Distribution and Abundance of Pelagic Tunicates in the North Yellow Sea
2014-04-20PietroFrancoCHENHongjuandLIUGuangxing
Pietro Franco, CHEN Hongju, and LIU Guangxing,
1) Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
2) College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, P. R. China
Distribution and Abundance of Pelagic Tunicates in the North Yellow Sea
Pietro Franco1),2), CHEN Hongju1),2), and LIU Guangxing1),2),*
1) Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China, Qingdao 266100, P. R. China
2) College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, P. R. China
In this paper, the distribution patterns and abundance of pelagic tunicates in the North Yellow Sea of China during the period 2006-2007 were analyzed. Zooplankton samples were obtained with vertical towing from bottom to surface using a WP2 plankton net (200 µm mesh size; mouth area: 0.25 m2). Five species belonging to two classes were identified: Oikopleura dioica, O. longicauda and Fritillaria borealis belonging to class Appendicularia; Salpa fusiformis and Doliolum denticulatum of class Thaliacea. O. dioica and O. longicauda were the dominant species, occurring in the samples of all four seasons, with different distribution patterns. Their maximum abundance were 1664.7 ind. m-3(spring) and 1031.7 ind. m-3(spring) respectively. Following Oikopleura spp. were D. denticulatum, which was found only in autumn with an average abundance of 149.6 ind. m-3, and S. fusiformis, which was detected all the year long except for autumn with low abundance (max. abundance 289.4 ind. m-3in summer). Only a very small amount of F. borealis was detected in summer samples, with an average abundance of 2.7 ind. m-3. The relationship between tunicates abundances and the environmental factors was analyzed using the stepwise regression model for each species. The variation of appendicularian abundance showed a significant correlation with the surface water temperature and with the concentration of Chl-a. No relationship was found between tunicates abundance and salinity, likely due to the slight changes in surface salinity of the studied area during the four seasons. Salps abundance and that of doliolids were significantly correlated to bottom water temperature, indicating that these two species (S. fusiformis and D. denticulatum) migrate vertically in the water column. In particular D. denticulatum, known to be a warm water species, showed not only an important correlation with water temperature, but also a spatial distribution connected to the warm currents in the North Yellow Sea. The occurrence of D. denticulatum represents an interesting result never found in past research work. Water temperature, algal distribution and currents were the most relevant environmental factors influencing the tunicate abundance and distribution in the North Yellow Sea. Further research is needed in order to get more information on the ecology of these organisms and to better understand their role in the ecosystem including the oceanic food web.
North Yellow Sea; pelagic tunicates; abundance; distribution; sea surface temperature
1 Introduction
The pelagic tunicates include the class Appendicularia (also called Larvacea) and the class Thaliacea, containing the doliolids, salps and pyrosomes. Thaliaceans are distributed worldwide (Deibel, 1998), mostly on continental shelves or coastal waters (Weibe et al., 1979). Appendicularians are also present in the world’s oceans (Fenaux et al., 1998), coastal waters and estuaries (Ritz et al., 2003). The ecological importance of pelagic tunicates has received little attention until recent years (Nakamura, 1998), the interest in tunicates, in their ecology and role in marine ecosystems is growing worldwide. First of all, they are planktonic grazers with high filtering rates and they can easily adapt to different environmental conditions because of their reproductive cycles and life histories (Alldredge and Madin, 1982); secondly they have an important role in pelagic food web, being preys for fishes (Sigler, 2001) but also consuming bacteria and plankton (Madin et al., 1997). They can also be used as indicators of ocean currents (Chen et al., 1988). Especially in recent years, the tunicates’ influence (appendicularians in particular) on the carbon flow through the planktonic food webs is one of the main topics of research. In fact, due to their body structure and their filter-feeding mechanism, they are able to retain large amounts of particulate material (Sommer et al., 2002). Pelagic tunicates tend to congregate in areas of high abundances of plankton (Nakamura, 1998), and they can feed on small particles. Thanks to that, they are considered to be a fundamental link in the microbial food web (Cristian and Madin, 2004). Moreover, fecal pellets, discarded houses of appendicularians and death bodies of pelagic tunicates sinking to the deep waters, contribute to the known marine snow and have astrong impact on the different processes of the ecosystem (Andersen, 1985; Alldredge, 2005). Main research has focused on pelagic tunicates’ growth rate (Nakamura et al., 1998), filtering, ingestion and clearance rates (Gibson and Paffenhöfer, 2000), reproduction and life cycles (Tebeau and Madin, 1994), transport of organic matter and vertical migration (Wiebe et al., 1979), and of course different studies on abundance and distribution (Tew and Lo, 2005; Deibel, 2009).
