Novel chemical permeation enhancers for transdermal drug delivery
2014-04-20PengQunXiochngLiuMnliWngLingFng
,Peng Qun,Xiochng Liu,Mnli Wng,Ling Fng,
aSchool of Pharmacy,Shenyang Pharmaceutical University,103 Wenhua Road,Shenyang,Liaoning 110016, People’s Republic of China
bSchool of Pharmacy,Beihua University,3999 Huashan Road,Jilin,Jilin 132013,People’s Republic of China
Review
Novel chemical permeation enhancers for transdermal drug delivery
Yang Chena,Peng Quana,Xiaochang Liua,Manli Wangb,Liang Fanga,*
aSchool of Pharmacy,Shenyang Pharmaceutical University,103 Wenhua Road,Shenyang,Liaoning 110016, People’s Republic of China
bSchool of Pharmacy,Beihua University,3999 Huashan Road,Jilin,Jilin 132013,People’s Republic of China
A R T I C L E I N F O
Article history:
Received 18 November 2013
Received in revised form
19 December 2013
Accepted 3 January 2014
Available online 23 January 2014
Transdermal
Transdermal drug delivery has been accepted as a potential non-invasive route of drug administration,with advantages of prolonged therapeutic action,decreased side effect, easy use and better patient compliance.However,development of transdermal products is primarily hindered by the low permeability of the skin.To overcome this barrier effect, numerous new chemicals have been synthesized as potential permeation enhancers for transdermal drug delivery.In this review,we presented an overview of the investigations in this f i eld,and further implications on selection or design of suitable permeation enhancers for transdermal drug delivery were also discussed.
© 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1. Introduction
For decades,utilization of skin as a route for delivering drugs has been an attracting alternative to conventional methods including injections and tablets.Its advantages include the avoidance of pain,elimination of hepatic f i rst-pass metabolism,sustained drug release for a prolonged period,easy use and withdrawal in case of side effects.However,the major limitation for transdermal drug delivery system(TDDS)is also the skin itself.As the outmost layer of the epidermis,the stratum corneum(SC)provides an outstanding barrier towards the absorption of chemical and biological toxins[1].It has a unique hierarchical structure,which is f i lled with multiple lipid bilayers and the embedded corneocytes[2,3].In theory,there are two main permeation routes through the SC, f i rst the intracellular route across the corneocytes as well as the lipid matrix,and second the intercellular route via the lipid domains between the corneocytes.The lipid phase is continuous throughout the SC,and therefore the penetrating substances must interact with this phase whether they penetrate transcelluarly or intercelluarly.
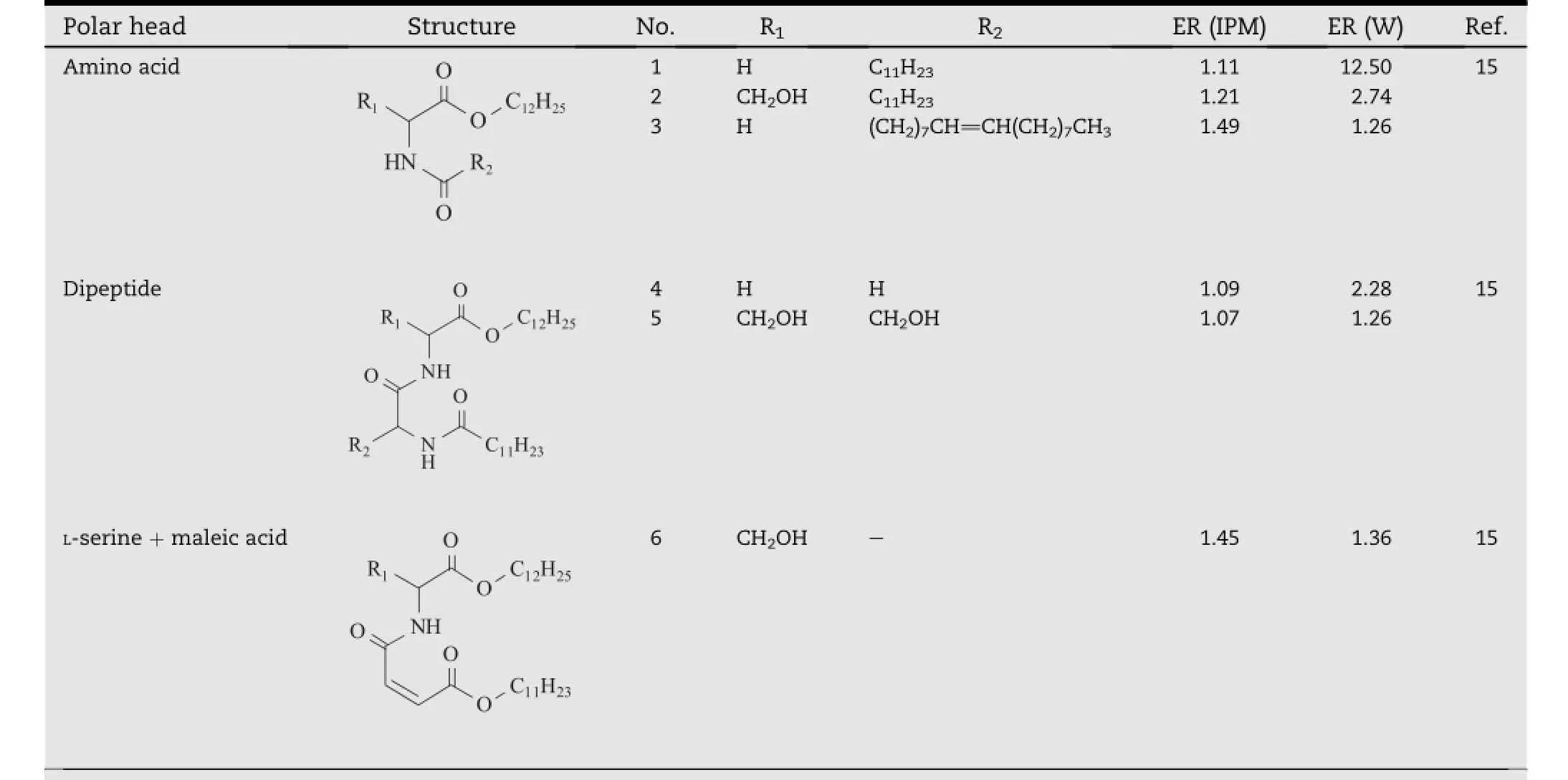
Table 1-Ceramide analogs with different polar heads.a
InordertofacilitatethepassageofmoleculesthroughtheSC, transdermal permeation enhancers have been extensively studied.Up to date,more than 360 chemicals have been demonstrated to enhance the skin permeability[4],including terpenes,sulphoxides,laurocapram,pyrrolidones,fattyacidand fatty alcohol,alcohol and glycol,surfactants,urea,and so on. However,as a result of their incompatibility in the formulation or local irritation issues,few to date have been routinely incorporated in the currently marketed transdermal products[5]. Therefore,exploration of chemicals to safely improve the skin permeabilityremainsanintensiveareaoftransdermalresearch.
There have been several reviews discussing the enhancement activity of the classic permeation enhancers that are commonly used in the transdermal products[5-8].However, a detailed introduction of those potential candidates newly developed is still lacking.Therefore,this paper provides a review on the current researches in this f i eld,which might be helpful the selection or development of a suitable absorption promoter in TDDS.
2. Novel permeation enhancers
2.1. Ceramide analogs
The lipids in the SC consist of ceramides,cholesterol and fatty acids,as well as a small quantity of cholesterol sulfate[9,10].It has been known that ceramides are the main components responsible for the barrier effect of the SC.Structurally,they are amphiphilic molecules,containing two long hydrocarbon tails and an acylamide polar head[11,12].Va´vrova´et al. believed that certain structure similarity might exist between enhancers and ceramides,so that the enhancer molecules could insert themselves between the hydrophobic tails of the ceramide bilayers and weaken the continuity of the lipid barrier[13].In their study,a series of ceramide analogs(Table 1)with two kinds of polar heads based onL-serine and glycine were respectively synthesized,and the relationship between the properties of the polar heads and enhancement activity was discussed.The result indicated that although these compounds were inactive in isopropyl myristate(IPM),signif i cant enhancement effects were observed when they were used in the water(W)suspensions.Especially,compound 1 with the simplest glycine group as the polar head structure was the most potent one,and its promoting activity was signif i cantly higher than compound 2,anL-serine based derivative which had a hydroxyl group in the polar head. Furthermore,these two compounds were both more active than their respective analogs with dipeptide as the polar heads,which were shown by compound 4 and 5.These results led to a hypothesis that the increasing size and H-bonding ability would decrease the activity of the ceramide analogs, and it might account for the little enhancing activity of compound 6,which possessed a large polar head with three hydroxyl groups.In addition,the study also suggested that the introduction of a double bond into the hydrocarbon chain did not contribute much to the activity of the present ceramide analog,which was demonstrated by the compound 3.
