Mesoporous carbon as a carrier for celecoxib:The improved inhibition effect on MDA-MB-231 cells migration and invasion
2014-04-20QinfuZhoZhiwenZhngTongyingJingYpingLiSilingWng
,Qinfu Zho,Zhiwen Zhng, Tongying Jing,Yping Li,Siling Wng,
aSchool of Pharmaceutics,Shenyang Pharmaceutical University,No.103 Wenhua Road,Shenyang,Liaoning Province 110016,China
bCenter of Pharmaceutics,Shanghai Institute of Materia Medica,Chinese Academy of Sciences,No.501 Haike Road, Shanghai 201203,China
Original Research Paper
Mesoporous carbon as a carrier for celecoxib:The improved inhibition effect on MDA-MB-231 cells migration and invasion
Wenquan Zhua,Qinfu Zhaoa,Xin Zhenga,Zhiwen Zhangb, Tongying Jianga,Yaping Lib,Siling Wanga,*
aSchool of Pharmaceutics,Shenyang Pharmaceutical University,No.103 Wenhua Road,Shenyang,Liaoning Province 110016,China
bCenter of Pharmaceutics,Shanghai Institute of Materia Medica,Chinese Academy of Sciences,No.501 Haike Road, Shanghai 201203,China
A R T I C L E I N F O
Article history:
Received 19 December 2013
Received in revised form
10 January 2014
Accepted 25 January 2014
Available online 21 February 2014
Mesoporous carbon
Carrier
Dissolution
Migration
Invasion
In the current study,mesoporous carbon(MC)with pore volume(1.53 cm3/g)and pore size (9.74 nm)was successfully prepared as a carrier for celecoxib(CEL).Celecoxib was loaded into the pore channels of MC using three different methods:solvent evaporation method, absorption method and physical mixing method.Solid-state characterization methods, such as SEM,TEM,BET,DSC and XRD were used to systematically investigate the process of the drug loading system.Dissolution tests were performed to examine the effects of MC on the release of CEL.Furthermore,the cytotoxicity,wound healing,migration and invasion experiments were carried out to measure the contribution of MC to the anti-tumor metastasis ability of celecoxib on MDA-MB-231 cells.The results showed that CEL could be kept in a non-crystalline state when they were incorporated into the MC using the solvent evaporation method or absorption method.The dissolution rate of CEL released from MCS(Mesoporous carbon-Celecoxib-Solvent evaporation method)and MCA (Mesoporous carbon-Celecoxib-Absorption evaporation method)was all signif i cantly higher than that of pure CEL.The cumulative release for MCS within the 5 min was up to 51.86%.MCS enhanced the inhibitory effect of CEL on the migration and invasion of MDAMB-231 cells.
© 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1. Introduction
The incidence of breast cancer is gradually increasing and it has recently become a major cause of cancer death in women. The high rate of metastasis of breast cancer remains a major obstacle to its treatment[1,2].Celecoxib(CEL),a selective cyclooxygenase-2 inhibitor,has been used in recent years to study anti-tumor metastasis[3-5].This was due to that cyclooxygenase-2 plays an important role in producing tumor metastasis[6,7].However,the poor solubility of CEL seriously affects its inhibitory effect on tumor metastasis[8].Many pharmaceutical techniques have been used to improve the poor solubility of CEL,such as microemulsions[9],nanoemulsion[10],and nanoparticles[11].The emergence of inorganic porous materials(including porous silica,porous carbon,and composites.)has opened up a new path for the development of drug delivery systems[12-14].Compared with traditional pharmaceutical carriers,an inorganic carrier has considerable advantages in terms of particle size,shape control,stability and surface functionalization[15,16].There have been several studies of drug delivery systems involving mesoporous carbon[17-19].Mesoporous carbon with a larger specif i c surface area,stronger adsorption capacity,greater pore volume,higher drug loading,and chemical inertness is more suitable for use as a drug delivery system,compared with other mesoporous materials.
To my best knowledge,only one paper has been published using mesoporous carbon as a carrier for CEL[20].However, accumulated release of CEL loaded into mesoporous carbon within the 5 min was just about 40%.The pore size of the mesoporous carbon reported in the study was 7.0 nm,and the porevolumewas1.09cm3/g.Itshouldbeemphasizedthatpore size and pore volume of mesoporous carbon were especially two important factors for release[21-23].Although mesoporous carbon with a larger pore size and pore volume had been synthesized,it had not been studied as a drug delivery system.So it was a challenge to explore mesoporous carbon with a larger pore size and volume to be a carrier for CEL.
The purposes of this study were to establish mesoporous carbon with a large pore volume and large pore size,which could improve the dissolution rate of CEL signif i cantly.CEL was loaded into the pore channels of MC using three different methods:solvent evaporation method,absorption method and physical mixing method.Solid-state characterization methods,such as SEM,TEM,BET,DSC and XRD,were used to systematically investigate the process of the drug loading system.The release prof i le of CEL test was examined to conf i rm the improved dissolution.Furthermore,the cytotoxicity,wound healing,migration and invasion tests were carried out to illustrate the contribution of MC to the enhanced anti-tumor metastasis ability of celecoxib.
2. Materials and methods
2.1. Materials
Pluronic block co-polymer F127 was kindly donated by BASF. Tetraethyl orthosilicate(TEOS),hydrof l uoric acid,sulfuric acid and sucrose were purchased from Yu Wang Reagent Company (Shandong,China).1,3,5-trimethylbenzene(TMB)was purchased from Sigma-Aldrich(St.Louis,MO,USA).Celecoxib (purity > 99.0%)was supplied from Shenyang Funning Pharmaceutical Co.,Ltd.All other chemicals were used in accordance with the requirements of analytical/spectroscopic/HPLC grade.Deionized water in all experiments was prepared by ion exchange.MDA-MB-231breastcancercells(humanorigin)were obtained from American Type Culture Collection(ATCC).3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide (MTT)and crystal violet were purchased from Sigma(St.Louis, MO,USA).
