Macroalgae Blooms and their Effects on Seagrass Ecosystems
2014-04-20HANQiuyingandLIUDongyan
HAN Qiuying, and LIU Dongyan
Key Laboratory of Coastal Zone Environmental Processes and Ecological Remediation, CAS, Shandong Provincial Key Laboratory of Coastal Zone Environmental Processes, CAS Experimental Station of Integrated Coastal Environment in Muping, Yantai Institute of Coastal Zone Research (YIC), Chinese Academy of Sciences, Yantai 264003, P. R. China
Macroalgae Blooms and their Effects on Seagrass Ecosystems
HAN Qiuying*, and LIU Dongyan
Key Laboratory of Coastal Zone Environmental Processes and Ecological Remediation, CAS, Shandong Provincial Key Laboratory of Coastal Zone Environmental Processes, CAS Experimental Station of Integrated Coastal Environment in Muping, Yantai Institute of Coastal Zone Research (YIC), Chinese Academy of Sciences, Yantai 264003, P. R. China
Seagrass decline caused by the macroalgae blooms is becoming a common phenomenon throughout temperate and tropical regions. We summarized the incidence of macroalgae blooms throughout the world and their impact on seagrass beds by direct and indirect ways. The competition for living space and using resources is the most direct effect on seagrass beds when macroalgae are blooming in an aquatic ecosystem. The consequence of macroalgae blooms (e.g., light reduction, hypoxia, and decomposition) can produce significant indirect effects on seagrass beds. Light reduction by the macroalgae can decrease the growth and recruitment of seagrasses, and decomposition of macroalgae mats can increase the anoxic and eutrophic conditions, which can further constrict the seagrass growth. Meanwhile, the presence of seagrass shoots can provide substrate for the macroalgae blooms. Controlling nutrient sources from the land to coastal waters is a general efficient way for coastal management. Researching into the synergistical effect of climate change and anthropognic nutrient loads on the interaction between searsasses and macroalgae can provide valuable information to decrease the negative effects of macroalgae blooms on seagrasses in eutrophic areas.
eutrophication; decline; seagrasses; macroalgae blooms
1 Introduction
Seagrass beds have been widely recognized as highly important coastal systems that provide valuable ecosystem services in trapping and storing nutrients and being food resource for animals (Orth et al., 2006). Recently, their vast carbon sink capacity has been quantified (Duarte et al., 2010). Seagrass beds worldwide can provide 19004 $ ha-1at least annually (Costanza et al., 1997). Seagrass decline has been reported in recent years over the world (Orth et al., 2006; Waycott et al., 2009). The environmental effects of excess nutrients are one of causes of seagrass losses (Orth et al., 2006; Burkholder et al., 2007). Nutrient can have different effects on seagrasses based on background concentration of nutrients and seagrass species. In oligotrophic environments, nutrient enrichment can facilitate seagrass growth and biomass (Short, 1983; Alcoverro et al., 1997; Peralta et al., 2003; Invers et al., 2004) or has no effects on seagrasses (Harlin and Thorne-Miller, 1981; Murray et al., 1992; Pederson and Borum, 1993; Pedersen, 1995; Lee and Dunton, 1997). Nutrient enrichment can negatively directly or indirectly impact on seagrasses in eutrophic waters (van Katwijk et al., 2010; Christianen et al., 2012). Nutrients such as nitrate and ammonia can directly impact Zostera marina by toxicity, resulting in decreased rhizome biomass, shoot biomass and density (Burkholder et al., 1992; Short et al., 1995; van Katwijk et al., 2010; Christianen et al., 2012). In eutrophic coastal areas, proliferation of phytoplankton, epiphytic microalgae and fast-growing drifting macroalgae usually occur, promoting light reduction, increasing the sediment organic matter load, which could induce the risk of anoxia and sulfide intrusion into meristematic areas of seagrasses (Pregnall et al., 1984; Holmer and Bondgaard, 2001; Greve et al., 2003), therefore restricting seagrasses growth (Twilley et al., 1985; Nelson and Lee, 2001).
Excessive growth of fastgrowing macroalgae has appeared in many coastal areas (Table 1). Macroalgae blooms are controlled by physical, chemical, and biological factors (Brush and Nixon, 2010). Generally, at sheltered locations where light is not limiting, nutrients control net primary production of macroalgae in most coastal systems and macroalgal biomass is therefore usually correlated to nutrient inputs (McGlathery et al., 2001). Worldwide, seagrasses experience negative effects from macroalgae blooms (Table 2), often leading to the decline of the seagrass beds. The succession from seagrasses to macroalgae can cause profound ecological changes, altering total system primary productivity, biogeochemical cycling and species composition (McGlathery, 2001). More specifically, sediments may become less stable, waters turn more turbid and nutrient turnover will increase and primary productivity, biomass as well asnursery functions will become more fluctuating (Mc-Glathery et al., 2001, 2007; Nelson, 2009). In a recent review, Thomsen et al., (2012) found that mass macroalgae have stronger affect on seagrasses than a small quantity of macroalgae, ‘rooted’ macroalgae has less effect than floating macroalgae, and bigger species of seagrass are more resistant than smaller species to macroalgae blooms. The negative effects of macroalgae blooms depend on the environmental variables in the region, impacting on the management of seagrass ecosystems subject to high nutrient loadings (Hessing-Lewis et al., 2011). Therefore, it is necessary to study the effect of macroalgae blooms on seagrass ecosystem to further understand the causes and mechanisms of seagrass decline. Our objective is to review the reported effects of macroalgae blooms on seagrass health and discuss the mechanisms leading to these effects. In addition, we discuss the effective measure to reduce the negative effects of macroalgae blooms on seagrass ecosystems and provide further research directions in the future.