Tofurtherexaminetheaforementionedhypothesis, ceramide analogs(Table 2)with six different chain lengths (compound 7-12)and eight different polar head groups (compound 7,13,15-20)were synthesized[14].When analogs of the same chain length were compared,the best enhancement effect was observed with compound 7 andcompound 18.These two compounds respectively had the polar head with the smallest size and that with the lowest H-bonding ability,which further conf i rmed the previous hypothesis that decreasing the size or H-bonding ability of the polar heads of ceramide analogs would contribute to their transdermal enhancement activity.Considering the fact that the polar head of compound 18 is relatively large,comparable to that of compound 15-17 which were inactive as permeation enhancers,it was concluded that H-bonding might be more signif i cant than the polar head size or other factors.In addition,the optimum chain length of the ceramide analogs was found to be 12 carbons(compound 7),which might be due to its favorable interaction with the intercellular skin lipids.
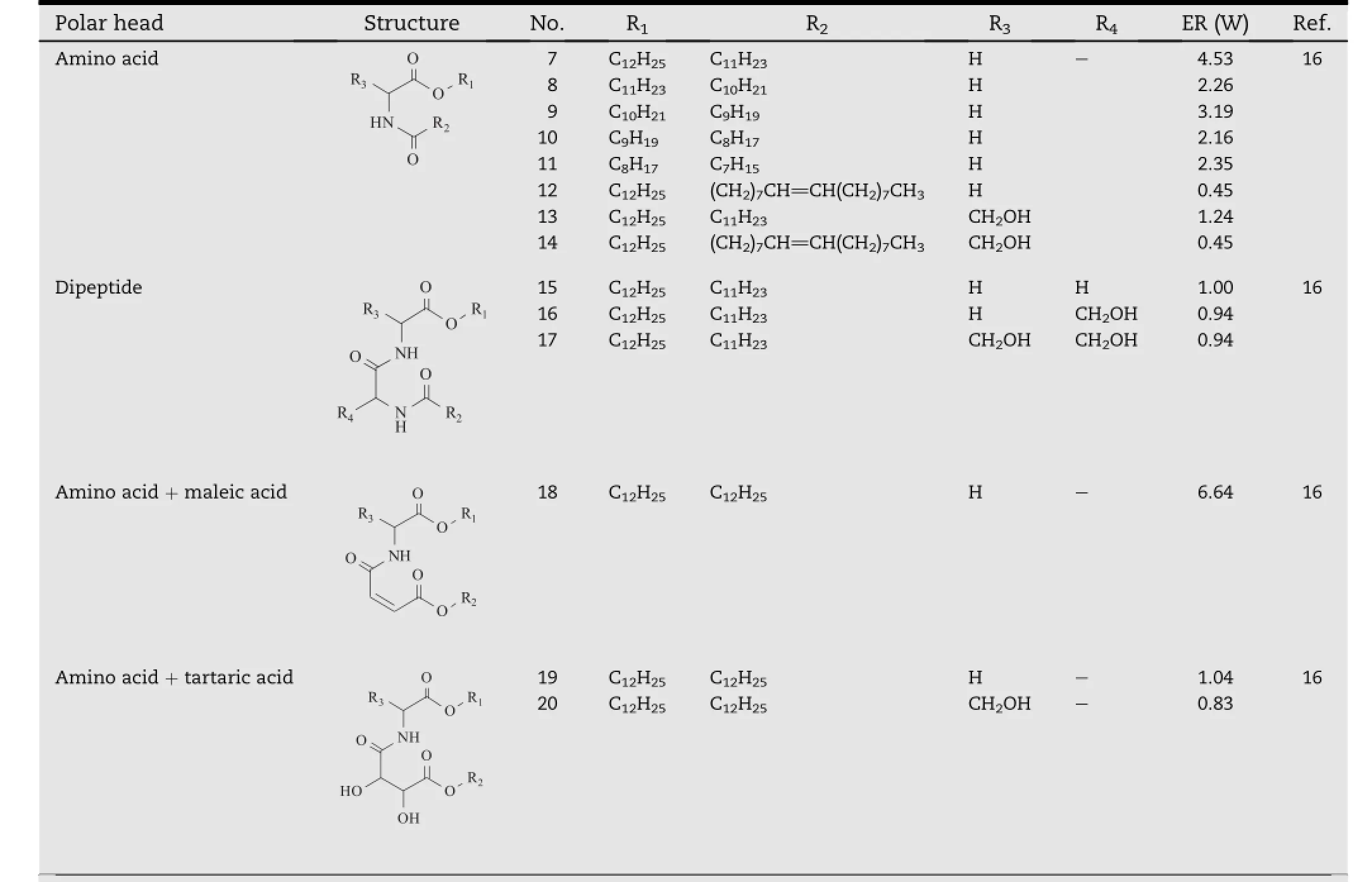
Table 2-Ceramide analogs with different polar head and hydrophobic chain length.a
2.2. Azone analogs
Azone(1-dodecylazacycloheptan-2-one or laurocapram,compound 21,Table 3)is the f i rst molecule specially designed as a skin permeation enhancer[10].It has been demonstrated to enhance the transdermal absorption of a wide variety of drugs [15].Azone probably exerts its permeation enhancing effects mainly via theinteraction with the lipid domains of the SC.The‘soup spoon’structure would make it more likely to partition into the bilayer lipids,where Azone might exist dispersed or in separated domains to disrupt the organized lipid packing[5]. Although FDA has not approved for its use in pharmaceutical products for its slight side effects,Azone is recorded in the Chinese Pharmacopoeia and widely used in China.To improve its properties,hundreds of Azone like compounds have been synthesized,which provide a huge amount of information about the molecular design for Azone analogs[16].
The structure of Azone can be divided into two parts,viz. the hydrophobic chain and the polar cycle head(the heterocycle and α carbon on the hydrophobic chain).Change of the chain length was the common structural modif i cation to the hydrophobic tails.By contrast,the modif i cations of the polar head group was complex,and usually carried out by alternation of heterocyclic ring size,isosteric replacements of N,O, and C atoms,removal of function groups,addition of other polar groups onto the α carbon of the hydrophobic chain[16]. Since the data was too large to easily pick out the information of interest,only key structural requirement for Azone like compounds were summarized here.To avoid the interlaboratory differences such as sample types and solvent systems for the enhancers,activity of Azone analogs were compared using the data from one research group.
2.2.1. The optimal hydrocarbon chain length
The enhancement activities of enhancers always increased as the length of the hydrophobic chains increased,and when thelength of the chain was longer than a certain limit,the activities rather decreased[17].This kind of parabolic dependence was frequently observed in the study of structureactivity relationship for amphiphilic permeation enhancer. The optimal hydrocarbon chain length for Azone analogs were always observed between8 and 14 carbons,which would vary according to their different polar heads(Table 3).The action of an enhancer was considered to be dependent on the balance between its hydrophilic and lipophilic nature,and therefore different length of alkyl chain was required for best incorporation of the enhancer with different polar heads into the skin lipid bilayers.This effect would produce a free space within the lipid lamellae,and thereby producing lateral fl uidization of the tightly packed lamellar lipids.In addition, these compounds could also form separate phases within the lipid lamellae,providing a more permeable shortcut for penetrating compounds.
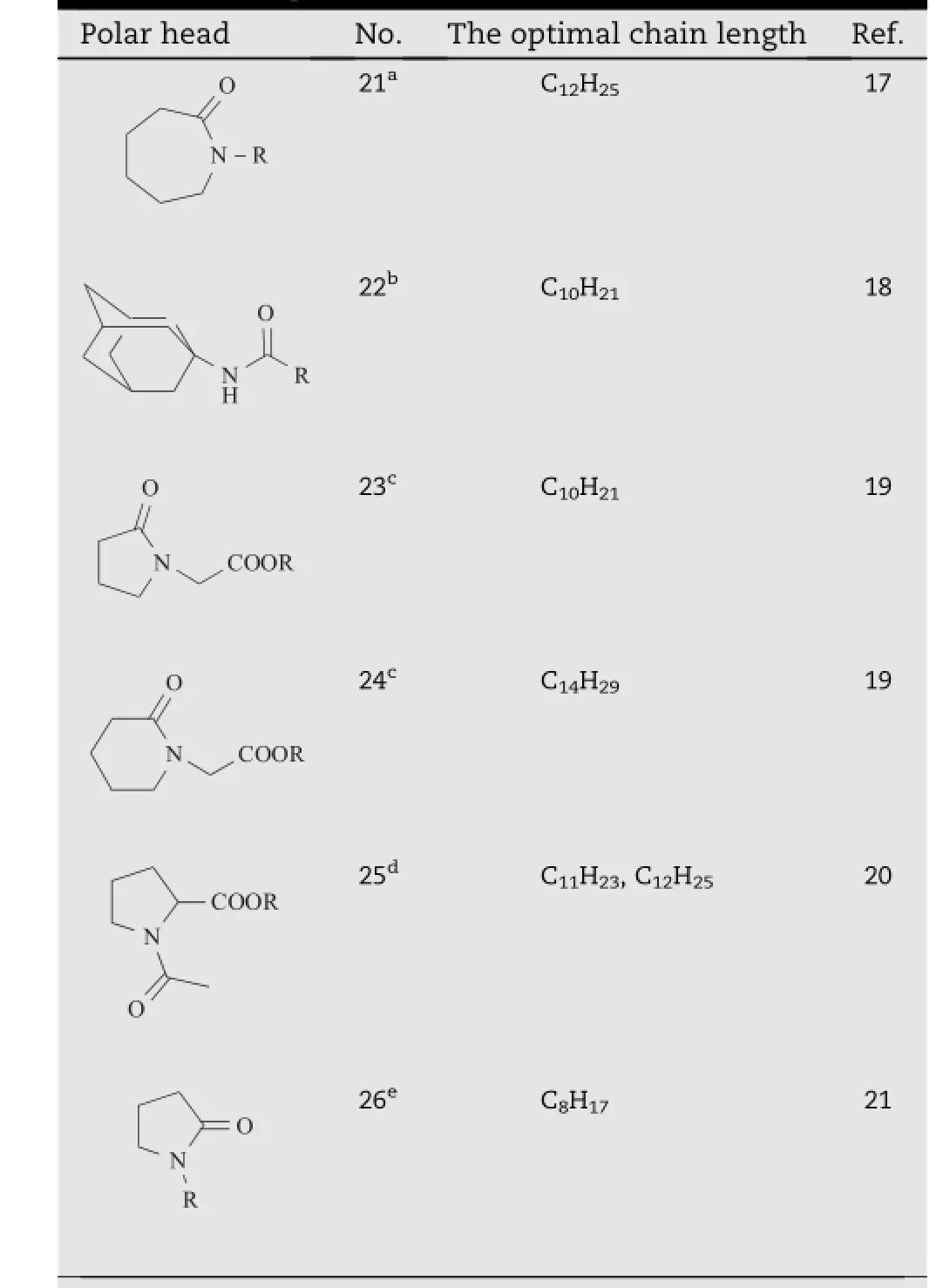
Table 3-The optimal chain lengths for Azone analogs with different polar heads.