2.2. Preparation and characterization of MC
2.2.1. Preparation of MC
The mesoporous carbon(MC)was prepared using mesoporous silica as template.Mesoporous silica was synthesized according to the following procedure.A solution was prepared by dissolving 1.25 g of KCl and 0.5 g of F127 in 30.0 ml of HCl (2.0 M)at 15°C with constant stirring,followed by the addition of 0.6 g of TMB.After 1 h of stirring at 15°C,2.0 g TEOS was added drop by drop to the solution under vigorous stirring. After 24 h of stirring at 15°C,the suspension was homogenized using an ATS AH100D homogenizer(ATS Engineer Inc., Shanghai,China).Then,the obtained mixture was placed into a Tef l on-lined autoclave for another 24 h at 100°C.Assynthesized material was f i ltered and dried at 60°C in the air.The resulting product was burned out at 600°C for 5 h to remove surfactant completely.The obtained product(1.0 g) was f i rst permeated in a mixed solution,which was composed of 1.0 g sucrose and 0.1 g H2SO4in 2.5 g H2O.The obtained gel was initially carbonized at 80°C for 6 h.Then,the temperature was increased to 160°C at the speed of 2°C/min for another 6 h.The obtained product was carbonized again at 80°C and 160°C for 6 h after the addition of an aqueous solution composed of 0.5 g sucrose,0.06 g H2SO4and 1.5 g H2O, respectively.The carbonization of the mixture was completed at 700°C for 3 h at the speed of 3°C/min under N2.Finally,the carbon-silica composite was mixed with 10%hydrof l uoric acid at 25°C for 48 h to remove the template.The mixture was f i ltered,washed with water,and dried at 100°C for 24 h.
2.2.2. Drug loading procedure
2.2.2.1.Solvent evaporation method.The solvent evaporation method involved soaking and solvent evaporation.MC was added in a methanol solution of CEL(20 mg/ml)according to a certain proportion(1:4).Then,the mixture was ultrasonicated for 30 min and stirred for 24 h at 25°C in a closed container. Finally,the solvent was evaporated and the precipitated powder was washed with methanol to remove the drugs on the surface of the carrier.At last,solid powder was dried at 40°C in the air until no organic solvent residue.Drug-loaded samples were labeled with MCS.
2.2.2.2.Absorption method.MC(20 mg)was added in a methanol solution of CEL(20 mg/ml).Then,the mixture was ultrasonicated for 30 min and stirred for 24 h at 25°C in a closed container.Finally,the mixture was centrifuged toisolate the solid power from it.The obtained samples were labeled with MCA.
2.2.2.3.Physical mixing method.CEL was mixed with MC according to a certain proportion(1:4).The obtained samples were labeled with MCP.
2.3. Characterization techniques
2.3.1. SEM study
The morphology of the samples plated with gold was characterized using a f i eld emission scanning electron microscope (JEOL-6700,Japan).
2.3.2. TEM study
The samples deposited on copper grids were characterized using TEM(Tecnai G2 20,FEI,USA).
2.3.3. Nitrogen adsorption analysis
Adsorption-desorption tests were performed on a nitrogen adsorption analyzer(V-Sorb 2800P,China).The carriers were degassed to removephysically adsorbed waterbefore analysis.
2.3.4. DSC analysis
DSC prof i les of the samples were recorded from a DSC instrument(TA Instruments,Q1000,USA).The temperature range was 50-200°C at a heating rate of 10°C/min.
2.3.5. XRD analysis
X-ray diffractometer (PW3040/60 PANALYII CALB.V Netherlands)was used to measure the crystalline characteristics of samples.The samples were scanned from 5°to 50°with a scan rate of 5°/min.
2.4. Analysis of drug content
The drug loading of CEL was analyzed using ultraviolet(UV) spectroscopy(UV-2000,Unico,USA),while the detection wavelength was 254 nm.CEL was separated from samples with methanol by ultrasonic method.The drug-loading was calculated using the following equation. Drugloading=Weight of CEL in sample/Weight of sample.
2.5. In vitro release behavior study
Dissolution tests were carried out using a USP II paddle method with a dissolution tester(KC-8D,Tianjin Guoming Medical Equipment Co.Ltd.).Phosphate buffer(pH 6.8)was used as the dissolution media.The dissolution procedure was as follows,900 ml of dissolution media in a basket was kept at 37°C and stirred at a rate of 100 rpm.200 mg samples (equivalent to 50 mg of CEL)wereadded and performedfor 1 h. 5 ml samples were collected at designated intervals(5,10,15, 20,30,45 and 60 min).The amount of CEL dissolved was measured by spectroscopy(UV-2000,Unico,USA)at 254 nm. All tests were performed for six times.
2.6. MDA-MB-231 cell experiments
2.6.1. Cellular cytotoxicity assays
MDA-MB-231 cells were propagated in DMEM(high glucose level)containing 10%fetal bovine serum(GIBCO),1%sodium pyrurate,100 μg/ml streptomycin and 100 units/ml penicillin. Cells trypsinized by trypsin-EDTA(0.25%,GIBCO)were used for subculture and plating.The cytotoxicity of CEL,MC and MCS against MDA-MB-231 cells was evaluated by MTT assay. Cellswereseeded into 96-well platesatadensityof 1 × 104cells/well and incubated for 24 h.Samples which contained CEL,MC and MCS with fresh culture media were added to the cells in different concentrations ranging from 0.1 μg/ml to 50 μg/ml and samples without these were used as a control.After a 24 h-incubation,the volumes removed were replaced with fresh culture medium followed by the addition of 20 μl/well of MTT solution(5 mg/ml).After another 4 h of incubation,the medium was replaced with DMSO.Then,the plates were gently shaken for about 10 min to ensure that the formazan was completely dissolved.The absorbance was determined at 570 nm with a microplate reader(ELX 800;BIOTEK Instrument,Inc.)and the cell viability(%)was calculated from the following equation:Cell viability(%)=(ODsample/ ODcontrol)× 100,ODsamplerepresents the results from the wells treated with different samples and ODcontrolrepresents the wells treated with DMEM alone.
