifouling compounds reported are fatty acids, terpenoids, polyethers and alkaloids (Qian et al., 2010), and they are biodegradable. Thus, marine microorganisms and macroorganisms are promising sources of biotechnologically interesting substances for ‘environmentally-friendly’ antifouling applications (Clare, 1996; Dafforn et al., 2011).
In marine environments, biofilms mainly consist of bacteria and diatoms (Marshall et al., 1971). Some biofilms of live microorganisms can produce chemical compounds that disrupt the formation of biofilms or pre-vent epibiosis. Therefore, the biofilms may themselves be the source of various bioactive natural products that are of applied biotechnological interest (Armstrong et al., 2000). This study was conducted to investigate the potential of the two bacterial strains isolated from marine biofilms to produce antifouling compounds and to identify these compounds.
2 Materials and Methods
2.1 Bacterial Strains
Biofilm cultivation was conducted in the Hong Kong harbor of Qingdao. When the biofilms matured, field samples were collected, and the microorganisms in the biofilms were isolated and purified. Identification of the strains was performed via 16S rRNA gene sequencing. The bacteria were isolated and purified according to the method described in Xu et al. (2007). During the primary screening, K. sedentarius QDG-B506 and B. cereus QDG-B509 showed significant repellent activity against other bacteria.
2.2 Test Organisms
Diatom and barnacle larvae are commonly used in antifouling biological activity tests (Hellio et al., 2002; Dobretsov et al., 2007). The marine diatom Skeletonema costatum was obtained from the Marine Eco-Pollution Chemistry Laboratory of the Ocean University of China. The microalgae were cultivated in 250 mL Erlenmeyer flasks containing Guillard’s F/2 medium under continuous illumination (5000 lux white fluorescent lamps) at 20℃ with a 12 h:12 h light:dark cycle. Culture media were all autoclaved (120℃, 20 min) and then inoculated with 20 mL cultivated microalgae in the exponential growth phase in 5 L Erlenmeyer flasks containing 3 L culture media under aseptic conditions. The cultures were shaken three times daily by hand to allow sufficient gas exchange until the microalgae were incubated to the exponential growth period (3 to 4 d) and reached a density of 105cells mL-1.
Adults of the Balanus amphitrete Darwin were collected from the intertidal zone in Zhanqiao, Qingdao. Larvae were obtained according to the method described in Thiyagarajan et al. (2003), and the newly released larvae were used for the experiment.
2.3 Anti-algal and Anti-larval Activity of K. sedentarius and B. cereus Supernatants
The bacterium broth culture was centrifuged at 8000 r min-1at 4℃ for 20 min, and the supernatant was collected. The diatom S. costatum was subjected to standard 96 h algal growth inhibition tests performed in 250 mL Erlenmeyer flasks filled with 100 mL S. costatum suspension in the exponential growth period. The test concentrations (volume fraction) chosen for the K. sedentarius and B. cereus supernatants were 0% (control), 2.0%, 3.0%, 4.0%, 5.0%, and 6.0%. All concentrations were tested using triplicate cultures.
Larval settlement bioassays were performed using sterile 3 mL 24-well polystyrene plates. K. sedentarius and B. cereus supernatants were prepared to the settled concentrations (volume fraction 0%, 2.0%, 2.5%, 5.0%, 7.5%, and 10.0%) in autoclaved filtered seawater (FSW). About 15 competent barnacle larvae were added to each well in 3 mL of the test solution. Three replicates of each treatment were used. The plates were incubated at 25℃ for 24 h in Plant Growth Chamber. The percentage of larval death was determined by counting the mortality and live individuals, and expressing the result as a proportion of the total number of larvae in the well.
2.4 Extraction and Algal Growth Inhibition Tests
The spent culture medium was processed with aerated, and then added with sodium thiosulfate. A toxicity test was performed after each treatment. The K. sedentarius (pH 8.4) and B. cereus (pH 8.6) supernatants were adjusted to a target pH (i.e., pH 2.0 or 10.0) using a strong acid (HCl) or base (NaOH). Supernatants of three different pH values (2.0, original value, and 10.0) were thoroughly extracted twice with equal volumes of petroleum ether. For each pH, all extracts were combined and then concentrated under reduced pressure at 40℃ until the volume of the extract residue was 5 mL to 10 mL. The extract was then transferred to a 50 mL centrifuge tube and blow-dried with N2. The obtained residue was then weighed.