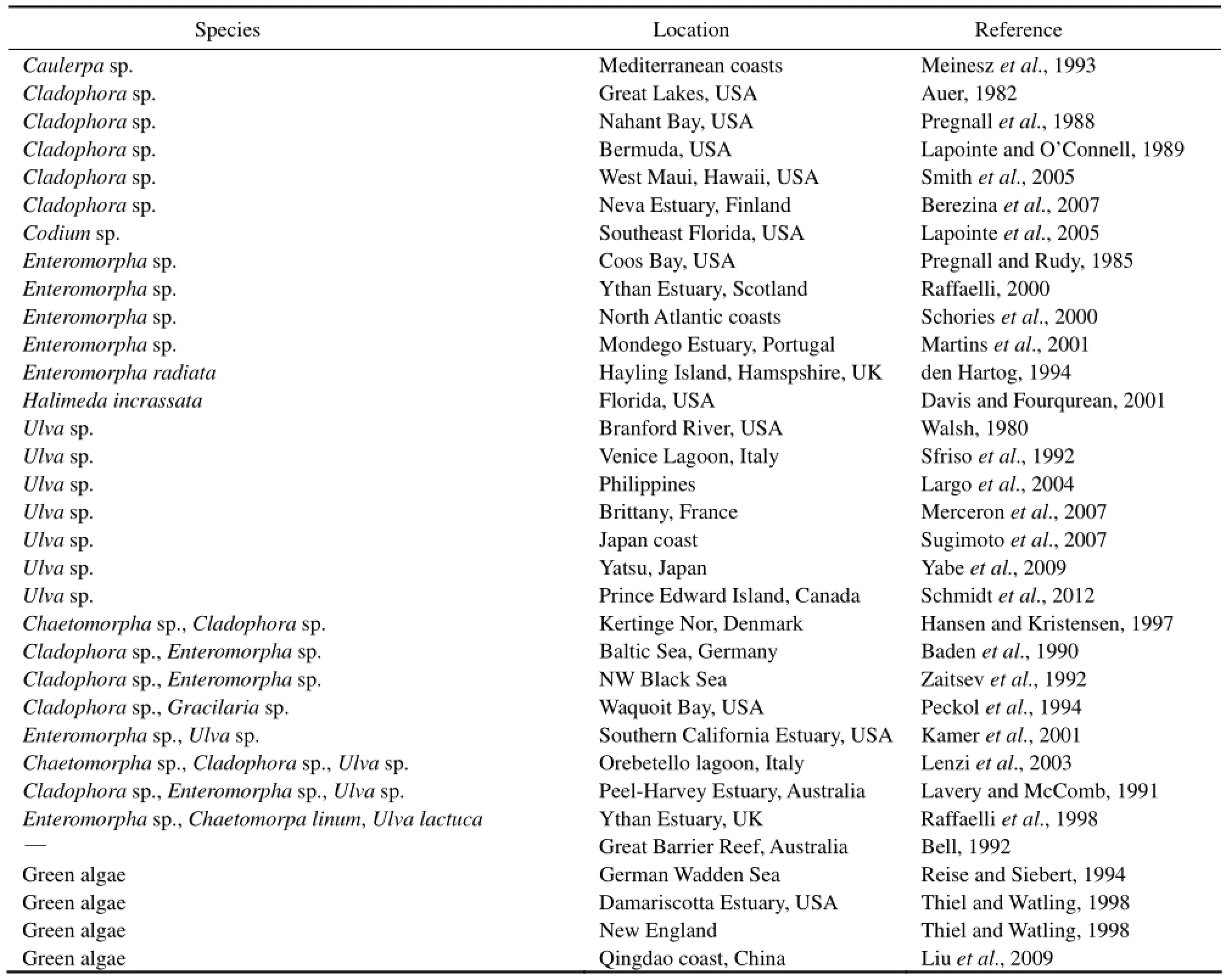
Table 1 List of macroalgae blooms recorded in literatures
2 Mechanisms Underlying the Negative Effect of Macroalgae Blooms on Seagrasses
2.1 Direct Effect of Macroalgae Blooms on Seagrasses
The direct effects of macroalgae on seagrass ecosystems include the competition for space or resources (Druehl, 1973; Williams, 1990; Fourqurean et al., 1995; Ceccherlli and Cinelli, 1997). Competition between seagrasses may have more effects than competition between seagrass and other species at high densities of seagrass (Rose and Dawes, 1999). Multer (1988) found that high biomass of macroalgae appears under the conditions of low and moderate seagrass shoot density, indirectly demonstrating the competitive relationship between seagrasses and macroalgae. When nutrient availability is low, competitive dominance of seagrasses over rhizophytic macroalgae occurs (Fourqurean et al., 1995), and rhizophytic macroalgae could even accelerate seagrass beds in their early successional stage, while in the later stage of bed development, the density of macroalgae declines as the seagrass bed is rebuilt, indicating the acceleration and competition for space between seagrasses and macroalgae (Williams, 1990). Davis and Fourqurean (2001) also demonstrated that under the condition of macroalgae removal, the ratio between C and N in leaf tissue of seagrass is significantly lower, suggesting competition mechanism for N between two species.