2.6.2. Migration and invasion assay
The migratory capacity of the cells was measured by the transwell chamber test using cell culture inserts(6.5 mm, Corning,NY).Cells(5 × 104cells)were suspended in serumfree medium and mixed with different test solutions(10 μg/ ml CEL,40 μg/ml MC and 40 μg/ml MCS),and then injected into the upper chamber.The complete DMEM medium with the above test solution in the bottom chamber was separated from the cells by a polycarbonate membrane(8.0 μm).After a 24 h-incubation,the cells which had not migrated to the undersurface of the membrane f i lter were completely removed using a cotton bud.The cells that had migrated through the membrane were f i xed with 75%ethanol and stained with crystal violet for 30 min.Then,they were washed with PBS three times.The stained cells were visualized and photographed using a f l uorescence microscope(Olympus,Japan). Finally,the stained cells were soaked in acetic acid for 20 min and stained with crystal violet solution.The absorbance of the crystal violet solutions was measured at 595 nm using a microplate reader(ELX800,BIO-TEX Instrument,Inc.).The inhibition ratio(%)was calculated from the following equation:The inhibiting ratio (%)= ODcontrol- ODsample/ ODcontrol× 100,ODsamplerepresents the results from cells treated with CEL,MC or MCS,and ODcontrolis from untreated cells.
Cell invasion behavior was analyzed using transwell cell culture inserts coated with matrigel.Cells(5 × 104cells)suspendedin serum-free mediumwere treatedwith differenttest solutions and transferred to the upper chamber.All the other operations were the same as those in the migration experiment.The stained cells were also photographed with a f l uorescence microscope and the absorbance was measured at 595 nm using a microplate reader.
2.6.3. Wound-healing assay
MDA-MB-231 cells were seeded at a density of 5 × 104cells in 24-well plates.The cells were given a uniform scratch using a micropipette tip when they grew into a monolayer f i lm and adhered to the well,and then washed with PBS until no dead cells remained.Fresh complete medium containing samples (CEL,MC and MCS)with the same concentration of CEL(10 μg/ ml)were added to the cells and those without added samples were used as a control.Cells were allowed to migrate to the scratch area and heal the wound for 0,12 and 24 h.Wound closure was photographed using a f l uorescence microscope (Olympus,Japan)at 0,12 and 24 h.
3. Results and discussion
3.1. Characterization of MC carrier
In the current test,the preparation of carbon material was carried out using the hard template method.Face-centered cubic mesoporous silica as a template was used to construct mesoporous carbon with converse pore structure.The pore channels of mesoporous carbon were transformed from the pore walls of templates.The morphology of the samples (carriers,drugs and drug loaded samples)was studied by SEM and TEM.As seen in Fig.1A,MC exhibited a hexagonal structure and about 5 μm of particle size.To study mesopore structure of MC,a part of MC was amplif i ed by TEM.The mesoporous structure of MC can be clearly seen from Fig.1C. MC had a clear 3-dimensional pore structure.Uniform pore channels of MC were arranged in a highly ordered array. Furthermore,the pore volume and the pore size of MC were high up to 1.51 cm3/g and 9.74 nm,respectively(Table 1).
3.2. Drug loading and characterization
As seen as in Fig.1B,bulk crystalline and irregular shape CEL beforedrugloadingwasshowedbySEM.AsseenasinFig.1D,in contrast with unloaded MC,most of the pore channels in MCS were visible,but they were not clear as MC,indicating that a large number of the pore-channels of MC had been f i lled with CEL.Moreover,disappearance of bulk crystalline drugs also indicatedthattheywereincorporatedintotheporesofcarriers. TheweightofCELloadedintheMCS,MCAandMCPwas18.1%, 15.5%and 19.9%using ultraviolet(UV)spectroscopy,respectively(Table 1).However,the practical drug loading(18.1%)of MCS was different from the expected drug loading(20%).The mainlyreasonforthisphenomenonwasthatsomedrugswere lost during drug loading and washing process.
3.3. Nitrogen adsorption analysis
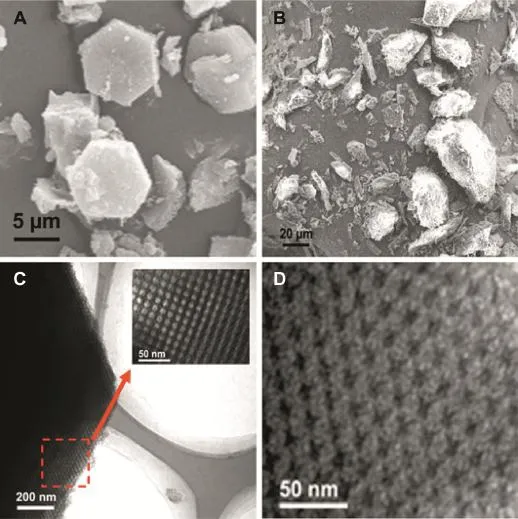
Fig.1-SEM images of(A)MC and(B)CEL.TEM images of(C)MC and(D)MCS.