2.2.2. Heterocyclic ring size
Michniak et al.compared the transdermal promoting activity of Azone and its analogs containing f i ve or six member cycle. As shown in Table 4,the presence of f i ve-member cycle (compound28)increasedtheactivity,whilesix(compound27) and seven member cycle(Azone)analogs exhibited lower activity[22].TheH-bondingnetformedbetweentheheadgroups is accepted as an important factor in stabilizing the lipid bilayers[23].Azone analogs with a small polar head might be expected to be situated well into the polar lipid domain,thus disrupting the ordered organization of the lipid layers.
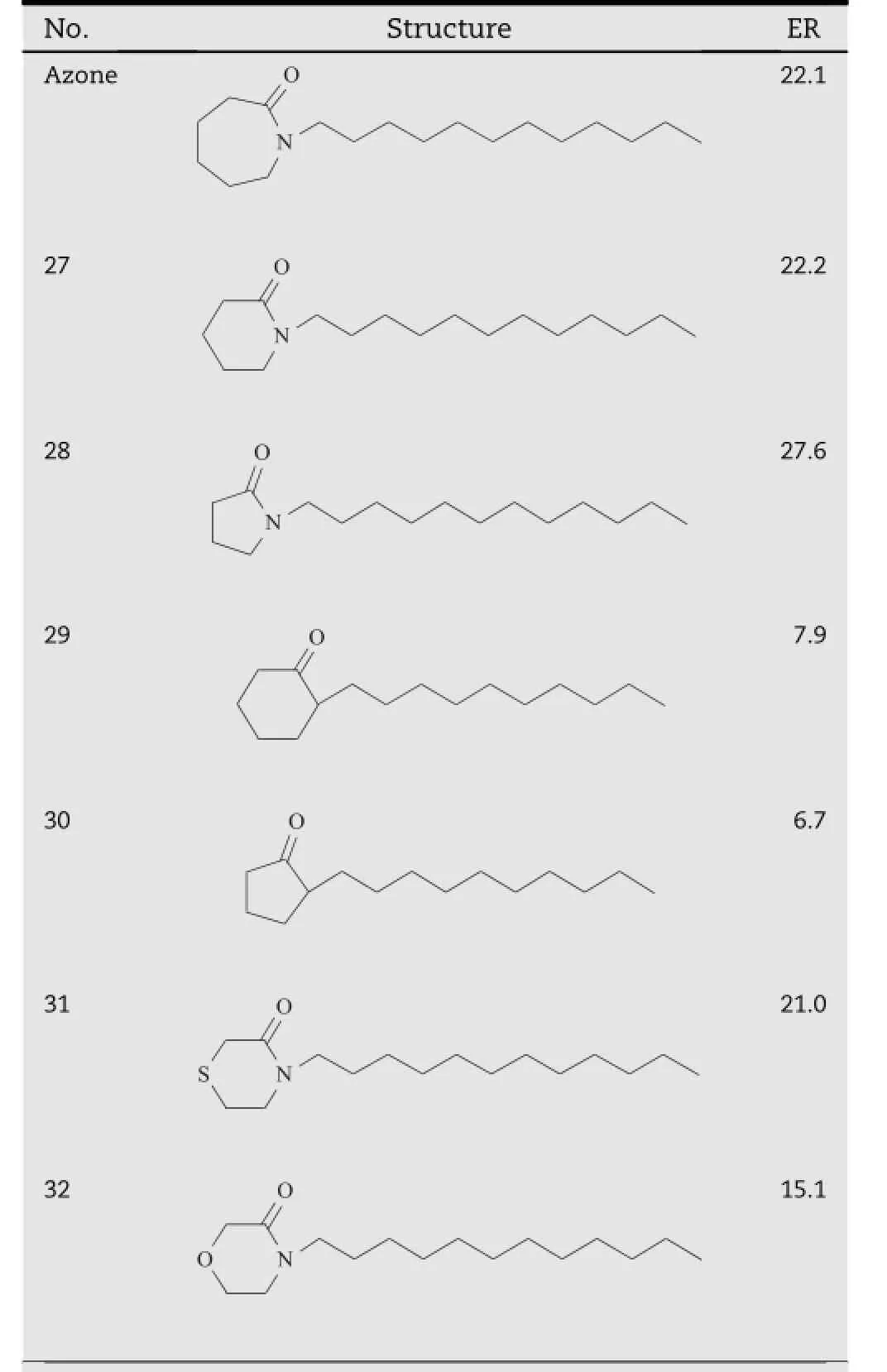
Table 4-Azone analogs with different polar cycle size and heteroatoms on the polar head.a
2.2.3. The heteroatoms in the polar head
The effect of the heteroatom in the polar head of Azone analogs was investigated in the study[22].As shown in Table 4, removal of the nitrogen atom in the polar heads of compound 27 and 28 resulted in a signif i cantly decrease in the promoting activity,which were demonstrated by compound 29 and 30. By contrast,in the polar cycle heads of compound 27 and 28, introduction of another heteroatom generated compounds with better promoting properties,i.e.compound 31 and 32. These f i ndings demonstrated that the heteroatom in the polar head was essential for the activity of Azone analogs.
2.2.4. The carbonyl in polar head
Hadgraft et al.evaluated the modifying effects of Azone and its several analogs on the transdermal absorption of metronidazole(Table 5)[23].Moving the carbonyl group of Azone from the cycle head to its α carbon of the alkyl chain gave the product of compound 32,which showed a comparable enhancement effect in comparison of Azone.However,isosteric substitution of carbonyl oxygen in Azone by sulfur led to a dramatically decreasing activity,which was demonstrated by compound 33.Additionally,when the carbonyl group of Azone was reduced into the alkyl,its enhancing activity also signif i cantly decreased,as shown by compound 34[24]. Therefore,it appeared that the carbonyl in polar head was needed for activity.
2.3. Menthol derivatives
Since its introduction,terpenes have attracted much interest as potential transdermal permeation enhancers[25,26].Ofthese compounds,menthol(compound 35,Table 6)represents a traditional,and arguably,the most common one that has been known as a lipid disordered agent to disrupt the molecular organization of the bilayer lipids in SC[9,27-29].In spite of its enhancement activity and relatively low skin irritation,application of menthol is often hindered by its undesirableodor and high volatility.Therefore,manyderivatives of menthol have been synthesized in the identif i cation of promising candidates with suitable properties for application in TDDS.