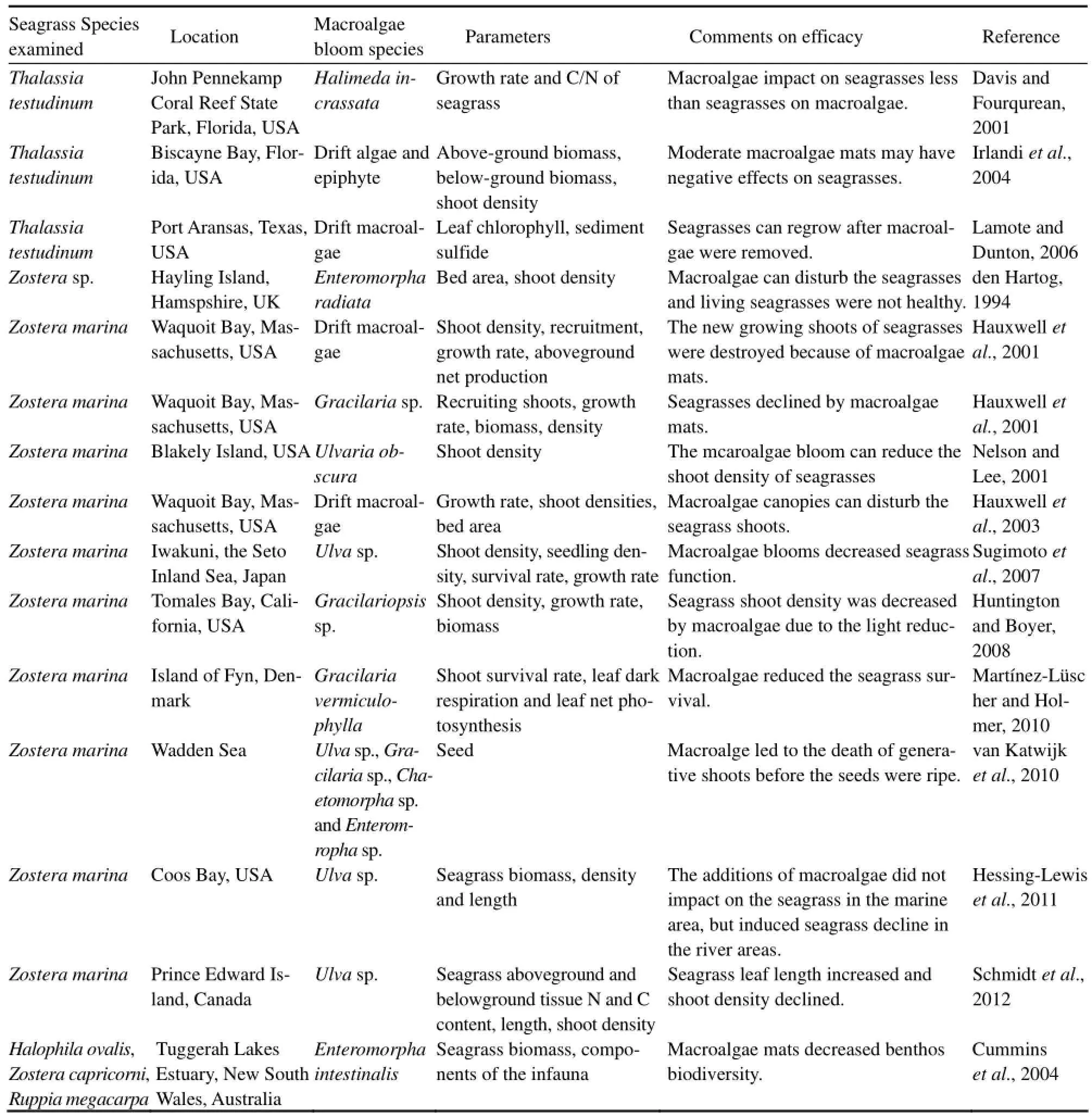
Table 2 Overview of effects of macroalgal blooms on seagrass
2.2 Indirect Effects of Macroalgae Blooms on Seagrass: Light Reduction
Light availability is a vital environmental factor affecting seagrass and macroalgae growth. Light reduction by the macroalgae may reduce the growth and recruitment of seagrasses and further affect seagrass ecosystem health. The result of light reduction induced by macroalgae blooms decreases the growth depth of seagrasses (Krause-Jensen et al., 2000; McGlathery, 2001). In highly eutrophic waters, macroalgae species can obtain the highest biomass of 0.5 kg m-2and 0.5 m canopy height (McGlathery, 2001). In Hiroshima Bay (Japan), floating Ulva canopy height can attain 20-30 cm (Sugimoto et al., 2007). Under the conditions of Cladophora vagabunda and Gracilaria tikvahiae blooms, more than 95% of light intensity is reduced from 6 to 8 cm cover (Krause-Jensen et al., 2000). In 3-4 cm ulvoid canopies, over 90% of photosynthetic photon flux density is attenuated (Sugimoto et al., 2007).
Light limitation may reduce photosynthetic activity of seagrasses. For example, light availability reduction because of macroalgae blooms leads to the shoot density decline of eelgrass in Hamblin Pond (Hauxwell et al., 2001). The macroalgae blooms leading to light reduction for seagrasses are correlated to the duration of the macroalgal cover. Shoot growth does not increase over 10 days after the macroalgae are removed from the seagrass bed, and growth of Thalassia testudinum does not decrease over 10 days when macroalgae are present, suggesting that improving and declining light availability by drift algae does not affect the short-term growth of seagrasses(Irlandi et al., 2004). It may be because the shaded and unshaded shoots of seagrass can utilize the resources together. But in a long period, the macroalgae can significantly decrease the seagrass biomass. For example, under the conditions of 100% macroalgae canopy for 2-3 months, 25% above-ground biomass of seagrass is reduced (Irlandi et al., 2004).
Carbon translocation and storage capacity between leaves, rhizomes and roots can control the ecological success of seagrasses under light reduction conditions (Lee and Dunton, 1996; Alcoverro et al., 1999; Touchette and Burkholder, 2000; Brun et al., 2002; Peralta et al., 2002). Ammonium assimilation into amino acids and other nutrogen-organic compounds requires carbon skeletons and energy, which are from photosynthesis or mobilized from carbon-reserves in seagrasses (Brun et al., 2008; Villazán et al., 2013). Light reduction from macroalgl blooms may increase the toxicity of ammonium on seagrasses (Brun et al., 2008; Villazán et al., 2013).
2.3 Indirect Effects of Macroalgae Blooms on Seagrasses: Increasing Water-Column and Sediment Hypoxia
Macroalgae may release 39% of gross production of themselves after most of the remaining fixed carbon is released (Albert and Valiela, 1994). Decomposition of macroalgae mats may release enormous dissolved organic matter into environment, which may increase biological oxygen demand and lead to the anoxia in eutrophicated waters. Extended hypoxia conditions increase energy requirements of seagrass for photosynthesis and further constrict the seagrass growth. The anoxia condition caused by dense macroalgae mats may change sulfide and nutrient cycles (McGlathery et al., 2007). High sulphide concentration resulting from the anoxia by macoralgae canopies in the seagrass beds can lead to sulphide intrusion into meristematic areas of seagrass, which decreases the maximum photosynthetic rate of seagrass and has an effect on leaf growth (Pedersen et al., 2004). In addition, sulfide concentrations in the pore water rise when plant photosynthesis decreases the oxygen supply to the roots (Jøgernsen, 1982). Sulfide accumulation in the sediments may further result in the decrease of the seagrass biomass or mortality (Koch et al., 2007; van der Heide et al., 2012).