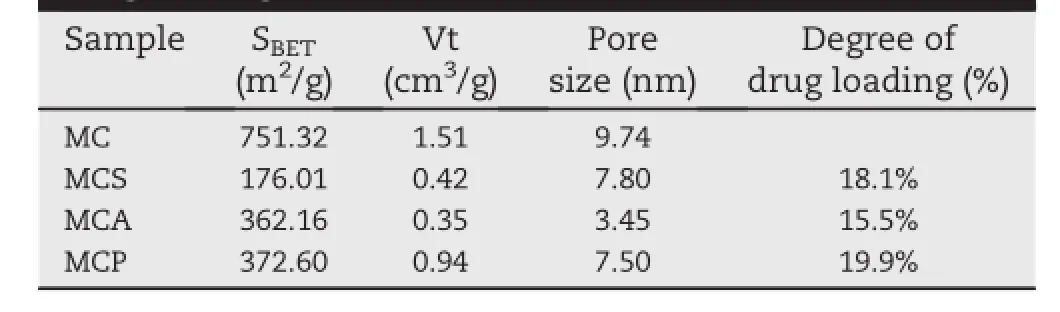
Table 1-Results of nitrogen adsorption analysis and drug loading.
The N2adsorption tests were carried out to investigate the pore structure characteristics of samples.As shown in Fig.2, their mesoporous structure was conf i rmed by the type H2 hysteresis loop and IV isotherms,according to the IUPAC classif i cation.Furthermore,the pore size distribution prof i les conf i rmed that MC had a uniform pore size(Fig.2).The result wasalsoconf i rmedby theBETanalysis.Table1showsthatMC had a uniform pore size of 9.74 nm.The shape of the hysteresis loop for MCP was almost the same as that of MC,indicating that physical mixing process did not produce a signif i cant effect on the pore structure.There were some changes of the shape of the hysteresis loop for MCS and MCA. This was due to that some pore spaces were occupied by drug molecules and their structure was changed after the drug loading.The data on the surface area(BET),pore volume(Vt) and BJH pore diameter(WBJH)were shown in Table 1.A signif i cant reduction of the pore volume and surface area for MCS and MCA indicated that some pore spaces were occupied by drug molecules and the successful drug loading was completed.However,the pore volume area for MCS did not show a signif i cant reduction due to that some of drugs were not loaded into the pore channels during the physical mixing process.Additionally,to study the relationship between the occupation volume and dispersion of drug molecules,the equation(Volume occupation ratio(%)=(wt%CEL/MMolarmass) × NAvogadro’s constant × VMolecule volume/VPore volume)was usedto calculatethe volumeoccupationratio of CEL loadedon MCS.The theoretical volume occupation ratio of CEL loaded on MCS is just 5.1%according to the practical drug loading (18.1%).However,the practical volume occupation ratio of CEL loaded on MCS and MCA was 72.2%and 76.8%,estimated from the results of nitrogen adsorption analysis(Table 1).It was worth noting that the practical volume occupation ratios of 72.2%and 76.8%were signif i cantly higher than the theoretical volume occupation ratio of 5.1%.It indicated that drug molecules of MCS and MCA were all not closely arranged inside the pores.Furthermore,some pore windows or pore channels may be blocked by them.Drug molecules did not closely pack in the pores,which resulted in the production of unoccupied space.This meant that their resistance in the release process may be reduced.
3.4. Solid state characterization
XRD measurements were carried out to study the crystal properties of samples.As shown in Fig.3,eight main characteristic peaks of pure CEL were foundat 14.8°,16.1°,19.7°21.5°, 23.4°,24.6°,25.3°and 29.4°,respectively.Comparatively,no any of the above-mentioned peaks was shown in the XRD patterns of MC.The main diffraction peaks of MCP were still preserved but weakened.This showed that the crystalline form of CEL was partly covered up by MC.However,MCA and MCS did not show any diffraction peaks.The drug was in a non-crystalline state if all the characteristic peaks of drugs were not present[24,25].So it was inferred that the crystalline form was completely covered up and transformed into a noncrystalline state.
DSC is a sensitive method for detecting crystalline characteristics of the samples.As shown in Fig.4,a sharp endothermic melting peak at about 161°C for CEL was shown due to its melting point.In contrast,there was no corresponding peak for MC at the same temperature.Additionally,the MCP also had a same but weak endothermic peak,which was due to that MC did not completely cover up the crystalline form of CEL.It is worth noting that the melting peak for MCA and MCS was both not present at about 161°C.The results were in good agreement with the above XRD results of MCA and MCS.This indicated that MC could successfully cover up the crystalline form of CEL,which was due to that CEL could be effectively loaded into the mesoporous channels of MC using the adsorption or solvent evaporated method.Furthermore,it has been reported that some drugs loaded by mesoporous materials could be maintained in the noncrystalline state due to space conf i nement of nanopores[26,27].
3.5. In vitro drug dissolution
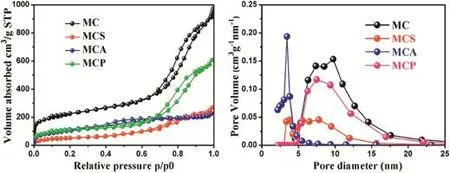
Fig.2-Adsorption-desorption isotherms and pore size distributions of MC,MCS,MCA and MCP.
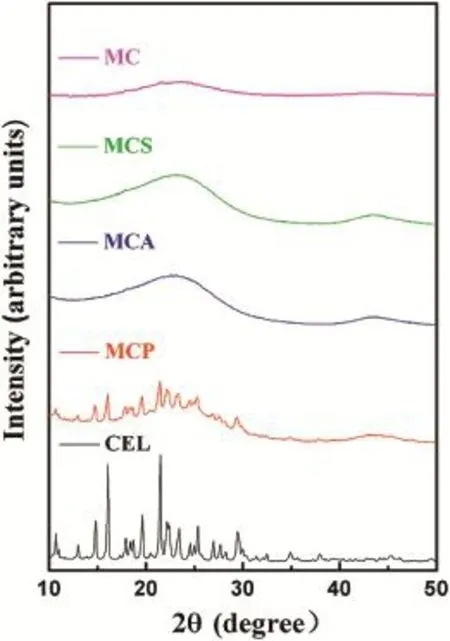
Fig.3-XRD patterns of MC,MCS,MCA,MCP and CEL.