The Yellow Sea (YS) is a shallow semi-enclosed marginal sea of the northwestern Pacific located between the mainland of China and the Korea Peninsula. The line connecting Chengshantou in the Shandong Peninsula of China and Chang San-got of Korean divides the YS into two parts, the North Yellow Sea (NYS) and South Yellow Sea (SYS). The main hydrographic features of the YS are the existence of a warm and salty water mass in the central YS in winter, namely the Yellow Sea Warm Water (YSWW), and in summer a water mass occupying the deep trough with temperatures lower than in the surrounding areas, namely the Yellow Sea Cold Water (YSCW) (Su, 1998) (Fig.1). These features influence the distributions of plankton in YS. The research about the specific groups of zooplankton in the YS has mainly focused on protozoa and copepods (Wang et al., 2003; Li et al., 2004; Xu et al., 2005; Huo et al., 2008;) except for the report of Liu et al. (2012), in which the ecology of pelagic tunicate Salpa fusiformis was studied. Different researches on tunicates have been carried out in the East China Sea, South China Sea and Taiwan Strait with regard to their abundance and distribution (Zhang et al., 2003; Xu et al., 2006). However, there is no detailed report about the species composition and seasonal variation of pelagic tunicates in NYS.
The aim of this study is to identify the different species of pelagic tunicates present in the NYS and to determine their abundance and distribution patterns according to different environmental features including main currents, temperature, salinity and chlorophyll a (Chl-a) distribution. One of the main points underlying this research work is the interesting results found about D. denticulatum. This species is known to be a warm water one. We report that spatial distribution of high densities of doliolids during autumn is closely linked to warm currents’ patterns (Fig.1), and we also found a significant correlation to surface and bottom temperatures. There are no studies for the NYS regarding warm water species and our research could be a starting point for further research.

Fig.1 Maps showing winter (a) and summer (b) main circulations in the China seas (from SU, 1998). The different currents are: Bohai Coastal Current (BC), Yellow Sea Coastal Current (YSCC), Tsushima Current (TC), Yellow Sea Warm Current (YSWC), Yellow Sea Cold Water (YSCW).
2 Materials and Methods
Sampling was carried out in the North Yellow Sea (Fig.2) during four cruises throughout the four seasons of 2006-2007. The four surveys were conducted at 78, 79, 81 and 83 stations during July-August 2006 (summer), January 2007 (winter), April-May 2007 (spring) and October 2007 (autumn) respectively. Zooplankton samples were obtained with vertical towing from the bottom to the surface using WP2 plankton net (200 µm mesh size; mouth diameter: 0.57 m; mouth area: 0.25 m2). The samples collected were preserved in a 5% formaldehyde solution. Data on temperature, conductivity and depth of the water were obtained using a CTD (Sea-Bird SBE 911) profiler. During the same cruises, chlorophyll-a concentration was measured as well. Later in the lab, identification and counting of the species for each sample were performed using a stereomicroscope. The abundance is expressed as ind. m-3.
All data mapping concerning abundance and distribution was made using the software SURFER 8.0. In order to determine the relationship between the abundance and distribution of pelagic tunicates and the environmental factors, the stepwise regression was carried out with sea surface and bottom temperature (SST, SBT), surface and bottom salinity (SSS, SBS) and Chl-a concentration as dependent variables and the total abundance of each species as independent variable during the four seasons (Tew and Lo, 2005). All the measured values were plotted to form scatter-plots graphs. The computer program Eviews 6.0 software was used for all the calculations.
The data concerning the environmental factors were taken as references from other scientific works whichhave already been published (Bao et al., 2009). As for Chl-a concentration values, the data were from Gao and Li (2009).

Fig.2 Map showing the location of the sampling stations in the North Yellow Sea.