Each weighed crude extract was first dissolved in methanol and then further diluted with Milli-Q water to obtain a 50 mg mL-1stock solution. The stock solution was further diluted with an S. costatum suspension that is in an exponential growth period to achieve the target concentrations for the toxicity tests. The final methanol concentration in the experimental vessels never exceeded 1%, a concentration that has no effect on algal growth. Five concentrations, namely, 0, 100, 250, 500, 750, and 1000 μg mL-1, were used to determine the anti-algal potential of the crude extracts. The effects of different concentrations of the crude extracts on S. costatum growth were investigated over a 96 h period. The experiments were performed in two replicates, and Chlorophyll a fluorescence was measured.
2.5 Purification and Algal Growth Inhibition Tests
Each crude extract was dissolved in 10 mL Milli-Q water to produce an aqueous suspension. Undissolved particles were removed by filtration through 0.45 μm filters. The 6 mL SPE HLB columns were preconditioned with methanol. Each filtrate was then passed through the columns at <5 mL min-1. Afterward, the columns were allowed to run dry. Methanol (10 mL) was slowly passed through each column and was then collected and stored at 4℃ prior to use.
Each elute was dried with N2at 35℃ and then redissolved in a small volume of Milli-Q water to obtain a concentration of 25 mg mL-1. The test solution was added into the S. costatum suspension in the exponential growthperiod at five concentrations: 0, 25, 50, 100, 200, and 400 μg mL-1. Each treatment had two replicates. The S. costatum was then incubated at 20℃ for 96 h. Inhibition of algal growth was determined by measuring the Chl-a fluorescence intensity at 48 and 96 h.
2.6 Identification of Antifouling Compounds
Based on the aforementioned procedure, the antifouling active substances of K. sedentarius and B. cereus should consist mainly of fatty acids and other acidic substances. Thus, the GC-MS conditions were set for acidic substances. The elution fraction was derivatized with N,O-bis(trimethylsilyl) trifluoroacetamide and trimethylchlorosilane for 1 h at 80℃. The derivatized sample was then analyzed by GC-MS (Agilent 7890A/5975C) using relatively nonpolar capillary columns (CP-Sil 88, 100 m length, 0.2 μm film thickness, 0.25 mm i.d.). The temperature was programmed from 65℃ to 150 ℃ at 10℃min-1, followed by a 12℃min-1ramp to 220℃, a 16℃min-1ramp to 310℃, and then a hold at 310℃ for 10 min. Helium was used as the carrier gas.
2.7 Chlorophyll Fluorescence Measurements and Statistical Analysis
Chlorophyll fluorescence was used for the rapid toxicity test. Chl-a fluorescence is a definite indicator of microalgal toxicity because of its simple measurement and rapid response. The Chl-a fluorescence intensities (FI) of the cultures were measured at room temperature (20℃ to 22℃) every 24 h by a F4500 fluorometer (Hitachi, Japan). The excitation wavelength was 450 nm, the emission wavelength was 685 nm, and the slit width was 5 nm, with a 0.05 s integration time. The anti-algal activities induced by the supernatant are presented as changes in the FI of the microalgae suspension. A decrease in FI indicated positive anti-algal activity of the test supernatant. The percent reduction of FI was calculated using the following formula:

where FIcontrolis the fluorescence intensities of the control and FItreatmentis the fluorescence intensities of each test concentration.
Data were analyzed using the Origin 8.0 statistical package. The EC50(concentration at which 50% of the maximal effect of S. costatum is observed in comparison with the control) levels of the samples were calculated by fitting the data to a sigmoidal inhibition model (Kooijman et al., 1996):
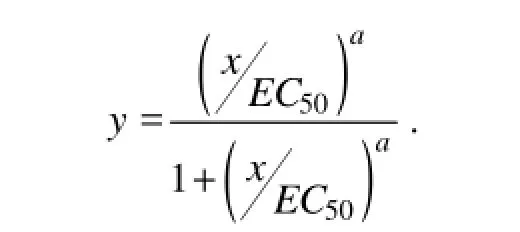
In this equation, x is the concentration of the samples, and y is the inhibited response.
3 Results and Discussion

Fig.1 Inhibitive activity of K. sedentarius and B. cereus supernatants. A, Inhibitive activity of K. sedentarius supernatant against S. costatum at 96 h; B, Inhibitive activity of B. cereus supernatant against S. costatum at 96 h; C, Inhibitive activity of K. sedentarius supernatant against barnacle larvae at 24 h; D, Inhibitive activity of B. cereus supernatant against barnacle larvae at 24 h.