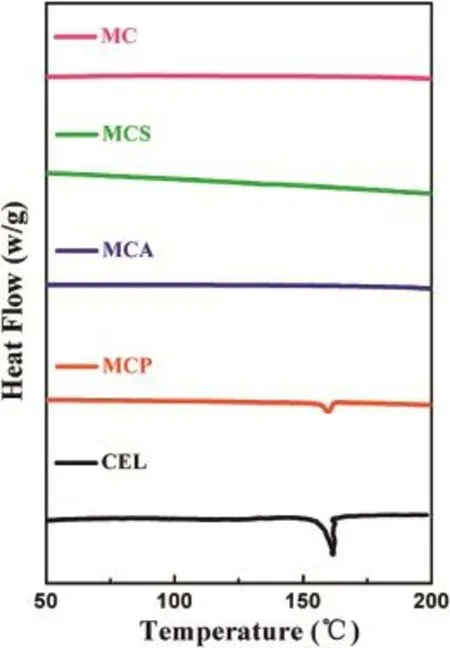
Fig.4-DSC patterns of MC,MCS,MCA,MCP and CEL.
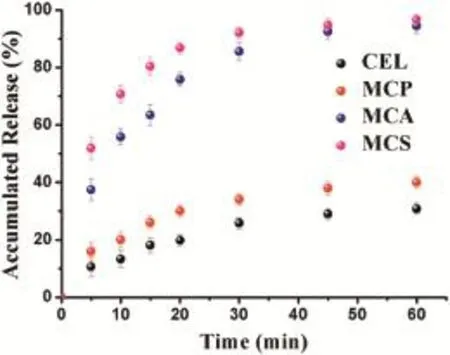
Fig.5-In vitro dissolution prof i les for MCS,MCA,MCP and CEL.
In vitro drug dissolution tests were conducted to investigate the impact of MC on the dissolution rate of CEL.As shown in Fig.5,the accumulated percentage of CEL released from pure CEL within 60 min was just 30.8%,ref l ecting its poor solubility. Dissolution behaviorofMCP exhibited no signif i cant improvement compared with pure CEL.Some drugs still stayed on the surface of MC when they were physically mixed, which resulted in the presence of the crystalline form of MCP. However,the cumulativerelease ofMCA and MCSafter 60 min was both about 3 times higher than that of pure CEL.This result was due to that CEL was conf i ned to the narrow spaces of the mesoporous carbon.Formation of a crystalline form was diff i cult,which maintained CEL in the non-crystalline or amorphous state,and this is shown in Fig.1B and D.In contrasttothecrystallineform,theformation ofthe noncrystalline state or amorphous form could signif i cantly increase the apparent solubility and dissolution rate of poorly water-soluble drugs[28].So,the noncrystalline state of CEL was one of the reasons for its improved dissolution.It was worth noting that the cumulative release for MCS within the 5 min was up to 51.86%,which was higher than that of CEL (lower than 45%)loaded into mesoporous carbon reported in previous studies.This could be due to that the pore size and pore volume both played a very important role in improving the dissolution for the poorly soluble drugs.The drug release process in the mesoporous materials can be described by the dissolved and released process.On one hand,the dissolved process was that the drug molecules were dissolved by the dissolution medium.The pore volume decided the volume of dissolution medium penetrated to the pore channels.So MC with pore volume(1.51 cm3/g)could dissolve more drug molecules,compared with other mesoporous carbon carriers with relatively small pore volume(1.09 cm3/g).On the other hand, the released process was that the drug molecules in the dissolution medium were released from the pores channels. The possibility of escaping from the pore channels for the drug molecules was determined by the pore size.Obviously, drug molecules in MC(9.74 nm)would have more chance to be released from the pore channels,compared with other mesoporous carbon carriers with relatively small pore size (7.0 nm).Additionally,MC with three dimensional interconnected pore structure could reduce the diffusion resistance of the dissolution medium and facilitate drugs release. So,MC with pore volume(1.51 cm3/g)and pore size(9.74 nm) can be used as an effective delivery vehicle for CEL to allow their controlled release.
3.6. MDA-MB-231 cell experiments
3.6.1. Cytotoxicity assays
The cytotoxicity of CEL,MC,MCS for MDA-MB-231 cells are shown in Fig.6.MC was essentially weakly cytotoxic to MDAMB-231 cells with a cell viability over 80%even at a concentration of 50 μg/ml,conf i rming that MC was not signif i cantly toxic to MDA-MB-231 cells.It was worth noting that the cell viability ofCEL and MCS gradually decreased (CEL:92.47-80.99%;MCS:91.65-80.02%)over the concentration range from 0.1 μg/ml to 50 μg/ml,but not signif i cantly.Moreover,cytotoxicity of MCS on cells was slightly stronger than that of CEL in the same concentration.
3.6.2. Migration and invasion analysis
In order to investigate the effect of CEL,MC and MCS on MDAMB-231 cell migration and invasion,a transwell migration test and invasion assay were carried out to assess the migration and invasionofcellsin responseto theiruse.Asshownin Figs. 7 and 8A,many cells migrated through the transwell monolayer were showed due to the full staining with crystal violet. It was concluded that the culture medium was assisted cell migration aftera 24h-incubation.MDA-MB-231cellsexhibited signif i cant migration and invasion activity on their own[29]. Then we examined the inhibitory effect of pure CEL on cell migration and invasion.Treatment with raw CEL reduced the crystalline purple area meaning that the number of migrated cells had decreased,compare with the control(Figs.7 and 8C). It has been previously reported that CEL inhibited the migration and invasion of MDA-MB-231 cells,accompanied by regulation of cyclooxygenase-2(COX-2)[4].We loaded CEL into the pore internal of MC and performed migration and invasion experiments using MCS to investigate whether MC can affect the inhibitory capacity of CEL on cell migration and invasion.In particular,the small crystal violet area showed that cell migration had been signif i cantly inhibited by MCS (Fig.7D).The number of MDA-MB-231 cells invaded through the f i lter containing matrigel also declined more signif i cantly in response to MCS treatment compared with that of cells treated with pure CEL(Fig.8D).Compared with the CEL treatment group,thesmallerareaofcrystalvioletshowed that MCS produced a more signif i cant inhibition than that of pure CEL.These results were also supported by data in Figs.7 and 8E,we also saw that the migration inhibition rate of MCS (77.1%)on cells was higher than that of CEL(33.9%)from the migration inhibition curve(Fig.7E).Invasive inhibition capacity of MCS(80.9%)on MDA-MB-231 cells was also higher than that of pure CEL(50.8%)(Fig.8E).The migration inhibition rate and the invasion inhibition rate of MDA-MB-231 cells treated with MCS was increased by 2.27-fold and 1.59-fold respectively compared with non-treated MDA-MB-231 cells. So,we concluded that MCS markedly inhibited the migration and invasion of MDA-MB-231 cells and its inhibitory effect was higher than that of CEL.