3 Result
3.1 Environmental Conditions


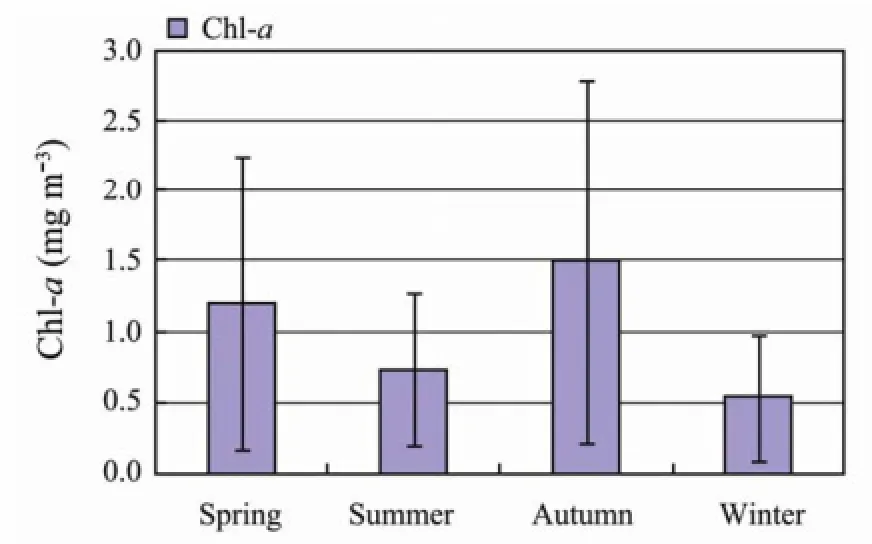
Fig.3 Seasonal variation of surface and bottom temperature, salinity and Chl-a (average values from bottom to surface of water column).
The variations of temperature, salinity (surface and bottom) and Chl-a (average values from bottom to surface of water column) during the four seasons in the NYS is showed in Fig.3.
According to Fig.3, the thermal stratification occurs during the summer period. In fact, the average surface water and bottom water temperatures increase respectively from 9℃ to a maximum of 23℃, and 7℃ to 16℃(in autumn). During winter until the beginning of the next spring there is no relevant difference between the average surface and bottom temperatures, which corresponds to well-mixed water. The average bottom salinity values are slightly higher than the surface ones all along the four seasons. The chl-a concentration first decreases from spring to summer, then reaches a peak of 1.5 mg m-3in autumn and finally decreases to a minimum value of 0.5 mg m-3in winter.
As for chl-a distribution patterns, the highest concentration values of Chl-a are found during autumn (6 mg m-3) and spring (4.8 mg m-3). During summer and winter high concentrations are distributed along the coasts.
3.2 Distribution and Abundance of Tunicates
A total of five different species were recorded in the NYS throughout the year 2006-2007: Oikopleura dioica, Oikopleura longicauda and Fritillaria borealis of class Larvaceans, the salp Salpa fusiformis and the doliolid Doliolum denticulatum (Table 1).
Table 1 shows the average seasonal abundance of different tunicates in the NYS. O. dioica and O. longicauda are the dominant species, occurring all the year long. S. fusiformis is present in every season except for autumn, with the highest value of 289.4 ind. m-3during summer. D. denticulatum is found only in autumn, with an average abundance of 149.6 ind. m-3. F. borealis is identified only in the samples collected in summer with an average abundance of 2.7 ind. m-3. Due to its low abundance, the latter was not taken into consideration for the analysis of distribution which follows.
The distributions of O. dioica and O. longicauda was similar throughout the year. The two most abundant spe-cies of tunicates appeared in most of the stations during autumn and summer, with maximum abundance 1664.7 ind. m-3(Spring) and 1031.7 ind. m-3(Spring) respectively. The two species were found mainly in the western part, in the coastal area close to the Bohai Sea. In winter, the densities measured were quite low (Figs.4 and 5).S. fusiformis appeared in the central trough during spring, summer and winter with a peak during summer (289.4 ind. m-3). The species occurred in at 3 stations during spring, at 2 stations in winter and at 7 stations during summer (Fig.6).