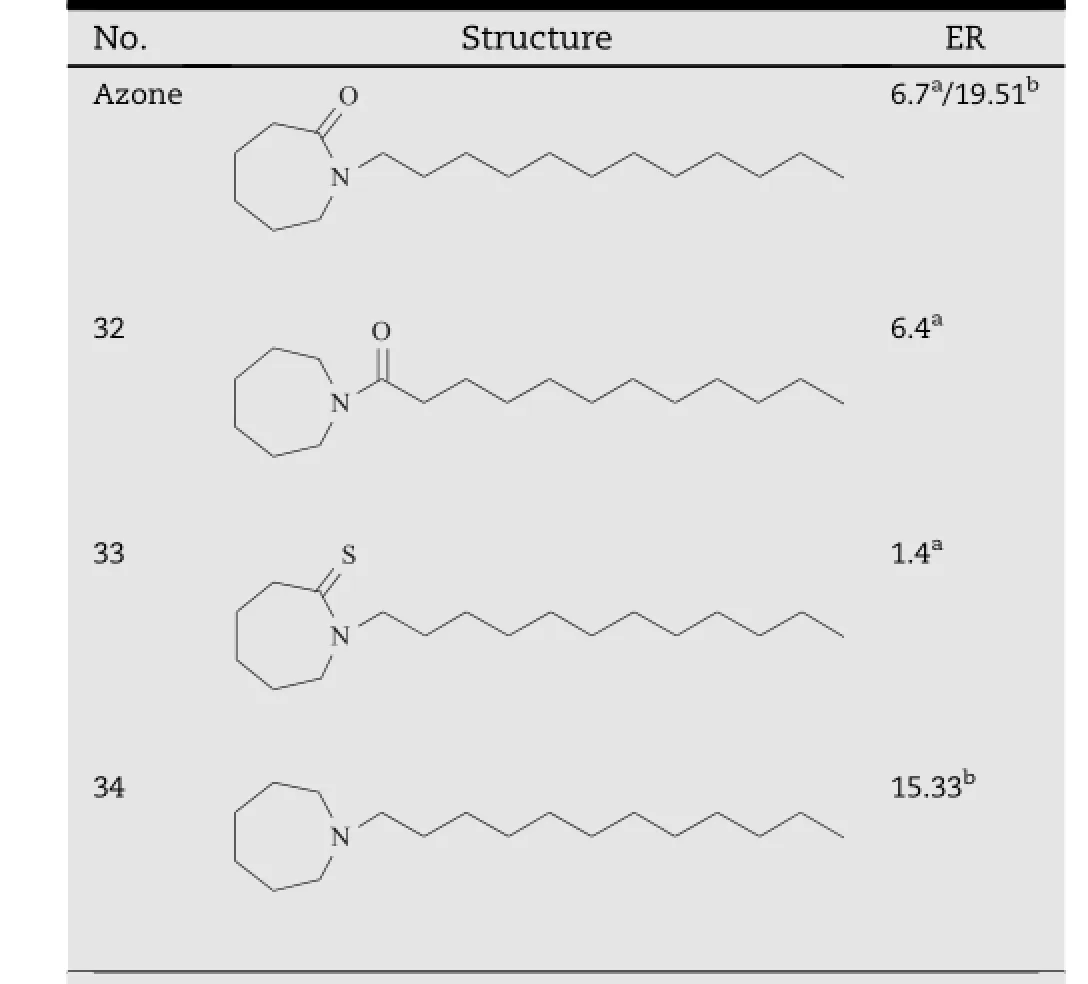
Table 5-Alternation of the carbonyl group in the polar head of Azone analogs.
2.3.1. Etherif i ed derivatives
Obata et al.f i rst found that an ether derivative of menthol,O-ethylmenthol(compound 36,Table 6),had an excellent activity toward the in vivo percutaneous absorption of ketoprofen alcoholic hydrogels through the rabbit skin[30-33].Based on this f i nding,a series of mono-and disubstituted derivatives of cyclohexane with an O-ethyl group were synthesized and evaluated for their absorption enhancement activity[34].As shown in Table 6,in the list of mono-substituted derivatives (compound 37-54),most of them acted better than O-ethylmenthol,except for methyl-substituted products(compound 37,43,49).Amongthe C-2 substitutedcompounds(compound 37-42),compound 40 which has an isopropyl group showed the highest activity.Among the C-3 substituted compounds (compound 43-48),isopropylated or butylated derivatives (compounds 46 and 47)showed the highest activity.Among the C-4 substituted compounds(compounds 49-54),compound 52 which also has an isopropyl group showed the highest activity.In the list of the di-substituted derivatives (compound 55-70),all of them had a similar or better effect than MET.The study also suggested that the irritation potency of these derivatives correlated well with their enhancing activity,and both of them were parabolically dependent with the log P of the compounds.As an exception,1-O-ethyl-3-butylcyclohexanol(OEBC,compound 47)was proved to be a safe and prominent candidate that promoted the transdermal absorption of ten different model drugs,yet exhibiting rather low irritation[35].In addition,thiomenthol derivatives(compound 71-83,Table 7)were also synthesized for more effective and safer permeation enhancer candidates.However,severe skin irritation was produced by these compounds,although some of them had strong promoting activity[36].
2.3.2. Esterif i ed derivatives
Zhao et al.employed menthol as a lead compound and synthesized O-acylmenthol derivatives(Table 8)with a series of saturated fatty acid(C2-C18)and pharmaceutical excipient acids[37,38].Indomethacin,ketoprofen,lidocaine,isosorbide dinitrate and 5-f l uorouracil were selected as model drugs based on their different physicochemical properties.In the case of saturated fatty acid esters,most of them exhibit good enhancing effect on the transdermal absorption of indomethacin,ketoprofen and isosorbide dinitrate,among which compound 88 and 89 respectively produced the greatest enhancing activity towards indomethacin and isosorbide dinitrate.For ketoprofen,compounds with shorter acylchains,such as compound 85 and 86 generally provided the best enhancing effect.However,most of these compounds had no enhancing activity towards5-f l uorouracilandlidocaine,except for compound 89 and 94.In the case of pharmaceutical excipient acids(lactic acid,cinnamic acid, salicylic acid and oleic acid),all of the compounds except for compound 99 promoted the transdermal absorption of isosorbide dinitrate very well.Compound 98 and 100 exhibited good promoting effect for ketoprofen.However,for 5-f l uorouracil and lidocaine,only compound 97 exhibited good activity,andforindomethacin,onlycompound100had enhancing effectiveness.The in vivo results obtained from the patches containing these compounds as permeation enhancers were in good agreement with the plasma concentration predicted form the in vitro data[39].The study also revealed that the drug lipophilicity had a signif i cant impact on the promoting activity of the O-acylmenthol derivatives,and a parabolic relationship between f l ux and log Ko/wof the drugs was observed.In recent investigations,the O-acylmenthol derivatives were demonstrated their enhancing activity towards several drugs with different physiochemical properties,including daphnetin[40]and granisetron[41].The mechanism for the enhancer action is still being studied.