3.1 Anti-algal and Anti-larval Activity of K. sedentarius and B. cereus Supernatants
The supernatants of K. sedentarius and B. cereus were tested for their anti-algae and anti-larval activity which showed the antibacterial activity in the primary screening.The two bacterial strains (Figs.1A and B) could inhibit the growth of S. costatum. Compared with the control, they decreased the FI of S.costatum by more than 50% at the concentrations of 4%, 5% and 6%; the EC50value of K. sedentarius against S. costatum growth was 3.4% ± 0.12%, and that for B. cereus was 3.05% ± 0.095%.
The anti-larval activity of K. sedentarius and B. cereus supernatants against barnacle cyprids of Balanus amphitrite was particularly noteworthy (Figs.1C and 1D). All the barnacle larvae died when the larvae were exposed to K. sedentarius supernatant at 10% or to B. cereus supernatant at 7.5%. The LC50value of K. sedentarius supernatant against barnacle larval was 3.95% ± 0.72%, while that of B. cereus was 3.11% ± 0.76%. The two bacterial strains showed an inhibitory effect on microalgae and barnacle larvae growth.
3.2 Anti-algal Activity of the Crude Extract
Each spent culture medium was treated with sodium thiosulfate or by aeration. The toxicity results show that the treatment with sodium thiosulfate or aeration had negligible effects on the toxicity of the fermentation broth, indicating that the causative agents of fermentation broth toxicity were not volatile or oxidative chemicals.
After adjusting the pH of the bacterial supernatant, the extracts were subjected to a toxicity test. The results show that the antifouling activities of the extracts were highest when the pH of the supernatant was adjusted to 2. The graduated pH changed the toxic effect. The experimental toxicity results and the literature suggest that the active substances are mainly fatty acids. The subsequent extracts were obtained at pH 2.
In the toxicity test, a decrease in FI of the suspended microalgal cells indicates the potency of the antifouling activity of the crude extract. Both test bacterial crude extracts induced a potent inhibitory effect in the target species (Figs.2A and 2B). Compared with the control, 750 and 1000 μg mL-1of the K. sedentarius and B. cereus crude extracts, respectively, decreased FI of S. costatum by more than 75%. EC50of the K. sedentarius crude extracts against S. costatum growth was 236.7 ± 14.08 μg mL-1, that of the B. cereus crude extracts being 290.6± 27.11 μg mL-1. The crude extract of K. sedentarius was more effective than that of B. cereus.
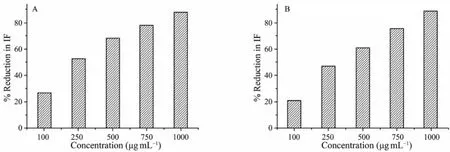
Fig.2 Inhibitory activity of the K. sedentarius and B. cereus crude extracts against S. costatum at 96 h. A, K. sedentarius; B, B. cereus.
3.3 Anti-Algal Activities of the Purified Compounds
HLB SPE columns were used to purify the crude extracts. The anti-algal activities of the purified compounds against S. costatum were then determined, and an increase in antifouling activities was found (Figs.3A and 3B). The purified compounds exhibited observable growth inhibition against S. costatum at 100 μg mL-1. EC50of K. sedentarius against S. costatum growth was 86.4 ± 3.71 μg mL-1, and that of B. cereus was 92.6 ±1.47 μg mL-1, and they were comparable with that of most natural antifouling compounds reported (Hellio et al., 2002; Qi et al., 2009). The results indicate the potential of bacterial metabolites as sources of antifouling agents (Table 1).

Fig.3 Inhibitory activity of the purified extract of K. sedentarius and B. cereus against S. costatum at 96 h. A, K. sedentarius; B, B. cereus.