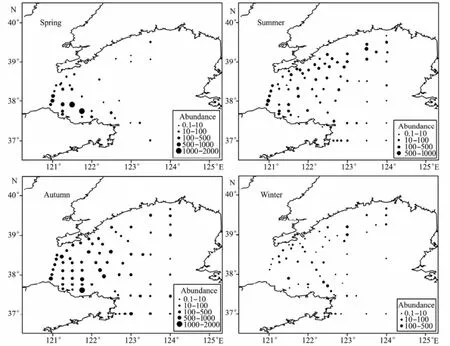
Fig.4 The abundance of O. dioica during the four seasons in 2006-2007.

Fig.5 The abundance of O. longicauda during the four seasons in 2006-2007.
D. denticulatum occurred only in autumn, with the highest abundance 543.5 ind. m-3. The distribution was concentrated mostly in the southern and western parts of the study area (Fig.7).

Table 1 Tunicates in the North Yellow Sea with relative average abundance (ind. m-3) during the four season

Fig.6 The abundance of S. fusiformis during the different seasons in 2006-2007.

Fig.7 The abundance of D. denticulatum during autumn in 2006-2007.
3.3 Influence of Environmental Factors on the Abundance and Distribution of Tunicates
The stepwise regression was used to determine which were the most significant environmental factors influencing the abundance and distribution of tunicates in the NYS. The surface and bottom temperatures, surface and bottom salinities and Chl-a concentration were selected as the independent variables, while the abundance of tunicates was selected as the dependent variable. The correla-tions were expressed by the function y = βx + c. Every species showed different results.
There was no correlation between the abundance of the five species and surface and bottom salinities. O. dioica abundance showed a correlation to surface water temperature (Fig.8), and to Chl-a concentration.
Fig.9 shows the correlation between O. longicauda abundance and surface water temperature.
D. denticulatum and S. fusiformis abundance were both correlated to the bottom water temperature (Fig.10). All the results are summarized in Table 2.

Fig.8 Relationship between the abundance of O. dioica and surface temperature and chl-a.
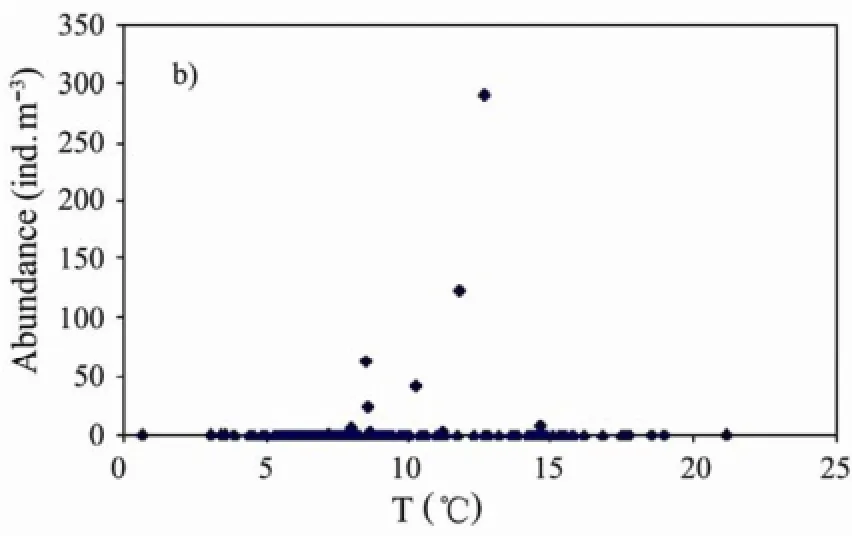
Fig.10 Relationship between the abundance of D. denticulatum (a) and S. fusiformis (b) and bottom temperature.

Table 2 Functions expressing the correlation between each species and the environmental factors (stepwise regression model)
4 Discussion
The present study focused on the distribution and abundance of appendicularians, salps and doliolids in the North Yellow Sea, and the relationship between their occurrence and the environmental factors. The obtained results revealed the distribution and seasonal variations of pelagic tunicates in the NYS. Taking into consideration the different relationships between every single species’abundance and the environmental factors, it is reasonable to describe the different classes of tunicates separately.