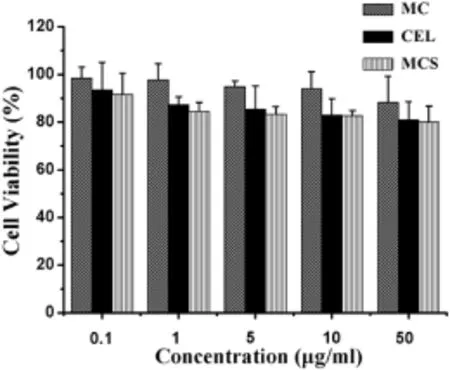
Fig.6-Cytotoxicity of MC,CEL and MCS on MDA-MB-231 cells at different concentrations(0.1,1,5,10 and 50 μg/ml).
Subsequently,the main reasons for the improved inhibitory effect of CEL on the migration and invasion of MDA-MB-231 cells were systematically studied.MTT tests were carried out to investigate the possibility that the inhibitory effect of MCS on cell migration and invasion was due to its toxic effect on cell viability.MDA-MB-231 cells were treated with MCS or CEL at a concentration of 10 μg/ml for 24 h.The results obtained showed that cell viability treated by MCS was not signif i cantly different from that by CEL when the concentration was 10 μg/ml(Fig.6).So,it can be excluded that their cytotoxicity was the main reason for inhibiting cell migration and invasion.Then,in order to study the relationship between MCS and MC for anti-tumor metastasis ability,we investigated the impact of MC on migration and invasion of MDAMB-231 cells.The results obtained showed that MC had almost no effect on cell migration and invasion due to its migration and invasion inhibitory rate of less than 20%(Figs.7 and 8B,E).So,we excluded the possibility that MC can inhibit the metastasis of MDA-MB-231 cells.Therefore,we can speculate that the main reason that the inhibition of MCS was higher than that of pure CEL for MDA-MB-231 cell metastasis may be due to the dissolution of raw CEL increased by MC.It is well known that the poor solubility of raw CEL limited its eff i cacy.Therefore,improvement in the solubility of CEL could play a crucialrole in the increasedpharmacodynamiceff i cacy. Improvement in the dissolution rate can increase the concentrations of drug in MDA-MB-231 cells.Furthermore,the surface area of the drug particles at the nano-level was markedly higher than that of the raw drug.In other words,the drug nanoparticles released from MC increased the contact area of the drug with the MDA-MB-231 cells,and thus produced a better inhibitory effect.In summary,these combined results indicate that MCS markedly inhibits migration and invasion of MDA-MB-231 cells and its inhibitory capacity was higher than that of CEL.Dissolution enhancement of CEL may be the main reason that MCS signif i cantly inhibited cell migration and invasion.Moreover,CEL was dispersed into nanoparticles after being loaded into the mesopores of MC. Then,the CEL nanoparticles were released from the MC and absorbed by MDA-MB-231 cells.It was well-known that nanoparticles could increase the absorption by cells[30].So the increased absorption for CEL may be another reason for improving the inhibition of cell migration and invasion.
In recent years,carbon materials have begun to be applied to the f i eld of drug delivery system[31-33].However,as far as we known,there have been almost no reports on the application of mesoporous carbon to treat anti-tumor metastasis. MC was used in our research into anti-tumor metastasis for the f i rst time.When MC was used as a drug carrier it effectively increased the dissolution of CEL and improved its therapeutic effect.So,MC appears to be a promising drug carrier for anti-tumor metastasis with signif i cant advantages such as low cytotoxicity,high adsorption and uniform pore size.
3.6.3. Wound-healing assay
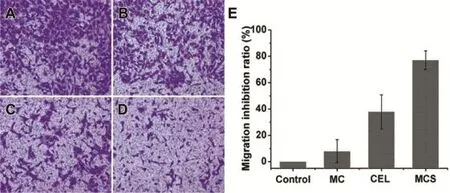
Fig.7-Fluorescence microscopy images of migrated MDA-MB-231 cells through transwell culture inserts membrane incubation with serum-free medium(A),serum-free medium mixed with MC(B),serum-free medium mixed with CEL(C), serum-freemediummixedwithMCS(D),ThemigrationinhibitingratioofMC,CEL,MCSonmigrationofMDA-MB-231cells(E).