Hypoxia and high sulphide may also decrease productivity of seagrasses by negatively affecting nutrient absorption (Pregnall et al., 1984). Because of the ability to fast absorb nutrients, fast-growing macroalgae can regenerate the nutrient from ambient environment, which can result in the temporary retention of nutrients (McGlathery et al., 1997). High respiration rates may cause a significant release of nutrients from both the sediments and decomposing algae (Sfriso et al., 1987). Under higher releasing rates of nutrients, macroalgae absorbing nutrients from water displace plants uptaking nutrients from sediments. The macroalgae bloom can bring more anthropogenic inputs of nitrogen into seagrass ecosystems, such as that from ammonia (Nelson, 2009). Higher ammonium concentration in ambient water has the deleterious effects on seagrasses (van der Heide et al., 2008). For example, the ammonia levels (>25 µmol L-1) may negatively impact on Zostera marina (van Katwijk et al., 1997), and high ammonium concentration (125 µmol L-1) can lead to the seagrass shoots reduction and seagrass death (Huntington and Boyer, 2008).
3 Other Effects
Because sediment is deposited on seagrass beds due to their ability to decrease current velocities and weaken wave energy (Gambi et al., 1990), seagrass presence may actually enhance macoalgae growth on the soft sediments (Tweedley et al., 2008). Macroalgae bloom can result in the sediment erosion, increasing the turbidiy of water under the condition of weak wind and wave action (Canal-Vergés et al., 2010). The turbidity and erosion may lead to the loss of eelgrass meadows by decreasing the light availability (van der Heide et al., 2008) and increasing the risk of seagrass rhizome being uprooted by water (Duarte, 2002; Han et al., 2012). In spring or summer, the degradation of huge amounts of macroalgae results in the break-down of the biological balance in the seagrass ecosystem. Macralgae with fast nutrient turnover rate can faster decompose than seagrass, which invokes nutrient releasing into water and consequently increases the concentration of nutrient, therefore further supporting macroalgae growth (McGlathery et al., 2001). All three effects above-mentioned will result in non-linear responses and favour macroalgae, thus accelerating the replacement of seagrasses by macroalgae. Thus, it is important to study the biomass thresholds of macroalgae that induces the seagrass beds decline for the seagrass ecosystem protection.
4 Future Needs and Management Implications
In the future, measures to decrease the negative effects of macroalgae blooms on seagrass ecosystems should be taken. Controlling nutrient sources from land to coastal waters is a general efficient way for management. Eutrophication is serious in many coastal areas due to agriculture, industry, aquaculture waste water. The occurrence of nutrients in excess is necessary for the macroalgae bloom (Hodgkin et al., 1980). There are some reports that seagrass ecosystem restoration is successful after reducing the nutrient loading into coastal areas, in Orbetello lagoon (Italy) (Lenzi et al., 2003), Mondego estuary (Portugal) (Cardoso et al., 2005) and southwest Florida (USA) (Tomasko et al., 2005).
Climate changes, such as increased temperature and sea level rise effects on seagrasses and macroalgae have been studied (Santos, 1993; Short and Neckles, 1999; Sousa-Dias and Melo, 2008). Higher temperature could facilitate certain macroalgal species growth, especiallyspecies inducing blooms (Sousa-Dias and Melo, 2008). Increased temperature has different effects on different seagrass species (Marsh et al., 1986). Warmer species increase photosynthesis and respiration with higher temperature, but temperate species reach their photosynthesis optimum below the highest seasonal temperature (Short and Neckles, 1999). Sea level rise may decrease the light level for seagrasses, therefore negatively impacting seagrass productivity and functional values (Short and Neckles, 1999; Hauxwell et al., 2001; Irlandi et al., 2004). Sea level rise may increase water flow or enhance tidal circulation, which could accelerate flushing of the macroalgae, and therefore reduce the negative effects of macroalgae bloom on seagrass ecosystems (Flindt et al., 1997; Lenzi et al., 2003). Sea level rise will draw in seawater into estuaries and rivers, resulting in salinity change, which in turn may alter competitive situation between seagrasses and macroalgae. Global climate change acceleration can be expected in the future. It is necessary to research into the synergistical effect of climate change and anthropogenic nutrient loads on the interaction between searsasses and macroalgae in eutrophic areas, which can provide valuable information to decrease the negative effects of macroalgae bloom on seagrasses.
Acknowledgements
This study was funded by the Natural Science Foundation of China (41106099), CAS Scientific Project of Innovation and Interdisciplinary, the Ministry of Science and Technology Project Foundation (2014FY210600), Yantai Science and Technology Bureau (2011061) and the Natural Science Foundation of Shandong Province (ZR 2009EQ006).
Alber, M., and Valiela, I., 1994. Production of microbial organic aggregates from macrophyte-derived dissolved organic material. Limnology and Oceanography, 39: 37-50.
Alcoverro, T., Romero, J., Duarte, C. M., and López, N. I., 1997. Spatial and temporal variations in nutrient limitation of seagrass Posidonia oceanica growth in the NW Mediterranean. Marine Ecoogy Progress Series, 146: 155-161.
Alcoverro, T., Zimmerman, R. C., Kohrs, D. G., and Alberte, R. S., 1999. Resouce allocation and sucrose mobilization in light-limited eelgrass Zostera marina. Marine Ecology Progress Series, 187: 121-131.
Auer, M. T., 1982. Ecology of filamentous algae. Journal of Great Lakes Research, 8: 1-237.
Baden, S. P., Loo, L. O., Pihl, L., and Rosenbergs, R., 1990. Effects of eutrophication on benthic communities including fish: Swedish west coast. Ambio, 19: 113-122.