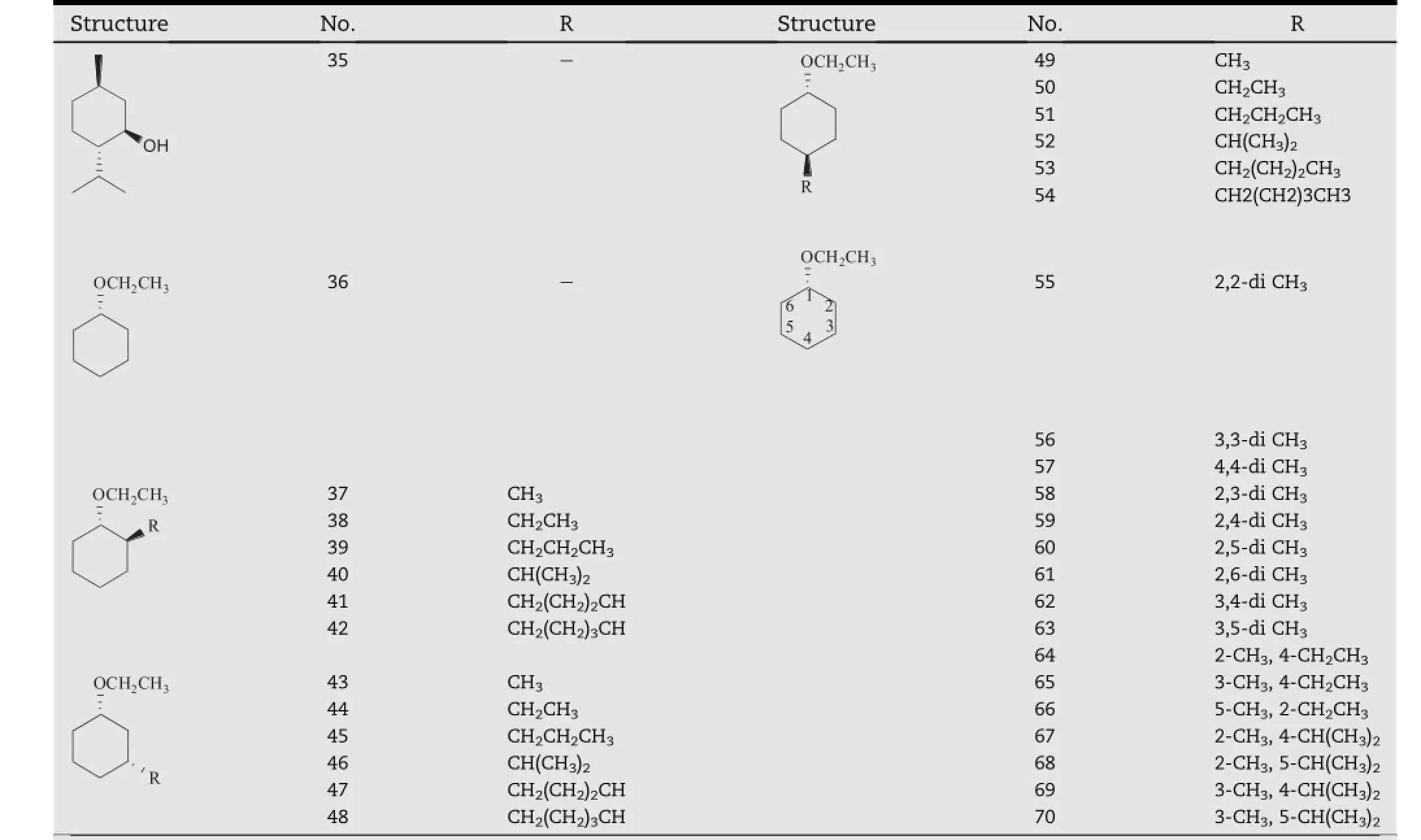
Table 6-Structural modif i cation on O-ethylmenthol.a
Table 7-Thiomenthol derivatives.a
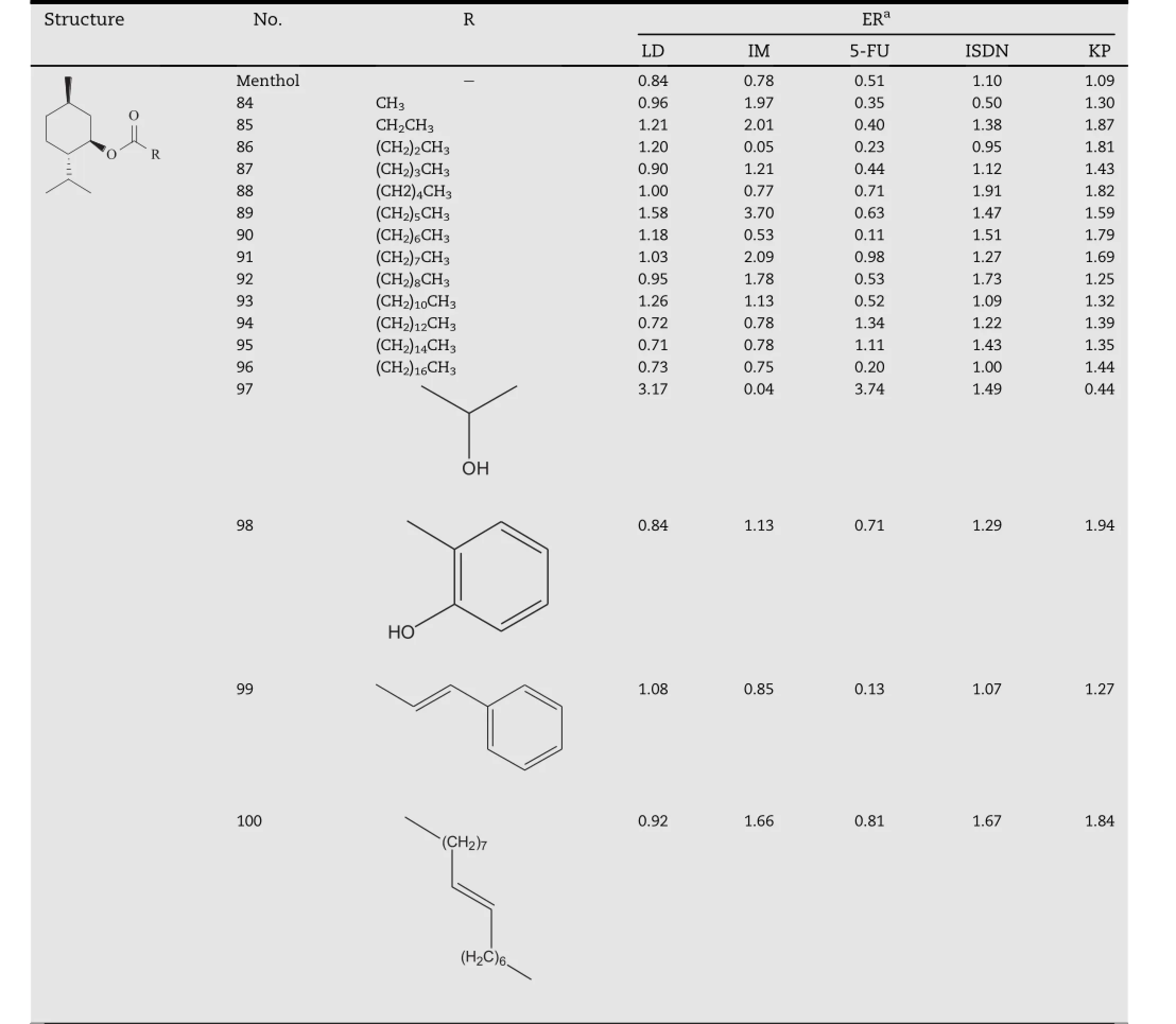
Table 8-Ester derivatives based on l-menthol.a
2.4. Transkarbams
Esters of amino acids were often synthesized as biodegradable permeation enhancers because of their safe and well-known metabolites.Doleˇzal et al.f i rst found that an acyclic Azone analog,i.e.dodecyl-6-aminohexanoate(DDEAC,Fig.1A),had a markedly higher activity and a lower toxicity than Azone[42]. However,it was indicated that the behavior of the enhancer did not correspond to that of an amino ester molecule.DDEAC would react with the carbon dioxide by the free amino group and form another two-chain enhancer with a carbamate salt forming its polar head,termed as Transkarbam 12(T12), which was suggested to be responsible for the enhancing effect[43].T12 has been demonstrated to be an effective and biodegradable skin permeation enhancer.In addition,lots of efforts have been made to explore its structure-activity relationshipby structural modif i cation atits threemolecular sites, which was illustrated in Fig.1B.
2.4.1. Carbamate salt
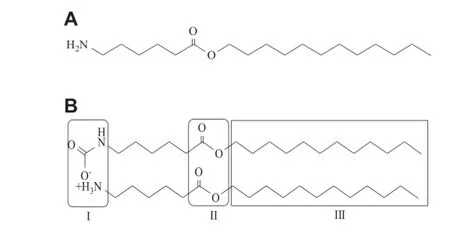
Fig.1-Chemical structures of DDEAC(A)and T12(B).Structural modif i cations of T12 were carried out mainly at three molecular sites as indicated in its chemical structure.
The authors believed that the carbamate salt(Part I)in the polar head of T12 played an essential role in its transdermal enhancement activity.To examine this hypothesis,a series of T12 derivatives with different polar heads(Table 9)were synthesized and studied,including the analogs with carbonic and carbamic acid esters in the polar heads,which were shown by compound 101-114[44].The results showed that although in aqueous suspension some of them were slightly more effective than T12;however,none of them could be comparable to T12 neither in PG/W nor IPM systems.Therefore,it was clear that the transdermal enhancing ability of T12 was closely related to the carbamate group,i.e.carbamic acid salt.A further study revealed that the possible mechanisms for T12 action could be that CO2was released from the polar moiety under the mildly acidic condition of the skin,causing the disruption of the highly ordered lipid lamellae.Additionally, its degradation product, protonated dodecyl-6-aminohexanoate(DDEAC)was also an active enhancer[45].
2.4.2. Ester linkage
The modif i cations of ester bonds(Part II)in the molecule of T12 were also taken into consideration and the corresponding carbonate(compound 115-120),carbamate(compound 121-130),ketone(compound 131-132),amide(compound 133-140),alkane(compound 141)analogs were synthesized, which were shown in Table 10[46,47].In IPM suspension, analogs of amides(compound 133-140)and compound 119 were comparable to that of T12.However,in PG/W vehicle,the activity of T12 was much better than all the synthesized compounds.The best chain length was further investigated among the amide analogs.As expected,a parabolic relationship was obtained with maximum at nonyl derivative(compound 137)in both vehicles and branching of the hydrocarbon chain decreased the activity dramatically.Based on these observations,it was found that ester bonds in T12 were indispensible for its enhancement effect.