The wound-healing test was also carried out to further evaluate the inhibition capability of MC,CEL and MCS on cell motility.As shown in Fig.9A-C(control group),the scratch width was signif i cantly reduced after a 12 h-incubation.It is worth noting that the scratch had completely healed after a 24 h-incubation,which suggested that the MDA-MB-231 cells themselves exhibited a high migration.As seen in Fig.9D-F (MC treatment group),the cells with MC treatments healed thoroughly after treatment for 24 h.These results were substantially the same as those of the control group,indicating that MC had no signif i cant effect on motility of cells in vitro. As shown in Fig.9G-I(CEL treatment group)and Fig.9J-L (MCS treatment group),wounds treated with CEL and MCS remained cracked and their width was signif i cantly wider than those of the control group after a 24 h-incubation,indicating that cell migration was signif i cantly inhibited after treatment with CEL and MCS.In addition,the scratch width treated with MCS was apparently wider than that of pure CEL, which demonstrated that migratory inhibitory effect of MCS on MDA-MB-231 cells was more potent than that of CEL.The result that the motility of MDA-MB-231 cells treated with MC could not be inhibited suggested that the anti-motile activity of MCS was no account of synergistic actions between MC and CEL.These results also supported the conclusions of the above migration and invasion experiment.
4. Conclusion
The results of this paper showed that mesoporous carbon with a large pore volume(1.53 cm3/g)and large pore size (9.74 nm)was successfully prepared.Celecoxib was successfully loaded into MC using three different methods.According to the results of DSC and XRD experiments,MCS and MCA could completely cover up the crystalline form of CEL,while the MCP could partly covered up the crystalline form of CEL. The dissolution study showed that the dissolution rate of CEL released from MCS and MCA was all signif i cantly higher compared with that of raw CEL.It was excited that the accumulated release for MCS within the 5 min was up to 51.86%. Furthermore,the results of the migration,invasion and wound-healing assays all indicated that MCS enhanced theinhibitory effect of CEL on the migration and invasion of MDAMB-231cells,whichmay be due to the improved dissolution.It was expected that the application for MC in the f i eld of antitumor metastasis could open up new opportunities for mesoporous carbon as a carrier.
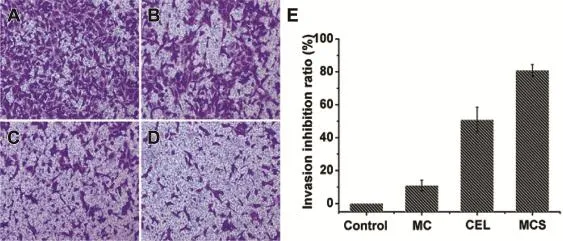
Fig.8-Fluorescence microscopy images of invaded MDA-MB-231 cells through transwell culture inserts matrigel membrane incubation with serum-free medium(A),serum-free medium mixed with MC(B),serum-free medium mixed with CEL(C),serum-free medium mixed with MCS(D),The invasion inhibiting ratio of MC,CEL,MCS on invasion of MDAMB-231 cells(E).
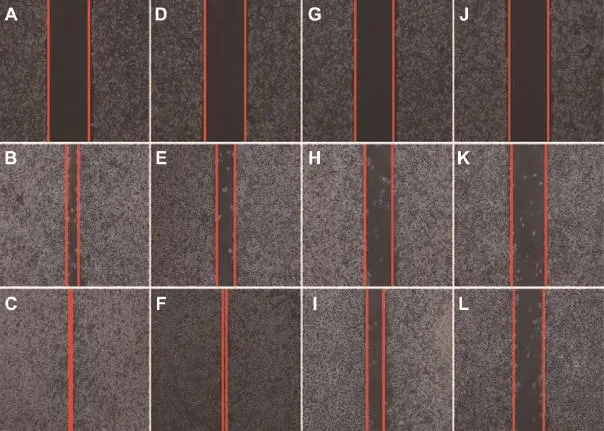
Fig.9-Fluorescence microscopy images of scratched MDA-MB-231 cells incubation with complete medium for 0 h(A),12 h (B),24 h(C),complete medium contained MC for 0 h(D),12 h(E),24 h(F),complete medium contained CEL for 0 h(G),12 h(H), 24 h(I),complete medium contained MCS for 0 h(J),12 h(K),24 h(L).
Acknowledgments
This work was supported by the National Basic Research Program of China(973 Program)(No.2009CB930300),National Natural Science Foundation of China(No.81273449).
R E F E R E N C E S
[1]Trenis DP,William JA,John DL.Targeting tumor cell motility to prevent metastasis.Adv Drug Deliv Rev 2011;63:568-581.
[2]Sharon M,Geoffrey L.Pharmacokinetics and pharmacogenomics in breast cancer chemotherapy.Adv Drug Deliv Rev 2009;61:381-387.
[3]Jong LR,Myung WS,Seok WP,et al.Celecoxib can prevent tumor growth and distant metastasis in postoperative setting.Cancer Res 2004;64:3230-3235.
[4]Wei HS,Guo SC,Xi LO,et al.Inhibition of COX-2 and activation of peroxisome proliferator-activated receptor γ synergistically inhibits proliferation and induces apoptosis of human pancreatic carcinoma cells.Cancer Lett 2009;275:247-255.
[5]Wei AY,Wang LW,He YJ,et al.Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity.Cancer Res 2004;64:2030-2038.
[6]Masashige T,Masakazu Y,Kazunori N,et al.A synergic inhibitory-effect of combination with selective cyclooxygenase-2 inhibitor and S-1 on the peritoneal metastasis for scirrhous gastric cancer cells.Cancer Lett 2006;244:247-251.
[7]Shi HY,Lv FJ,Zhu ST,et al.Dual inhibition of 5-LOX and COX-2 suppresses esophageal squamous cell carcinoma. Cancer Lett 2011;309:19-26.
[8]Sokbom K,Moon HK,In AP,et al.Elevation of cyclooxygenase-2 is related to lymph node metastasis in adenocarcinoma of uterine cervix.Cancer Lett 2006;237:305-311.
[9]Natesan S,Subhabrata R,Saroj KG,et al.Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib.Biol Pharm Bull 2004;27:1993-1999.
[10]Faiyaz S,Sanjula B,Alka A,et al.Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion.J Nanobiotech 2008;6:1-8.
[11]Michael M,Corey B,Ron B,et al.Polymeric nanoparticles for increased oral bioavailability and rapid absorption using celecoxib as a model of a low solubility,high-permeability drug.Pharm Res 2012;29:427-440.