Bell, P. R. F., 1992. Eutrophication and coral reefs-some examples in the Great Barrier Reef Lagoon. Water Research, 26: 553-568.
Berezina, N. A., Tsiplenkina, I. G., Pankova, E. S., and Gubelit, J. I., 2007. Dynamics of invertebrate communities on the stony littoral of the Neva Estuary (Baltic Sea) under macroalgal blooms and bioinvasions. Transitional Waters Bulletin, 1: 65-76.
Brun, F. G., Hernández, I., Vergara, J. J., Peralta, G., and Pérez-Lloréns, J. L., 2002. Assessing the toxicity of ammonium pulses to the survival and growth of Zostera noltii. Marine Ecology Progress Series, 225: 177-187.
Brun, F. G., Olivé, I., Malta, E., Vergara, J. J., Hernández, I., and Pérez-Llorénans, J. L., 2008. Increased vulnerability of Zostera noltii to stress caused by low light and elevated ammonium levels under phosphate deficiency. Marine Ecology Progress Series, 365: 67-75.
Brush, M. J., and Nixon, S. W., 2010. Modeling the role of macroalgae in a shallow sub-estuary of Narragansett Bay, RI (USA). Ecological Modelling, 221: 1065-1079.
Burkholder, J. M., Mason, K. M., and Glasgow Jr, H. B., 1992. Water-column nitrate enrichment promotes decline of eelgrass Zostera marina: Evidence from seasonal mesocosm experiments. Marine Ecology Progress Series, 81: 163-178.
Burkholder, J. M., Tomasko, D. A., and Touchette, B. W., 2007. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology, 350: 46-72.
Canal-Vergés, P., Vedel, M., Valdemarsen, T., Kristensen, E., and Flindt, M. R., 2010. Resuspension created by bed load traport of macroalgae: implications for ecosystem functioning. Hydrobiologia, 649: 69-76.
Cardoso, P. G., Brandão, A., Pardal, M. A., Raffaelli, D., and Marques, J. C., 2005. Resilience of Hydrobia ulvae populations to anthropogenic and natural disturbances. Marine Ecology Progress Series, 289: 191-199.
Ceccherlli, G., and Cinelli, F., 1997. Short-term effects of nutrient enrichment of the sediment and interactions between the seagrass Cymodocea nodosa and the introduced green alga Caulerpa taxifolia in a Mediterranean Bay. Journal of Experimental Marine Biology and Ecology, 217: 165-177.
Christianen, M. J. A., Govers, L. L., Bouma, T. J., Kiswara, W., Roelofs, J. G. M., Lamers, L. P. M., and van Katwijk, M. M., 2012. Marine megaherbivore grazing may increase seagrass tolerance to high nutrient loads. Journal of Ecology, 100: 546-560.
Costanza, R., d’Aege, R., de Groot, R., Farber, S., Grasso, M., Hannon, B., Limburg, K., Naeem, S., O’Neill, R. V., Jose, P., Raskin, R. G., Sutton, P., and van den Belt, M., 1997. The value of the world’s ecosystem services and natural capital. Nature, 387: 253-260.
Cummins, S. P., Roberts, D. E., and Zimmerman, K. D., 2004. Effects of the green macroalga Enteromorpha intestinalis on macrobenthic and seagrass assemblages in a shallow coastal estuary. Marine Ecology Progress Series, 266: 77-87.
Davis, B. C., and Fourqurean, J. W., 2001. Competition between the tropical alga, Halimeda incrassate and the seagrass, Thalassia testudinum. Aquatic Botany, 71: 217-232.
den Hartog, C., 1994. Suffocation of a littoral Zostera bed by Enteromorphy radiate. Aquatic Botany, 47: 21-28.
Druehl, L. D., 1973. Marine transplantations. Science, 179: 12.
Duarte, C. M., 2002. The future of seagrass meadows. Environmental Conservation, 29: 192-206.
Duarte, C. M., Marbà, N., Gacia, E., Fourqurean, J. W., Beggins, J., Barrón, C., and Apostolaki, E. T., 2010. Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows. Global Biogeochemical Cycles, 24: GB4032, DOI: 10.1029/2010GB003793.
Flindt, M. R., Salomonsen, J., Carrer, M., Bocci, M., and Kamp-Nielsen, L., 1997. Loss, growth and transport dynamics of Chaetomorpha aerea and Ulva rigida in the Lagoon of Venice during an early summer field campaign. Ecological Modelling, 102: 133-141.
Fourqurean, J. W., Powell, G. V. N., Kenworthy, W. J., and Zieman, J. C., 1995. The effects of long-term manipulation of nutrient supply on competition between the seagrasses Thalassia testudinum and Halodule wrightii in Dlorida Bay. Oikos, 72: 349-358.
Gambi, M. C., Nowell, A. R. M., and Jumars, P. A., 1990. Flume observations on flow dynamics in Zostera marina (eelgrass) beds. Marine Ecology Progress Series, 61: 159-169.
Greve, T. M., Borum, J., and Pedersen, O., 2003. Meristematic oxygen variability in eelgrass (Zostera marina). Limnology and Oceanography, 48: 210-216.
Han, Q. Y., Bouma, T. J., Brun, F. G., Suykerbuyk, W., and van Katwijk, M. M., 2012. Resilience of Zostera noltii to burial or erosion disturbances. Marine Ecology Progress Series, 449: 133-143.
Hansen, K., and Kristensen, E., 1997. Impact of macrofaunal recolinization on benthic metabolism and nutrient fluxes in a shallow marine sediment previously overgrown with macroalgal mats. Estuarine, Coastal and Shelf Science, 45: 613-628.
Harlin, M. M., and Thorne-Miller, B., 1981. Nutrient enrichment of seagrass beds in a rhode island coastal lagoon. Marine Biology, 65: 221-229.
Hauxwell, J., Cebrian, J., and Baliela, I., 2001. Macroalgal canopies contribute to eelgrass (Zostera marina) decline in temperate estuarine ecosystems. Ecology, 82: 1007-1022.