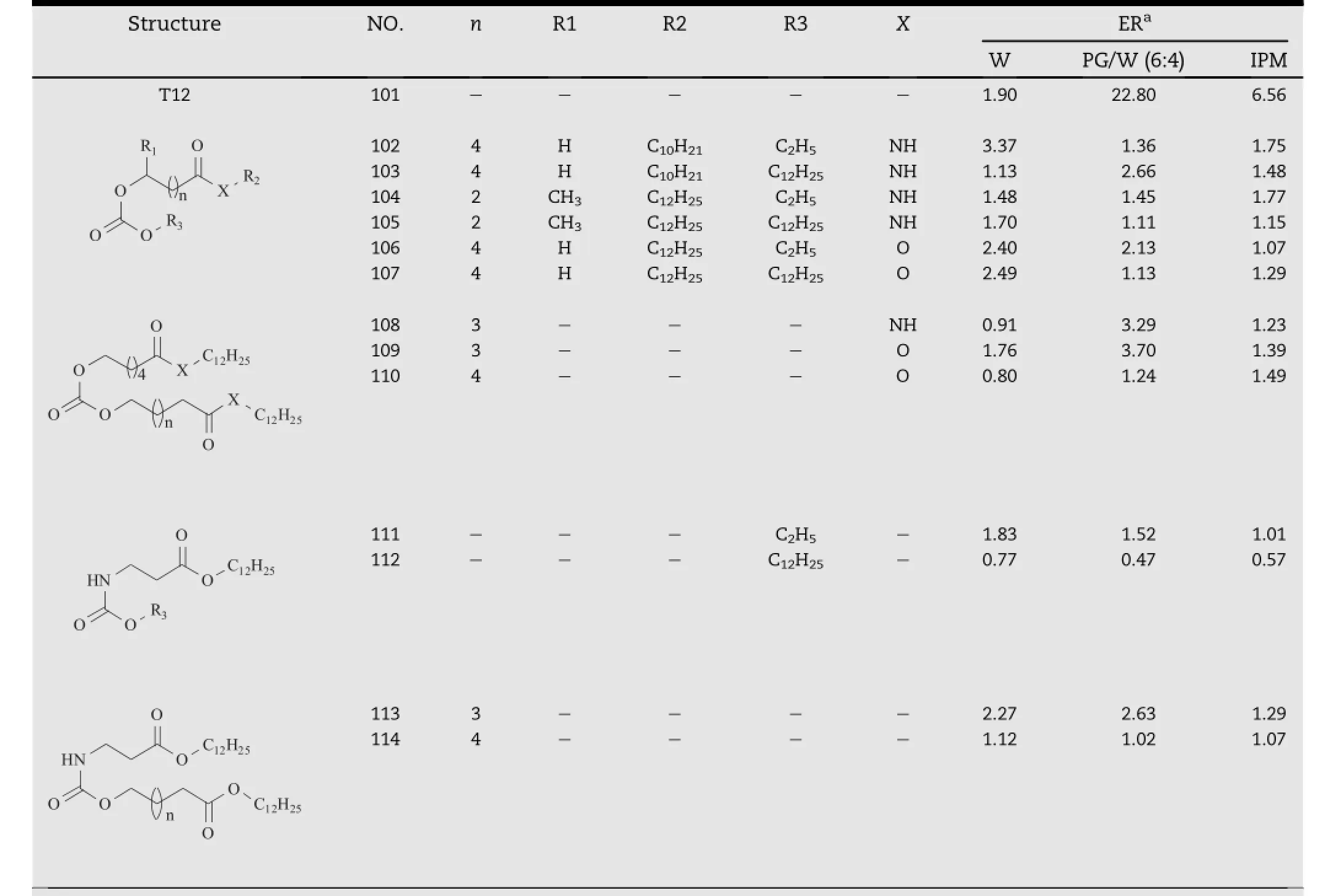
Table 9-T12 analogs based on carbonic and carbamic acid esters.a
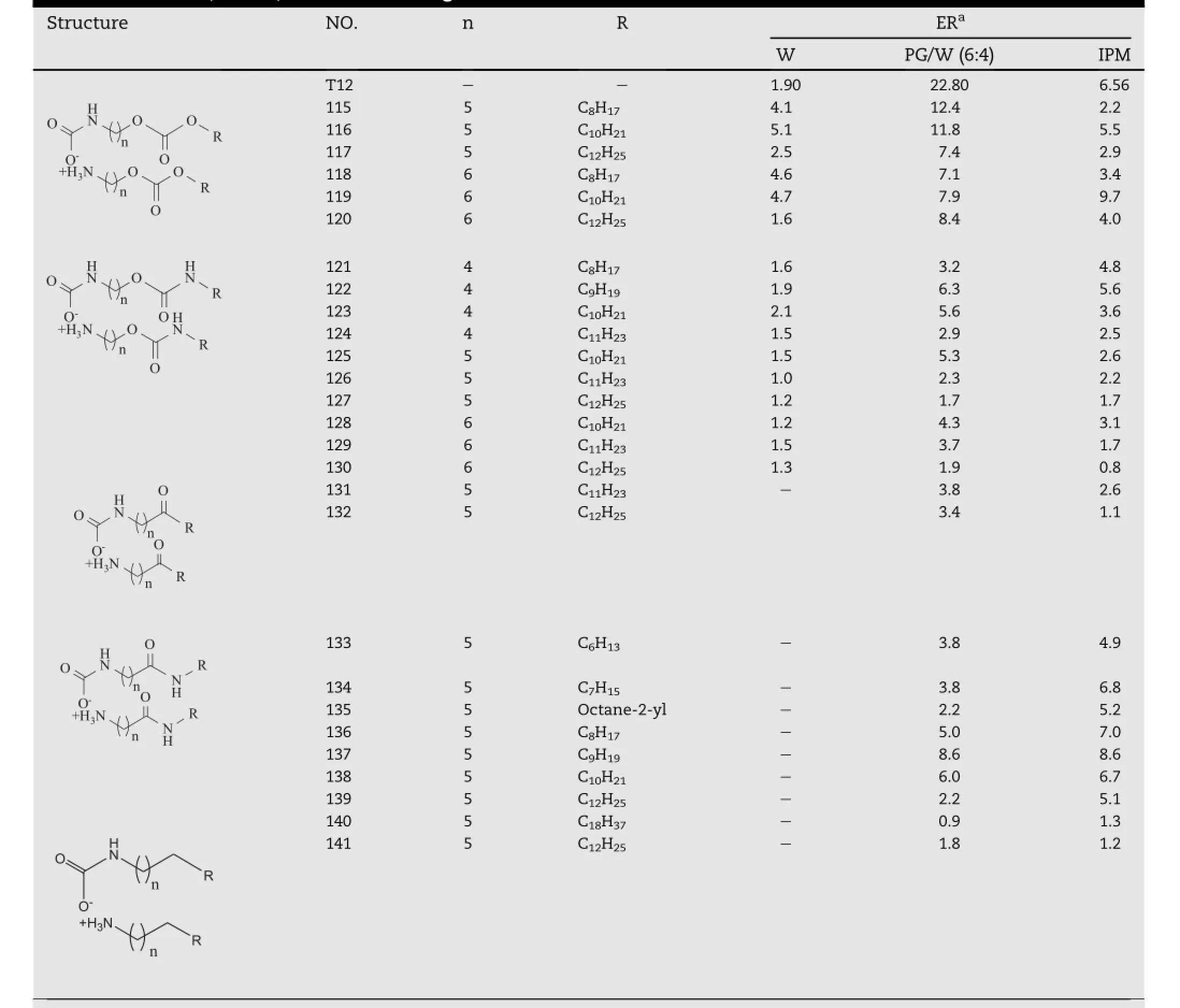
Table 10-Ketone,amide,and alkane analogs of T12.a

Table 11-T12 isomers with varying ester positions.a
In addition,a series of T12 isomers with varying ester positions(compound 142-149,Table 11)were studied on their enhancing activity in a latest investigation[48].The result suggested that the activity of these derivatives highly depended on the positions of the ester bonds,and the optimum performance was observed when the distance of the ester group from the carbamate polar head reached 6-7 carbons (compound 146-147),permitting no longer or shorter.An intramolecular hydrogen bond between the ester carbonyl and N-H of transkarbams might lead to the formation of a pseudo cyclic head similar to that of Azone,which would be one possible explanation of this result[49].The study also suggested that the ammonium salt(ammonium chlorides) formed upon decomposition of the carbamate was also an active enhancer species(compound 150-157,Table 12), though not as effective as the corresponding Transkarbams.
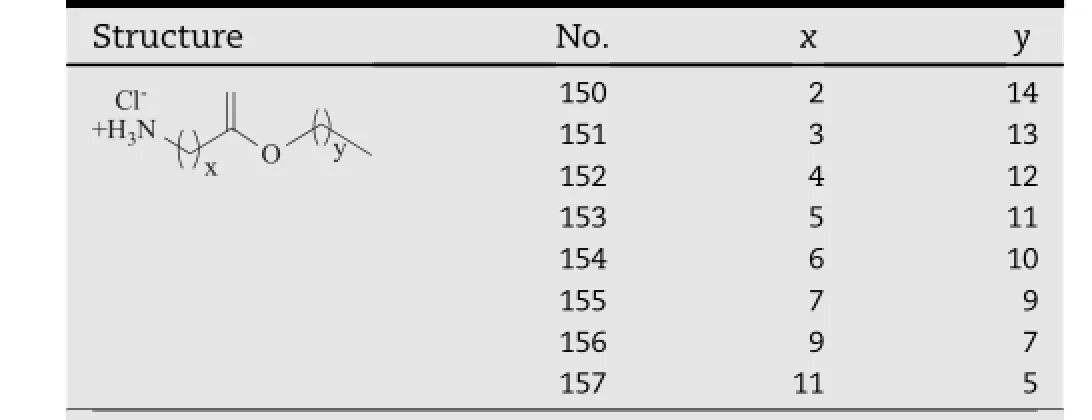
Table 12-Ammonium chloride analogs of T12.a
2.4.3. Hydrophobic tails
Transkarbamswithterminalmethylorethylbranchingin Part III(compound 153-169,Table 13)were also prepared and their promoting activity was evaluated by comparison with that of their linear analogs(compound 158-162)[50].Both of these two kinds of transkarbams exhibited a parabolic relationship between the chain length and the permeation enhancingactivity.Thebestenhancementeffectwas observed at around 10 carbons in the alkanol moiety in both series,namely compound 160 and 165.No signif i cant difference could be observed between the linear group and the methyl-branched group with the same chain length,such as compound 161 and 166.In the case of the ethyl-branched analogs,the activity of compound 169 did not change significantly by comparison with its linear analog(compound 162), however,compound 168 showed a lower activity than its linear analog,compound 160.