[12]Cao X,Deng WW,Fu M,et al.Seventy-two-hour release formulation of the poorly soluble drug silybin based on porous silica nanoparticles:In vitro release kinetics andin vitro/in vivo correlations in beagle dogs.Eur J Pharm Sci 2013;48:64-71.
[13]Hu YC,Zhi ZZ,Wang TY,et al.Incorporation of indomethacin nanoparticles into 3-D ordered macroporous silica for enhanced dissolution and reduced gastric irritancy. Eur J Pharm Biopharm 2011;79:544-551.
[14]Hu YC,Zhi ZZ,Jiang TY,et al.Spherical mesoporous silica nanoparticles for loading and release of the poorly watersoluble drug telmisartan.J Control Release 2010;145:257-263.
[15]Sang JS,Bai X,Sang BL.Inorganic hollow nanoparticles and nanotubes in Nanomedicine:part 1.Drug/gene delivery applications.Drug Discov Today 2007;12:650-656.
[16]Sang JS,Bai X,Sang BL.Inorganic hollow nanoparticles and nanotubes in Nanomedicine:part 2:Imaging,diagnostic,and therapeutic applications.Drug Discov Today 2007;12:657-663.
[17]Wang XF,Liu P,Tian Y.Ordered mesoporous carbons for ibuprofen drug loading and release behavior.Micropor Mesopor Mat 2011;142:334-340.
[18]Zhao P,Wang LH,Sun CS,et al.Uniform mesoporous carbon as a carrier for poorly water soluble drug and its cytotoxicity study.Eur J Pharm Biopharm 2012;80:535-543.
[19]Ji SI,Byong CB,Young-Seak L.The effect of carbon nanotubes on drug delivery in an electro-sensitive transdermal drug delivery system.Biomaterials 2010;31:1414-1419.
[20]Zhao P,Jiang HT,Jiang TY,et al.Inclusion of celecoxib into f i brous ordered mesoporous carbon for enhanced oral bioavailability and reduced gastric irritancy.Eur J Pharm Sci 2012;45:639-647.
[21]Zhu WQ,Wan L,Zhang C,et al.Exploitation of 3D facecentered cubic mesoporous silica as a carrier for a poorly water soluble drug:inf l uence of pore size on release rate.Mat Sci Eng C 2014;34:78-85.
[22]Le JJ,Jing YS,Zhen YL,et al.In vitro and in vivo evaluation of paclitaxel-loaded mesoporous silica nanoparticles with three pore sizes.Int J Pharm 2013;445:12-19.
[23]Shyamal KD,Shobhna K,Hirotoshi Y,et al.Effects of surface acidity and pore size of mesoporous alumina on degree of loading and controlled release of ibuprofen.Micropor Mesopor Mat 2009;118:267-272.
[24]Shobhna K,Rajesh H,Aninda JB.Inf l uence of surface chemistry of mesoporous alumina with wide pore distribution on controlled drug release.J Control Release 2009;140:34-39.
[25]Salonen J,Laitinen L,Kaukonen AM,et al.Mesoporous silicon microparticles for oral drug delivery:loading and release of f i ve model drugs.J Control Release 2005;108:362-374.
[26]Zhang YZ,Zhang JH,Jiang TY,et al.Inclusion of the poorly water-soluble drug simvastatin in mesocellular foam nanoparticles:drug loading and release properties.Int J Pharm 2011;410:118-124.
[27]Zhang YZ,Jiang TY,Zhang Q,et al.Inclusion of telmisartan in mesocellular foam nanoparticles:drug loading and release property.Eur J Pharm Biopharm 2010;76:17-23.
[28]Xiao HP,Thomas J,Larry A.Increasing the dissolution rate of a low-solubility drug through a crystalline-amorphous transition:a case study with indomethicin.Drug Dev Ind Pharm 2008;34:221-231.
[29]Abhik B,Yong Z,Michael LC,et al.A soluble transforming growth factor β type III receptor suppresses tumorigenicity and metastasis of human breast cancer MDA-MB-231 cells. Cancer Res 1999;59:5041-5046.
[30]Yuka F,Yasunori I,Shu JN,et al.Lipid nanoparticles with no surfactant improve oral absorption rate of poorly watersoluble drug.Int J Pharm 2013;451:92-94.
[31]Sandeep KV,Dan Z,Giorgia P,et al.Delivery of drugs and biomolecules using carbon nanotubes.Carbon 2011;49:4077-4097.
[32]Ruibin L,Ren AW,Liang Z,et al.Folate and iron difunctionalized multiwall carbon nanotubes as dualtargeted drug nanocarrier to cancer cells.Carbon 2011;49:1797-1805.
[33]Elena H,Vera N,Constanze L,et al.Drug loading,dispersion stability,and therapeutic eff i cacy in targeted drug delivery with carbon nanotubes.Carbon 2012;50:622-632.
*Corresponding author.Tel.:+86 24 23986348,+86 15904019144(mobile);fax:+86 24 23986348.
E-mail address:silingwang@syphu.edu.cn(S.Wang).
Peer review under responsibility of Shenyang Pharmaceutical University
Production and hosting by Elsevier
http://dx.doi.org/10.1016/j.ajps.2014.02.001
1818-0876/© 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Novel chemical permeation enhancers for transdermal drug delivery
- Current prodrug strategies for improving oral absorption of nucleoside analogues
- Application of sialic acid/polysialic acid in the drug delivery systems
- Development and evaluation of lafutidine solid dispersion via hot melt extrusion:Investigating drug-polymer miscibility with advanced characterisation
- Determination of azithromycin in raw materials and pharmaceutical formulations by HPLC coupled with an evaporative light scattering detector
- Rapid and sensitive analysis of cyclobenzaprine by LC-MS/MS:Application to a pharmacokinetic study of cyclobenzaprine in dog