Hauxwell, J., Cebrian, J., and Valiela, I., 2003. Eelgrass Zostera marina loss in temperate estuaries: relationship to land derived nitrogen loads and effect of light limitation imposed by algae. Marine Ecology Progress Series, 247: 59-73.
Hemminga, M. A., 1998. The root/rhizome system of seagrasses: An asset and a burden. Journal of Sea Research, 39: 183-196. Hessing-Lewis, M. L., Hacker, S. D., Menge, B. A., and Rumrill, S. S., 2011. Context-dependent eelgrass-macroalage interactions along an estuarine gradient in the pacific northwest, USA. Estuaries and Coasts, 34: 1169-1181.
Hodgkin, E. P., Birch, P. B., Black, R. E., and Humphries, R. B., 1980. The Peel-Harvey estuarine system study (1976-1980). Report N. 9. Department of Conservation and Environment, Perth, Western Australia.
Holmer, M., and Bondgaard, E. J., 2001. Photosynthetic and growth response of eelgrass to low oxygen and high sulphide concentrations during hypoxic events. Aquatic Botany, 70: 29-38.
Huntington, B. E., and Boyer, K. E., 2008. Effects of red macroalgal (Gracilariopsis sp.) abundance on eelgrass Zostera marina in Tomales Bay, California, USA. Marine Ecology Progress Series, 367: 133-142.
Invers, O., Kraemer, G. P., Pérez, M., and Romero, J., 2004. Effects of nitrogen addition on nitrogen metabolism and carbon reserves in the temperate seagrass Posidonia oceanica. Journal of Experimental Marine Biology and Ecology, 303: 97-114.
Irlandi, E. A., Orlando, B. A., and Biber, P. D., 2004. Drift algae-epiphyte-seagrass interactions in a subtropical Thalassia testudinum meadow. Marine Ecology Progress Series, 279: 81-91.
Jøgernsen, B. B., 1982. Mineralization of organic matter in the sea bed-the role sulphate reduction. Nature, 296: 643.
Kamer, K., Boyle, K. A., and Fong, P., 2001. Macroalgal bloom dynamics in a highly eutrophic southern California Estuary. Estuaries, 24: 623-635.
Koch, M. S., Schopmeyer, S., Kyhn-Hansen, C., and Madden, C. J., 2007. Synergistic effects of high temperature and sulphide on tropical seagrass. Journal of Experimental Marine Biology and Ecology, 341: 91-101.
Krause-Jensen, D., Middelboe, A., and Christensen, P. B., 2000. Eelgrass, Zostera marina, growth along depth gradients: Upper boundaries of the variation as a powerful predictive tool. Oikos, 91: 233-244.
Lamote, M., and Dunton, K. H., 2006. Effects of drift macroalgae and light attenuation on chlorophyll fluorescence and sediment sulfides in the seagrass Thalassia testudinu. Journal of Experimental Marine Biology and Ecology, 334: 174-186.
Lapointe, B. E., and O’Connell, J., 1989. Nutrient-enhanced growth of Cladophora prolifera in Harrington Sound, Bermuda: Eutrophication of a confined, phosphorus-limited marine ecosystem. Estuarine, Coastal and Shelf Science, 28: 347-360.
Lapointe, B. L., Barile, P. J., Littler, M. M., Littler, D. S., and Bedford, B. J., 2005. Macroalgal blooms on southeast Florida coral reefs: I. Nutrient stoichiometry of the invasive green alga Codium isthmocladum in the wider Caribbean indicates nutrient enrichment. Harmful Algae, 4: 1092-1105.
Largo, D. B., Sembrano, J., Hiraoka, M., and Ohno, M., 2004. Taxonomic and ecological profile of ‘green tide’ species of Ulva (Ulvales, Chlorophyta) in central Philippines. Hydrobiologia, 512: 247-253.
Lavery, P. S., and McComb, A. J., 1991. Macroalgal-sediment nutrient interactions and their importance to macroalgal nutrition in a eutrophic estuary. Estuarine, Coastal and Shelf Science, 32: 281-295.
Lee, K. S., and Dunton, K. H., 1997. Effects of in situ light reduction on the maintenance, growth and partitioning of carbon resources in Thalassia testudinum Banks ex König. Journal of Experimental Marine Biology and Ecology, 210: 53-73.
Lenzi, M., Palmieri, R., and Porrello, S., 2003. Restoration of the eutrophic Orbetello Lagoon (Tyrrhenian Sea, Italy): Water quality management. Marine Pollution Bulletin, 46: 1540-1548.
Liu, D. Y., Keesing, J. K., Xing, Q. G., and Shi, P., 2009. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Marine Pollution Bulletin, 58: 888-895.
Marsh Jr., J. A., Dennison, W. C., and Alberte, R. S., 1986. Effects of temperature on photosynthesis and respiration in eelgrass (Zostera marina L.). Journal of Experimental Marine Biology and Ecology, 101: 257-267.
Martínez-Lüscher, J., and Holmer, M., 2010. Potential effects of the invasive species Gracilaria vermiculophylla on Zostera marina metabolism and survival. Marine Environmental Research, 69: 345-349.
Martins, I., Pardal, M. A., Lillebø, A. I., Flindt, M. R., and Marques, J. C., 2001. Hydrodynamics as a major factor controlling the occurrence of green macroalgal blooms in a eutrophic estuary: A case study on the influence of precipitation and river management. Estuarine, Coastal and Shelf Science, 52: 165-177.
McGlathery, K. J., Krause-Jensen, D., Rysgaard, S., and Christensen, P. B., 1997. Patterns of ammonium uptake within dense mats of the filamentous macroalga Chaetomorpha linum. Aquatic Botany, 59: 99-115.
McGlathery, K. J., 2001. Macroalgal blooms contribute to the decline of seagrass in nutrient-enriched coastal waters. Journal of Phycology, 37: 453-456.
McGlathery, K. J., Anderson, C. I., and Tyler, A. C., 2001. Magnitude and variability of benthic and pelagic metabolism in atemperate coastal lagoon. Marine Ecology Progress Series, 216: 1-15.