4.1 Appendicularian
The organisms belonging to the family Oikopleuridae are known to be eurythermal and euryhaline (Alldredge,1976). This property is also exhibited by the species O. dioica, O. longicauda in the NYS, which occurred with temperature and salinity values of about 1-26℃ (Figs.9-10) and about 28-33, respectively. The two species are also known to prefer surface water for spawning (Alldredge, 1976). According to Fig.8, the optimal range of surface temperature for these two species is about 8 to 19℃. In the graph the central values are absent because our survey was not continuously carried out during the different seasons but only at some particular dates. Therefore, we also supposed the concentration to be high where lacking data was situated. There was no correlation with salinity, which may be explained as follows: the salinity in the NYS is quite stable all along the year and the high or low abundance of appendicularians is not influenced by the same irrelevant salinity changes.
The reproduction of appendicularians is proved to increase with the rise of temperature (Paffenhöfer, 1976). Considering the circulation in the NYS and the different temperatures measured, it is possible to give an explanation to the highest abundance during summer and autumn, especially along the coastal margins of the Shandong and Liaoning provinces and at the front created between the different water masses in the region bordering the Bohai Sea (Chen, 2009).
There was a correlation between the O. dioica abundance and the concentration of Chl-a (see Fig.8). This species of large numbers occurred when the chl-a concentration was lower than 2 mg m-3, but at higher concentrations we also found the presence of the organisms. This means that the high concentration of algae was not a limiting factor for the life of O. dioica. Their filter-feeding structure, the ‘house’, can be replaced when it gets clogged by high concentration of food particulates (Tiselius et al., 2003). Even though no correlation was found for O. longicauda and chl-a, most of this species also occurred with chl-a concentration lower than 2 mg m-3.
4.2 Thaliaceans
4.2.1 Doliolid
D. denticulatum shows alternation between sexual and asexual reproduction. Every life stage tends to stay at different water depths (Tew and Lo, 2005). Gonozooids and phorozooids mainly inhabit shallow waters (18-36 m), while oozoids prefer deeper waters (59-78 m). Although this species does not show clear diel vertical migration (Gibson and Paffenhöfer, 2000), it can live in different depths of water. Berner et al. (1961) showed the range of temperature in which D. denticulatum usually occurs (14-15℃).
As our results showed, the species occurred only during autumn, and it was distributed all along the coastal waters of Shandong province and in the central area of the NYS (Fig.7). Starting during autumn and all along the winter season, the YSWC, characterized by waters of high temperature and salinity, flows northward in the central trough of the YS, reaching the northern part and the Bohai Sea (Naimie et al., 2001). The high abundance of the species during this season and its absence during the rest of the year may be explained by the relationship with environmental factors. Analyzing Fig.10a, which shows a clear correlation between bottom temperature and D. denticulatum abundance, we supposed that this species could reproduce better as a result of favorable temperatures. During autumn, the variation in surface and bottom temperatures were characterized by a similar trend (changes going from about 8℃ to 20℃). As we said, the bloom reached quite high abundance levels (max. value 543.5 ind. m-3) but most of the organisms were characterized by small gonozooids. Our interpretation of this fact is that D. denticulatum bloom occurred after asexual reproduction, the release of many small gonozooids from phorozooids, which allows rapid increase in abundance (Alldredge and Madin, 1982). This is a significant result in our work. In fact, there are no reports on this warm water species in the NYS and it would be interesting in future to do more research in this direction. If we take into consideration the reports from Xu et al. (2007) and Feng et al. (2009) and their work for the seas of eastern China, we can formulate an interesting hypothesis. Feng et al. (2009) described the water temperature conditions in the seas of eastern China during a period of 62 years (1945-2006). They showed an important rise in the water temperature (0.015℃ year-1). Xu et al. (2007) analyzed the relationship between the distribution of Thaliacea and water mass in the East China Sea, stating that these organisms are good indicators for warm currents. There is an intimate relationship between the distribution of D. denticulatum and water temperature. As we mentioned, our study also showed the correlation between warm bottom water and the spatial distribution of the species. In this case, it might be important to carry on more field work in the following years in the North Yellow Sea, trying to understand whether the global rise in water temperature will affect the distribution and abundance of doliolids and other species as well.
Although we found no correlation between the doliolids and Chl-a concentration using the stepwise regression, we supposed the high concentrations of the latter may also have enhanced the elevated numbers of these organisms. That also may explain their presence only during autumn, which, as we have already described, is the season for which Chl-a was more concentrated. No correlation with salinity was found; it seems that the small changes of salinity did not affect the organism’s reproduction and distribution.