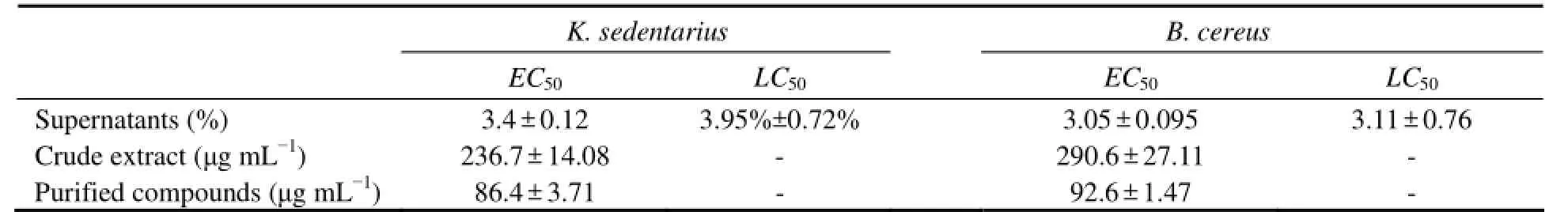
Table 1 Summary of anti-algal and anti-larval activity of K. sedentarius and B. cereus, EC50± SD values and LC50± SD values
3.4 Identification of Antifouling Compounds
After HLB SPE purification, the active fractions (methanol elution) were analyzed using GC-MS. The active antifouling compounds of K. sedentarius were identified as myristic and palmitic acids, and those of B. cereus were identified as myristic and octadecanoic acids (Fig.4). Fatty acid has been considered as one of the most promising marine natural antifouling compounds (Faulkner, 2000). Some fatty acid compounds, including 12-methyl-tetradecanoid acid, 2-hydroxy myristic acid, and cis-9-oleic acid isolated from marine bacteria (Xu et al., 2008; Bhattarai et al., 2006), palmitic acid from macroalgae (Bazes et al., 2009), and linolenic acid from microalgae (Ohta et al., 1994; Bhagavathy et al., 2011), were reported as potential natural antifouling compounds.

Fig.4 GC-MS analysis of the purified extract of K. sedentarius and B. cereus. A, K. sedentarius; B, B. cereus.
4 Conclusions
In this study, the bacteria K. sedentarius and B. cereus, which were isolated from marine biofilms, were found to produce potential antifouling compounds. These compounds were identified by GC-MS as myristic, palmitic, and octadecanoic acids. These fatty acids either chemically synthesized or in the form of crude extracts, would have high application potential as a source of useful compounds for antifouling technology.
Acknowledgements
This study was supported by the National Basic Research Program of China (973 program, No. 2010CB735806). We are thankful to the Marine Genetics and Gene Resource Exploitation Laboratory of the Ocean University of China for isolating and identifying bacteria strains.
Alzieu, C., 1998. Tributyltin: Case study of a chronic contaminant in the coastal environment. Ocean and Coastal Management, 40: 23-26.
Armstrong, E., Boyd, K. G., and Burgess, J. G., 2000. Prevention of marine biofouling using natural compounds from marine organisms. Biotechnology Annual Review, 6: 221-241.
Bazes, A., Silkina, A., Douzenel, P., Fa, F., Kervarec, N., Morin, D., Berge, J. P., and Bourgougnon, N., 2009. Investigation of the antifouling constituents from the brown alga Sargassum muticum (Yendo) Fensholt. Journal of Applied Phycology, 10: 1573-1576.
Bhagavathy, S., Sumathi, P., and Jancy, S. B., 2011. Green algae Chlorococcum humicola-A new source of bioactive compounds with antimicrobial activity. Asian Pacific Journal of Tropical Biomedicine, 1: S1-S7.
Bhattarai, H. D., Ganti, V. S., and Paudel, B., 2006. Isolation of antifouling compounds from the marine bacterium, Shewanella oneidensis SCH0402. World Journal of Microbiology and Biotechnology, 23: 243-249.
Buma, A. G. J., Sjollema, S. B., van de Poll, W. H., Klamer, H. J. C., and Bakker, J. F., 2009. Impact of the antifouling agent Irgarol 1051 on marine phytoplankton species. Journal of Sea Research, 61: 133-139.
Callow, M. E., and Callow, J. A., 2002. Marine biofouling: A sticky problem. Biologist, 49: 1-5.
Clare, A. S., 1996. Marine natural product antifoulants: Status and potential. Biofouling, 9: 221-229.
Dafforn, K. A., Lewis, J. A., and Johnston, E. L., 2011. Antifouling strategies: History and regulation, ecological impacts and mitigation. Marine Pollution Bulletin, 62 (3): 453-465.
Dahlstrom, M., Martensson, L., Jonsson, P., Amebrant, T., and Elwing, H., 2002. Surface active adrenoreceptor compounds prevent the settlement of cyprid larvae of Balanus improvisus. Biofouling, 16: 191-198.
Dobretsov, S., Xiong, H. R., Xu, Y., Levin, L. A., and Qian, P. Y., 2007. Novel antifoulants: Inhibition of larval attachment by proteases. Marine Biotechnology, 9: 388-397.
Faulkner, D. J., 2000. Marine natural products. Natural Product Reports, 17: 7-55.
Hellio, C., Berge, J. P., Beaupoil, C., Gal, Y. L., and Bourgougno, N., 2002. Screening of marine algal extracts for anti-settlement activities against microalgae and macroalgae. Biofouling, 18: 205-215.