McGlathery, K. J., Sundbäck, K., and Anderson, I. C., 2007. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Marine Ecology Progress Series, 348: 1-18.
Meinesz, A. J., De Vaugelas, J., Hesse, B., and Mari, X., 1993. Spread of the introduced tropical green algae Caulerpa taxifolia in northern Mediterranean waters. Journal of Appllied Phycology, 5: 141-147.
Merceron, M., Antoine, V., Auby, I., and Morand, P., 2007. In situ growth potential of the subtidal part of green tide forming Ulva spp. stocks. Science of the Total Environment, 384: 293-305.
Morand, P., and Merceron, M., 2005. Macroalgal population and sustainability. Journal of Coastal Research, 21: 1009-1020.
Multer, H. G., 1988. Growth, ultrastructure and sediment contribution of Halimeda incrassate and Halimeda monile, Nonsuch and Falmouth Bays, Antigua W. I. Coral Reefs, 6: 179-186.
Murray, L., Dennison, W. C., and Kemp, W. M., 1992. Nitrogen versus phosphorus limitation for growth of an estuarine population of eelgrass (Zostera marina L.). Aquatic Botany, 44: 83-100.
Nelson, T. A., and Lee, A., 2001. A manipulative experiment demonstrates that blooms of the macroalga Ulvaria obscura can reduce eelgrass shoot density. Aquatic Botany, 71: 149-154.
Nelson, W. G. (ed.), 2009. Seagrasses and protective criteria: A review and assessment of research status. Office of research and development, National Health and Environmental Effects Research Laboratory, EPA/600/R-09/050.
Orth, R. J., Carruthers, T. J. B., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Heck, Jr., K. L., Hughes, A. R., Kendrick, G. A., Kenworthy, W. J., Olyarnik, S., Short, F. T., Waycott, M., and Williams, S. L., 2006. A global crisis for seagrass ecosystems. BioScience, 56: 987-996.
Peckol, P., DeMeo-Anderson, B., Rivers, J., Valiela, I., Maldonado, M., and Yates, J., 1994. Growth, nutrient uptake capacities and tissue constituents of the macroalgae, Cladophora vagabunda and Gracilaria tikvahiae, related to sitespecific nitrogen loading rates. Marine Biology, 121: 175-185.
Pederson, M. F., and Borum, J., 1993. An annual nitrogen budget for a seagrass Zostera marina population. Marine Ecology Progress Series, 101: 169-177.
Pederson, M. F., 1995. Nitrogen limitation of photosynthesis and growth: Comparison across aquatic plant communities in a Danish Estuary (Roskilde Fjord). Ophelia, 41: 261-272.
Pedersen, O., Binzer, T., and Borum, J., 2004. Sulphide intrusion in eelgrass (Zostera marina L.). Plant, Cell & Environent, 27: 595-602.
Peralta, G., Pérez-Lloréns, J. L., Hernández, I., and Vergara, J. J., 2002. Effects of light availability on growth, architecture and nutrient content of the grass Zostera noltii Hornem. Journal of Experimental Marine Biology and Ecology, 269: 9-26.
Peralta, G., Bouma, T. J., van Soelen, J., Pérez-Lloréns, J. L., and Hernández, I., 2003. On the use of sediment fertilization for seagrass restoration: A mesocosm study on Zostera marina L. Aquatic Botany, 75: 95-110.
Pregnall, A. M., Smith, R. D., Kursar, T. A., and Alberte, R. S., 1984. Metabolic adaptations of Zostera marina (eelgrass) to diurnal periods of root anoxia. Marine Biology, 83: 141-147.
Pregnall, A. M., and Rudy, P. P., 1985. Contribution of green macroalgal mats (Enteromorpha spp.) to seasonal production in an estuary. Marine Ecology Progress Series, 24: 167-176.
Pregnall, A. M., and Miller, S. L., 1988. Flux of ammonium from surf-zone and nearshore sediments in Nahant Bay, Massachusetts, USA, in relation to free-living Pilayella littoralis. Marine Ecology Progress Series, 24: 167-176.
Raffaelli, D. G., Raven, J. A., and Poole, L. J., 1998. Ecological impact of green macroalgal blooms. Oceanography and Marine Biology: An Annual Review, 36: 97-125.
Raffaelli, D., 2000. Interactions between macro-algal mats and invertebrates in the Ythan estuary, Aberdeenshire, Scotland. Helgoland Marine Research, 54: 71-79.
Reise, K., and Siebert, I., 1994. Mass occurrence of green algae in the German Wadden Sea. German Journal of Hydrography, (Supplement), 1: 171-180.
Rose, C. D., and Dawes, C. J., 1999. Effects of community structure on the seagrass Thalassia testudinum. Marine Ecology Progress Series, 184: 83-95.
Santos, R., 1993. A multivariate study of biotic and abiotic relationships in a subtidal algal stand. Marine Ecology Progress Series, 94: 181-190.
Schmidt, A. L., Wysmyk, J. K. C., Craig, S. E., and Lotze, H. K., 2012. Regional-scale effects of eutrophication on ecosystem structure and services of seagrass beds. Limnology and Oceanography, 57: 1389-1402.
Schories, D., Anibal, J., Chapman, A. S., Herre, E., Isaksson, I., Lillebo, A. I., Pihl, L., Reise, K., Sprung, M., and Thiel, M., 2000. Flagging greens: Hydrobiid snails as substrata for the development of green algal mats (Enteromorpha spp.) on tidal flats of North Atlantic coasts. Marine Ecology Progress Series, 199: 127-136.
Sfriso, A., Marcomini, A., and Pavoni, B., 1987. Relationships between macroalgal biomass and nutrient concentrations in a hypertrophic area of Venice Lagoon. Marine Environmental Research, 22: 297-312.
Sfriso, A., Pavoni, B., Marcomini, A., and Orio, A. A., 1992. Macroalgae, nutrient cycles, and pollutants in the lagoon of Venice. Estuaries, 15: 517-528.
Short, F. T., 1983. The seagrass, Zostera Marina L.: Plant morphology and bed structure in relation to sediment ammonium in izembed lagoon, Alaska. Aquatic Botany, 16: 149-161.