4.2.2 Salp
S. fusiformis is a warm water species. Chen (1978) proved that S. fusiformis occurs not frequently in the YS. In our study, this species was present from winter to summer. the YSWC flowing northwards in the central through (Naimie et al., 2001), that is where S. fusiformis occurred. This current might be the main reason for its distribution in the NYS. Although referring to a warm water species, several studies proved that temperaturesabove 20℃ could affect the organism (Liu et al., 2012); in fact, the filtration rate under these conditions showed a decrease while the excretion rate increased, proving a higher energy consumption (Andersen and Nival, 1986). From analyzing our results (Fig.10b), the correlation between bottom water temperature and the salp abundance was clear. As a matter of fact, we supposed that the summer surface relatively high temperature was not suitable for the spawning of these organisms. Moreover, these tunicates effectuate a diel vertical migration, which may allow the organisms to approach the surface in order to feed and then migrate to deeper waters which are suitable for their life conditions.
According to Liu et al. (2012) the salps are distributed mainly in oligotrophic waters with low chl-a concentration. These organisms are filter-feeders and their apparatus could be clogged and damaged because of abundant level of particulate material (Zeldis et al., 1995). This could be the reason why in our research their distribution occurred in areas where the Chl-a was lower than 1 mg m-³ and no organisms were found in autumn, the season of highest Chl-a concentration.
In summary, our results show that high abundance of tunicates occurred in the research area throughout the year. It is important to analyze the community structure and its connection to the oceanic environment: firstly, in order to describe the biodiversity of the same area; secondly, and most important, to be able to research into and understand the role of zooplankton in their ecological niche and in the oceanic food chain, and the links with the other oceanic organisms. Moreover, we hypothesized that the warm water species D. denticulatum would occur more frequently and be more abundant in the NYS in the case of future rise in water temperature caused by global warming.
However, there are some limitations to our findings. In fact, our research concerned a period of four seasons within a two-year span (2006-2007) and not throughout one single year. This could cause some problems for the comparison with other kind of work of the same nature. Moreover, as we have already mentioned, our study in the NYS is innovative and it is still not possible to make comparisons with other scientists’ findings. Lastly, the mesh size of the WP2 plankton net (200 µm) is not narrow enough to collect the small-sized organisms, including the new-born and juveniles. This fact could influence the results of our research.
This work could be a starting point for further research, especially about the ecology of these organisms. For instance, it would be interesting to measure the effect of predation by fish larvae or other organisms on tunicates to determine the efficiency of their grazing and filter-feeing, to analyze their behavior in laboratory or in situ, and to consider their reactions to food limitation or changes in physical environmental conditions. Moreover, further work on abundance and distribution in different periods could be helpful to make comparisons and to understand better the changes the pelagic tunicates’ community structure would undergo. These and other topics should be taken into consideration in order to have a better understanding of the ecosystem in the NYS.
Acknowledgements
This study was supported by the National Key Basic Research Project (2005CB422306) and National Natural Science Foundation of China (40876066). We are grateful to the captain and crew of R/V Dong-Fang-Hong 2, and also to Mr. Xu Donghui, Mr. Zhu Yanzhong and Mr. Huang Yousong for their help on board. Thanks to Professor Li Zhengyan of CESE, OUC, for the data of Chl-a in the North Yellow Sea.
Alldredge, A., 1976. Appendicularians. Scientific American, 235: 95-102.
Alldredge, A. L., 2005. The contribution of discarded appendicularian houses to the flux of particulate organic carbon from oceanic surface waters. In: Response of Marine Ecosystems to Global Change: Ecological Impact of Appendicularian, Gorsky, G., et al., eds., GB Scientific Publisher, Paris, 309-326.
Alldredge, A. L., and Madin, L. P., 1982. Pelagic tunicates unique herbivores in the marine plankton. Bioscience, 32 (8): 655-663.
Andersen, V., 1985. Filtration and ingestion rate of Salpa fusiformis Cuvier (Tunicata: Thaliacea): Effects of size, individual, weight and algal concentration. Journal of Experimental Marine Biology and Ecology, 87: l3-29.
Andersen, V., and Nival, P., 1986. Ammonia excretion rate of Salpa fusiformis Cuvier (Tunicata: Thaliacea): Effects of individual weight and temperature. Journal of Experimental Marine Biology and Ecology, 99: 121-132.