Hellio, C., Simon-Colin, C., Clare, A. S., and Deslandes, E., 2004. Isethionic acid and floridoside isolated from the red alga, Grateloupia turuturu, inhibit settlement of Balanus amphitrite cyprid larvae. Biofouling, 20: 139-145.
Holmstroem, C., and Kjelleberg, S., 1999. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiology Ecology, 30: 285-293.
Iyapparaj, P., Revathi, P., Ramasubburayan, R., Prakash,S., Anantharaman, P., Immanuel, G., and Palavesam, A., 2013. Antifouling activity of the methanolic extract of Syringodium isoetifolium, and its toxicity relative to tributyltin on the ovarian development of brown mussel Perna indica. Ecotoxicology and Environmental Safety, 89: 231-238.
Kitano, Y., Akima, C., Yoshimura, E., and Nogata, Y., 2011. Anti-barnacle activity of novel simple alkyl isocyanides derived from citronellol. Biofouling, 27: 201-205.
Kooijman, S. A. L. M., Hanstveit, A. O., and Nyholm, N., 1996. No-effect concentration of algal growth inhabitation tests. Water Research, 30: 1625-1632.
Little, B. J., and Wagner, P. A., 1997. Spatial relationships between bacteria and mineral surfaces. Reviews in Mineralogy and Geochemistry, 35: 123- 160.
Li, X. C., Dobretsov, S., Xu, Y., Xiao, X., Hung, O. S., and Qian, P. Y., 2006. Antifouling diketopiperazines produced by a deep-sea bacterium, Streptomyces fungicidicus. Biofouling, 22: 187-194.
Marshall, K. C., Stout, R., and Mitchell, R., 1971. Mechanism of the initial events in the sorption of marine bacteria to surfaces. Journal of General Microbiology, 68: 337-341.
Ohta, S., Chang, T., Kawashima, A., Nagate, T., Murase, M., Nakanishi, H., Miyata, H., and Kondo, M., 1994. Anti Methicillin-Resistant Staphylococcus aureus (MRSA) activity by linolenic acid isolated from the marine microalga Chlorococcum HS-101. Bulletin of Environmental Contamination and Toxicology, 52: 73-680.
Qian, P. Y., Xu, Y., and Fusetani, N., 2010. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling, 26: 223-234.
Qi, S. H., Xu, Y., Xiong, H. R., Qian, P. Y., and Zhang, Z., 2009. Antifouling and antibacterial compounds from a marine fungus Cladosporium sp. F14. World Journal of Microbiology and Biotechnology, 25: 399-406.
Silkina, A., Bazes, A., Mouget, J. L., and Bourgougnon, N., 2012. Comparative efficiency of macroalgal extracts and booster biocides as antifouling agents to control growth of three diatom species. Marine Pollution Bulletin, 64: 2039-2046.
Thiyagarajan, V., Harder, T., and Qian, P. Y., 2003. Combined effect of temperature and salinity on larval development and attachment of the subtidal barnacle Balanus trigonus Darwin. Journal of Experimental Marine Biology and Ecology, 287: 223-236.
Xu, Y., Li, H. L., Li, X. C., Xiao, X., and Qian, P. Y., 2008. Inhibitory Effects of a branched-chain fatty acid on larval settlement of the polychaete hydroides elegans. Marine Biotechnology, 11: 495-504.
Yebra, D. M., Kiil, S., and Dam-Johansen, K., 2004. Antifouling technology – past, present and future steps towards efficient and environmentally friendly antifouling coatings. Progress in Organic Coatings, 50: 75-104.
Yang, L. H., Lee, O. O., Jin, T., Li, X. C., and Qian, P. Y., 2006. Antifouling properties of 10b-formamidokalihinol-A and kalihinol A isolated from the marine sponge Acanthella cavernosa. Biofouling, 22: 23-32.
(Edited by Ji Dechun)
(Received August 28, 2013; revised March 22, 2014; accepted April 4, 2014)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-66781815
E-mail: surongguo@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- Sedimentary Evolution of the Holocene Subaqueous Clinoform off the Southern Shandong Peninsula in the Western South Yellow Sea
- Identification of Fucans from Four Species of Sea Cucumber by High Temperature1H NMR
- Experimental Study on the Flow Around Two Tandem Cylinders with Unequal Diameters
- Revision of P-wave Velocity and Thickness of Hydrate Layer in Shenhu Area, South China Sea
- Study on Internal Waves Generated by Tidal Flow over Critical Topography
- Isolation and Characterization of Fucoidans from Five Brown Algae and Evaluation of Their Antioxidant Activity