Short, F. T., Burdick, D. M., and Kaldy, III., 1995. Mesocosm experiments quantify the effects of eutrophication on eelgrass, Zostera marina. Limnology and Oceanography, 40: 740-749.
Short, F. T., and Neckles, H. A., 1999. The effects of global climate change on seagrasses. Aquatic Botany, 63: 169-196.
Smith, J. E., Runcie, J. W., and Smith, C. M., 2005. Characterization of a large-scale ephemeral bloom of the green alga Cladophora sericea on the coral reefs of West Maui, Hawai’i. Marine Ecology Progress Series, 302: 77-91.
Sousa-Dias, A., and Melo, R. A., 2008. Long-term abundance patterns of macroalgae in relation to environmental variables in the Tagus Estuary (Portugal). Estuarine, Coastal and Shelf Science, 76: 21-28.
Sugimoto, K., Hiraoka, K., Ohta, S., Niimura, Y., Terawaki, T., and Okada, M., 2007. Effects of ulvoid (Ulva spp.) accumulation on the structure and function of eelgrass (Zostera marina L.) bed. Marine Pollution Bulletin, 54: 1582-1585.
Thiel, M., and Watling, L., 1998. Effects of green algal mats on infaunal colonization of a New England mud flat-long-lasting but highly localized effects. Hydrobiologia, 375/376: 177-189.
Thomsen, M. S., Wernberg, T., Engelen, A. H., Tuya, F., Vanderklift, M. A., Holmer, M., McGlathery, K. J., Arenas, F.,Kotta, J., and Silliman, B. R., 2012. A meta-analysis of seaweed impacts on seagrasses: generalities and knowledge gaps. PLoS One, 7: 1-8.
Touchette, B. W., and Burkholder, J. M., 2000. Overview of the physiological ecology of carbon metabolism in seagrasses. Journal of Experimental Marine Biology and Ecology, 250: 169-205.
Tomasko, D. A., Corbett, C. A., Greening, H. S., and Raulerson, G. E., 2005. Spatial and temporal variation in seagrass coverage in Southwest Florida: assessing the relative effects of anthropogenic nutrient load reductions and rainfall in four contiguous estuaries. Marine Pollution Bulletin, 50: 797-805.
Twilley, R. J., Kemp, W. M., Staver, K. W., Stevenson, J. C., and Boynton, W. R., 1985. Nutrient enrichment of estuarine submersed vascular plant communities. 1. Algal growth and effects on production of plants and associated communities. Marine Ecology Progress Series, 23: 179-191.
Tweedley, J. R. M., Jackson, E. L., and Attrill, M. J., 2008. Zostera marina seagrass beds enhance the attachment of the invasive alga Sargassum muticum in soft sediment. Marine Ecology Progress Series, 354: 305-309.
van der Heide, T., Smolders, A. J. P., Rijkens, B. G. A., van Nes, E. H., van Katwijk, M. M., and Roelofs, J. G. M., 2008. Toxicity of reduced nitrogen in eelgrass (Zostera marina) is highly dependent on shoot density and pH. Oecologia, 158: 411-419.
van der Heide, T., Govers, L. L., de Fouw, J., Olff, H., van der Geest, M., van Katwijk, M. M., Piersma, T., van de Koppel, J., Silliman, B. R., Smolders, A. J. P., and van Gils, J. A., 2012. Three-stage symbiosis forms the foundation of seagrass ecosystem. Science, 336: 1432-1434.
van Katwijk, M. M., Vergeer, L. H. T., Schmitz, G. H. W., and Roelofs, J. G. M., 1997. Ammonium toxicity in eelgrass Zostera marina. Marine Ecology Progress Series, 157: 159-173.
van Katwijk, M. M., Bos, A. R., Kennis, P., and de Vries, R., 2010. Vulnerability to eutrophication of a semi-annual life history: A lesson learnt from an extinct eelgrass (Zostera marina) population. Biological Conservation, 143: 248-254.
Villazán, B., Brun, F. G., Jiménez-Ramos, R., Pérez-Lloréns, J. L., and Vergara, J. J., 2013. Interaction between ammonium and phosphate uptake rate in the seagrass Zostera noltii. Marine Ecology Progress Series, 488: 133-143.
Walsh, B. L., 1980. Comparative nutrient dynamics of a marsh mudflat ecosystem. Estuarine, Coastal and Shelf Science, 10: 143-164.
Waycott, M., Duarte, M., Carruthers, T. J. B., Orth, R. J., Dennison, W. C., Olyarnik, S., Calladine, A., Fourqurean, J. W., Heck, Jr., K. L., Hughes, A. R., Kendrick, G. A., Kenworthy, W. J., Short, F. T., and Williams, S. W., 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America, 106: 12377-12381.
Williams, S. L., 1990. Experimental studies of Caribbean seagrass bed development. Ecological Monographs, 60: 449-469.
Yabe, T., Ishii, Y., Amano, Y., Koga, T., Hayashi, S., Nohara, S., and Tatsumoto, H., 2009. Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology, 10: 239-245.
Zaitsev, Y. P., 1992. Recent change in the trophic structure of the Black Sea. Fisheries Oceanography, 1: 180-189.
(Edited by Ji Dechun)
(Received September 2, 2013; revised November 25, 2013; accepted March 10, 2014)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-535-2109112
E-mail: qyhan@yic.ac.cn
杂志排行
Journal of Ocean University of China的其它文章
- Sedimentary Evolution of the Holocene Subaqueous Clinoform off the Southern Shandong Peninsula in the Western South Yellow Sea
- Identification of Fucans from Four Species of Sea Cucumber by High Temperature1H NMR
- Experimental Study on the Flow Around Two Tandem Cylinders with Unequal Diameters
- Revision of P-wave Velocity and Thickness of Hydrate Layer in Shenhu Area, South China Sea
- Study on Internal Waves Generated by Tidal Flow over Critical Topography
- Isolation and Characterization of Fucoidans from Five Brown Algae and Evaluation of Their Antioxidant Activity