Bao, X. W., Li, N., Yao, Z. G., and Wu, D. X., 2009. Seasonal variation characteristics of temperature and salinity of the North Yellow Sea. Periodical of Ocean University of China. 39 (4): 553-562 (in Chinese with English abstract).
Berner, L. D., and Reid Jr., J. L., 1961. On the response to changing temperature of the temperature-limited plankter Doliolum denticulatum Quoy and Gaimard 1835. Limnology and Oceanography, 6 (2): 205-215.
Chen, J. K., 1978. Two salps observed in the northern Yellow Sea. Chinese Journal of Zoology, 2: 13-16 (in Chinese with English abstract).
Chen, R. X., Chai, B. J., and Lin, M., 1988. Notes on vertical distribution of zooplankton in center of South China Sea. Acta Oceanologica Sinica, 10 (3): 337-341 (in Chinese with English abstract).
Chen, C. T. A., 2009. Chemical and physical fronts in the Bohai, Yellow and East China seas. Journal of Marine Systems, 78: 394-410.
Cristian, A. V., and Madin, L. P., 2004. Zooplankton feeding ecology: Clearance and ingestion rates of the salps Thalia democratic, Cycosalpa affinis and Salpa cylindrica on naturally occurring particles in the Mid-Atlantic Bight. Journal of Plankton Research, 26 (7): 827-833.
Deibel, D., 1998. The abundance, distribution, and ecological impact of doliolids. In: The Biology of Pelagic Tunicates. Bone, Q., ed., Oxford University Press, Oxford, 171-186.
Fenaux, R., Bone, Q., and Deibel, D., 1998. Appendicularia distribution and zoogeography. In: The Biology of Pelagic Tunicates. Bone, Q., ed., Oxford University Press, Oxford, 253-264.
Feng, L., and Lin, X. P., 2009. Long-term trend of the east china sea surface temperature during 1945-2006. Periodical of Ocean University of China, 39 (1): 13-18 (in Chinese with English abstract).
Gao, S., and Li, Z. Y., 2009. Spatial and seasonal variation of chlorophyll and primary productivity in summer and winter in the Northern Yellow Sea. Periodical of Ocean University of China, 39 (4): 604-610 (in Chinese with English abstract).
Gibson, D. M., and Paffenhöfer, G. A., 2000. Feeding and growth rates of the doliolid, Dolioletta gegenbauri Uljanin (Tunicata, Thaliacea). Journal of Plankton Research, 22 (8): 1485-1500.
Huo, Y. Z., Wang, S. W., Sun, S., Li, C. L., and Liu, M. T., 2008. Feeding and egg production of the planktonic copepod Calanus sinicus in spring and autumn in the Yellow Sea, China. Journal of Plankton Research, 30 (6): 723-734, DOI: 10. 1093/plankt/fbn034.
Li, C., Sun, S., Wang, R., and Wang, X., 2004. Feeding and respiration rates of a planktonic copepod (Calanus sinicus) oversummering in Yellow Sea cold bottom waters. Marine Biology, 145: 149-157, DOI: 10.1007/s00227-004-1306-x.
Liu, Y. C., Sun, S., and Zhang, G. T., 2012. Seasonal variation in abundance, diel vertical migration and body size of pelagic tunicate Salpa fusiformis in the Southern Yellow Sea. Chinese Journal of Oceanology and Limnology, 30 (1): 92-104.
Madin, L. P., Purcell, J. E., and Miler, C. B., 1997. Abundance and grazing effects of Cydosalpa bakeri in the subarctic Pacific. Marine Ecology Process Series, 157: 175-183
Naimie, C. E., Blain, C. A., and Lynch, D. R., 2001. Seasonal mean circulation in the Yellow Sea - A model-generated climatology. Continental Shelf Research, 21: 667-695.
Nakamura, Y., 1998. Blooms of tunicates Oikopleura spp. and Dolioletta gegenbauri in the Seto Inland Sea, Japan, during summer. Hydrobiologia, 385 (1-3): 183-192.
Paffenhöfer, G. A., 1976. On the biology of appendicularia of the south eastern North Sea. In: 10thEuropean Marine Biology Symposium. Personne, G., and Jaspers, E., eds., Universal Press, Ostend, Wetteren, Belgium, 2: 437-455.