2.5. TXA Derivatives
Tranexamic acid(TXA)is known to improve the skin barrier homeostasis as well as the skin conditionas a whole.Basedon the studies of T12,a series of TXA derivatives(compound170-173,Table 14)were synthesized as permeation enhancers,which were expected to be metabolized into TXA in viable epidermis,and hereby restore the skin barrier function [51].The structure of TXA is similar to 6-aminohexanoic acid, which constitutes the polar head of T12.The only difference between transkarbams and TXA derivatives is that TXA possesses a cyclohexane ring instead of the aliphatic chain.
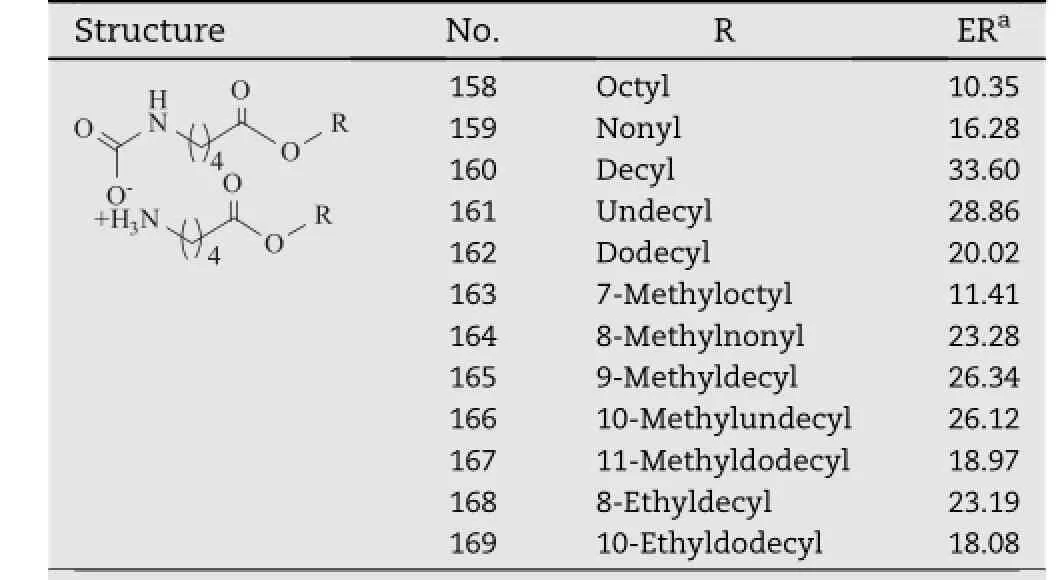
Table 13-Transkarbams analogs with terminal methyl or ethyl branching.a
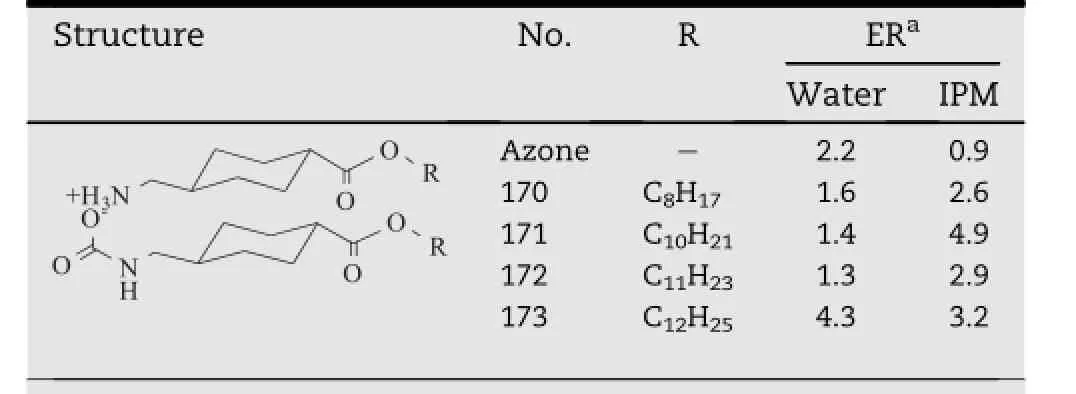
Table 14-TXA derivatives with different hydrocarbon chain.a
TX12(compound 173)showed an excellent activity both in aqueous and IPM donor vehicle,which was almost two times higher than that of Azone.However,as a result of its relative rigidity of the cyclohexane cycle and energetically unfavorable change of the conformation,its activity was still lower than T12.Degradation of the carbamate salt of TX12 was verif i ed in a slightly acidic environment by FTIR.Enzymolysis of the pertinent ester bond by porcine esterase was monitored by TLC and HPLC for qualitative and quantitative investigation,proving the release of TXA in the skin.In consequence,easier and faster recovery of the skin would be expected as a result of a favorable inf l uence of TXA on skin repair process.
2.6. DDAIP And DDAK
DDAIP(dodecyl 2-(dimethylamino)propanoate,compound 174,Table 15),was a potent and biodegradable enhancer that had excellent transdermal enhancing activity towards severaltypes of drugs,including indomethacin,clonidine,hydrocortisone and adefovir[52-55].DDAK(compound 178,Table 15), was synthesized by combination of the ionizable dimethylamino polar head from DDAIP and 5-carbon linking group from T12[56,57].The only difference between DDAK and DDAIP lies in the linking group,in which DDAK has a longer chain length of f i ve carbon atoms while DDAIP has a branched linking group.To compare these two compounds in detail,four model drugs,including theophylline,hydrocortisone,adefovir and indomethacin were selected to evaluate the activity of the enhancers.The results revealed that DDAK exhibited signif icantly better activity than DDAIP for theophylline,hydrocortisoneandadefovir,whileDDAIPwasonlybetterfor indomethacin.Subsequently the effect of different linking chain length was studied with compound 175-179,which werealso shownin Table 15.An optimumlinking chain length (n=4-6)was suggested to play a signif i cant role in the enhancing activity of DDAK and its derivatives.By comparison of DDAIP and its linear isomer(compound 175,Table 15), it was found that the branching linking group played a negative effect on the activity of DDAIP[53].DDAK analogs with different alky chains were also prepared(compound 178 and 180-183,Table 15)and their promoting effect on theophylline was studied.The most potent enhancement effect was provided by compound 183.The relationship between the chain length and the enhancement effect has a bell-like shape[58].
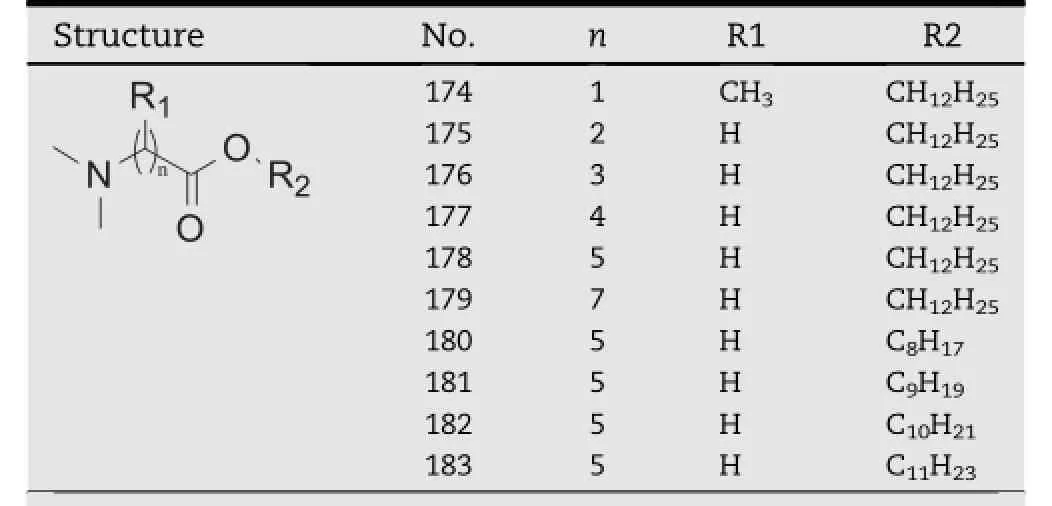
Table 15-DDAK and its derivatives.a
2.7. Natural essential oils
Currently,considerable research is now in progress on the use of natural essential oils as potential permeation enhancers to improvethe drugpermeationthroughthe skin[59].Up to date, many natural oils,such as niaouli oil[60],eucalyptus oil[61], peppermint oil[62],chuanxiong oil[63],turpentine oil[64], chlorophytum borivilianum oil[65],sweet basil oil[66]and so on,have all been demonstrated their potential ability in transdermal permeation enhancement for a number of hydrophilic and lipophilic drugs.