Ritz, D., Swadling, K., Hosie, G., and Cazassus, F., 2003. Guide to the zooplankton of south eastern Australia. In: Fauna of Tasmania Committee, University of Tasmania, Hobart, 22-24.
Sigler, M. F., 2001. Young of the year sablefish abundance, growth and diet in the Gulf of Alaska. Alaska Fishery Research Bulletin, 8 (1): 57-70.
Sommer, U., Beminger, U. G., and Bottger-Schnack, R., 2002. Grazing during early spring in the Gulf of Aqab and the northern Red Sea. Marine Ecology Progress Series, 239: 251-261.
Su, J., 1998. Circulation dynamics of the China Seas North of 18˚N. In: The Sea. Robinson, A. R., and Brink, K. H., eds., Wiley, New York, 11: 483-505.
Tebeau, C. M., and Madin, L. P., 1994. Grazing rates for three life history stages of the doliolid Dolioletta gegenbauri Uljanin (Tunicata, Thaliacea). Journal of Plankton Research, 16 (8): 1075-1081.
Tew, K. S., and Lo, W. T., 2005. Distribution of Thaliacea in SW Taiwan coastal water in 1997, with special reference to Doliolum denticulatum, Thalia democratica and T. oriental. Marine Ecology Progress Series, 292: 181-193.
Tiselius, P., Petersen, J. K., Nielsen, T. G., Maar, M., Møller, E. F., Satapoomin, S., Tonnesson, K., Zervoudaki, T., Christou, E., Giannakourou, A., Sell, A., and Vargas C., 2003. Functional response of Oikopleura dioica to house clogging due to exposure to algae of different sizes. Marine Biology, 142: 253-261, DOI: 10.1007/s00227-002-0961-z.
Wang, R., Zuo, T., and Wang, K., 2003. The Yellow Sea cold bottom water - An oversummering site for Calanus sinicus (Copepoda, Crustacea). Journal of Plankton Research, 25 (2): 169-183, DOI: 10.1093/plankt/25.2.169.
Wiebe, P. H., Madin, L. P., Haury, L. R., Harbison, G. R., and Philibin, L. M., 1979. Diel vertical migration by Salpa aspera and its potential for large-scale particulate organic matter transport to the deep-sea. Marine Biology, 53: 249-255.
Xu, Z. L., Lin, M., and Zhang, J. B., 2006. Changes in dominant species of Thaliacea in the East China Sea. Acta Zoologica Sinica, 52 (1): 53-62.
Xu, Z. L., Lin, M., and Zhang, J. B., 2007. Relationship of water environment and abundance, distribution of Thaliacea in the East China Sea. Oceanologia et Limnologia Sinica, 38 (6): 549-554
Xu, Z. L., Song, W. B., and Hu, X. J., 2005. Notes on two marine ciliates from the Yellow Sea, China: Placus salinus and Strombidium opalatum (Protozoa, Ciliophora). Journal of Ocean University of China, 4 (2): 137-144.
Zeldis, J. R., Davis, C. S., James, M. R., Ballaral, S. L., Booth, W. E., and Chang, F. H., 1995. Salp grazing: Effects on phytoplankton abundance, vertical distribution and taxonomic composition in a coastal habitat. Marine Ecology Progress Series, 126: 267-283.
Zhang, J. B., Huang, J. X., and Lian, G. S., 2003. Species composition and abundance distribution of Thaliacea in late autumn and early winter in the Nanwan Bay of Taiwan, China. Marine Science Bulletin, 22 (6): 9-16.
(Edited by Ji Dechun)
(Received April 24, 2013; revised December 20, 2013; accepted January 11, 2014)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-66782672
E-mail: gxliu@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- Sedimentary Evolution of the Holocene Subaqueous Clinoform off the Southern Shandong Peninsula in the Western South Yellow Sea
- Identification of Fucans from Four Species of Sea Cucumber by High Temperature1H NMR
- Experimental Study on the Flow Around Two Tandem Cylinders with Unequal Diameters
- Revision of P-wave Velocity and Thickness of Hydrate Layer in Shenhu Area, South China Sea
- Study on Internal Waves Generated by Tidal Flow over Critical Topography
- Isolation and Characterization of Fucoidans from Five Brown Algae and Evaluation of Their Antioxidant Activity