Natural oils from plants are mixtures of many diverse and unique chemical compounds.Their constitutions are complex and variable,depending on the growing place,season and extraction process of the origin plants.Therefore,how to clarify the action of natural oils remains a great challenge for the researchers in the f i eld of natural skin permeation enhancers.Gradual separation might provide a promising method to elucidate the individual effect of each component in the natural oils[67].Additionally,this work would also contribute to the formulation of certain multi-component mixturesactingmoreeff i cientlyandreproduciblywith respect to the natural essential oils[60].Mechanistic study revealed that natural essential oils,such as tulsi oil,eucalyptus oil,clove oil and black cumin oil increased the percutaneous absorption of carvedilol by extraction of lipids from SC as well as by loosening the hydrogen bonds between ceramides subsequently leading to f l uidization of the lipid bilayers[61].In addition,further research is still desirable to scale up the natural oils for transdermal enhancement use and implement manufacturing of f i nal dosage forms on commercial scale[68].
3. Mechanisms of permeation enhancement and selection of an enhancer
The mechanisms of action of permeation enhancers are complex.According to the lipid protein partitioning(LPP) theoryproposed by Barry,enhancerswould act by one or more of the three main mechanisms:(a)disruption of the highly ordered structure of the lipids in SC,(b)interaction with the epidermal keratin and(c)improvement in partitioning of the drug into the skin[69].Actions on the intercellular keratin might result in the denaturation of the protein,which was an irreversible biological process[5].Enhancers targeting the keratin in SC would also lead to great irritation to the skin[70]. Therefore,the skin irritation of a permeation enhancer would likely be more reversible if it only interacted with the intercellular lipid of the SC.In terms of lipid disruptors,they often fall into two categories:ones that exact the lipids from the SC, labeled “extractors,” and ones that partition into the SC lipid bilayers exhibiting a f l uidizing effect on the lipid bilayers, labeled “f l uidizers”.Generally,the lipid f l uidizers regularly outperform the lipid extractors for their little effect on the normal constitution of the SC.Karande et al.introduced two equations(1)and(2)to assess the safety and eff i cacy of the lipid f l uidizers and the lipid extractors[4].In the equations, the values of ER and IP respectively represent the enhancement ratio and irritation potential of a CPE,and their ratio indicates the quality to be an ideal permeation enhancer. Obviously,for the lipid extractors,ER/IP is bound by a theoretical limit of one.However,no theoretical upper limit is evident in the case of the lipid f l uidizers,which indicated their eff i cacy and safety as potential transdermal permeation enhancers.
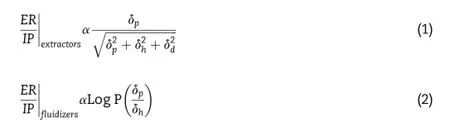
Amphiphilicity was an important determinant for the enhancing activity of the lipid f l uidizers.In general,most of the above mentioned permeation enhancers have the similar structures with the SC lipids,i.e.containing a polar head and one or two hydrophobic chains.The long chains of the amphiphilic enhancers enable themselves to be inserted into the intercellular lipids of the SC,and their polar heads could interact with the polar lipid region via H-bonds and Van der Waals force.The dual effects would effectively disturb the orderly organization of the lipid packing,thus increasing the diffusion of drugs with low selectivity[71].From the analysis of the above listed materials,there is usually an optimum length of the hydrocarbon chain(Cn≥ 7)that is neither branching nor cyclization to obtain the best promoting activity.Alternatively,the changes of polar head also affect the enhancing activity,likely by modifying its ability of H-bonding with the intercellular lipids.
Although LPP theory suggested a general scheme for the mechanismsofpermeationenhancement,itcouldnot explain the specif i c effectiveness of an enhancer towards oneor a certain kind of drugs.It is likely that the precise enhancer activity will depend on the physicochemical properties of the enhancer as well as the penetrant,and the possible complex formation between drugs and enhancers might be of particular importance in disruption of the transdermal barrier.For example,Drakuli′c et al.has introduced a modeling method to prove the formation of complexes between different drugs and terpenes,and indicated their different behavior in the process of transdermal permeation[72].
Most of the current studies have been aimed at gaining better insights into the relationship between the structures of the enhancersand theireffectivenessin transdermal enhancement.However,it has been conf i rmed that thereis no evident correlation between the permeation enhancement effect and the skin irritation[73].Studies of structureirritation relationship and mechanisms of the irritation of the enhancer are important as well[74-76].
4. Conclusion
The paper has given an overview of the investigations on the design of novel permeation enhancers for transdermal drug delivery systems.The list of these materials in the paper is clearly not exhaustive,but represents the general strategies involving in the identif i cation of agents that can facilitate drug delivery through the skin barrier.At the same time further studies are also needed in the areas of skin irritation and acting mechanisms of the skin permeation enhancers.
Acknowledgment
This work was supported by National Natural Science Foundation of China(No:30973654 and No:81173007).
R E F E R E N C E S
[1]Hadgraft J.Skin deep.Eur J Pharm Biopharm 2004;58:291-299.
[2]Karande P,Mitragotri S.Enhancement of transdermal drug delivery via synergistic action of chemicals.Biochim Biophys Acta 2009;1788:2362-2373.
[3]Menon GK.New insights into skin structure:scratching the surface.Adv Drug Deliv Rev 2002;54(Suppl.1):S3-17.
[4]Karande P,Jain A,Ergun K,et al.Design principles of chemical penetration enhancers for transdermal drug delivery.Proc Natl Acad Sci USA 2005;102:4688-4693.
[5]Williams AC,Barry BW.Penetration enhancers.Adv Drug Deliv Rev 2004;56:603-618.
[6]Walker RB,Smith EW.The role of percutaneous penetration enhancers.Adv Drug Deliv Rev 1996;18:295-301.
[7]Sinha VR,Kaur MP.Permeation enhancers for transdermal drug delivery.Drug Dev Ind Pharm 2000;26:1131-1140.
[8]Pathan IB,Setty CM.Chemical penetration enhancers for transdermal drug delivery systems.Trop J Pharm Res 2009;8:173-179.
[9]Narishetty ST,Panchagnula R.Effect of l-menthol and 1,8-cineole on phase behavior and molecular organization of SC lipids and skin permeation of zidovudine.J Control Release 2005;102:59-70.
[10]Wertz PW,van den Bergh B.The physical,chemical and functional properties of lipids in the skin and other biological barriers.Chem Phys Lipids 1998;91:85-96.
[11]Obata Y,Utsumi S,Watanabe H,et al.Infrared spectroscopic study of lipid interaction in stratum corneum treated with transdermal absorption enhancers.Int J Pharm 2010;389:18-23.
[12]Hatta I,Nakazawa H,Obata Y,et al.Novel method to observe subtle structural modulation of stratum corneum on applying chemical agents.Chem Phys Lipids 2010;163:381-389.
[13]Va´vrova´K,Hraba´lek A,Dolezal P,et al.l-Serine and glycine based ceramide analogues as transdermal permeation enhancers:polar head size and hydrogen bonding.Bioorg Med Chem Lett 2003;13:2351-2353.
[14]Va´vrova´K,Hraba´lek A,Dolezal P,et al.Synthetic ceramide analogues as skin permeation enhancers:structure-activity relationships.Bioorg Med Chem 2003;11:5381-5390.
[15]Ahad A,Aqil M,Kohli K,et al.Chemical penetration enhancers:a patent review.Expert Opin Ther Pat 2009;19:969-988.
[16]Jampilek J,Brychtova K.Azone analogues:classif i cation, design,and transdermal penetration principles.Med Res Rev 2012;32:907-947.
[17]Hoogstraate AJ,Verhoef J,Brussee J,et al.Kinetics, ultrastructural aspects and molecular modelling of transdermal peptide f l ux enhancement by N-alkylazacycloheptanones.Int J Pharm 1991;76:37-47.
[18]Han SK,Park YH,Kim CK.Preparation of N-adamantyl nalkanamides and evaluation of their transdermal penetration in the rabbit.Int J Pharm 1995;126:35-40.
[19]Michniak BB,Player MR,Sowell JW.Synthesis and in vitro transdermal penetration enhancing activity of lactam N-acetic acid esters.J Pharm Sci 1996;85:150-154.
[20]Tenjarla SN,Kasina R,Puranajoti P,et al.Synthesis and evaluation of N-acetylprolinate esters-novel skin penetration enhancers.Int J Pharm 1999;192:147-158.
[21]Yoneto K,Ghanem AH,Higuchi WI,et al.A mechanistic study of the effects of the 1-Alkyl-2-pyrrolidones on bilayer permeability of stratum corneum lipid liposomes:a comparison with hairless mouse skin studies.J Pharm Sci 1995;84:312-317.
[22]Michniak BB,Player MR,Godwin DA,et al.In vitro evaluation of azone analogs as dermal penetration enhancers:V. Miscellaneous compounds.Int J Pharm 1998;161:169-178.
[23]Hadgraft J,Peck J,Williams DG,et al.Mechanisms of action of skin penetration enhancers/retarders:azone and analogues.Int J Pharm 1996;141:17-25.
[24]Michniak BB,Player MR,Godwin DA,et al.In vitro evaluation of a series of azone analogs as dermal penetration enhancers:IV.Amines.Int J Pharm 1995;116:201-209.
[25]Aqil M,Ahad A,Sultana Y,et al.Status of terpenes as skin penetration enhancers.Drug Discov Today 2007;12:1061-1067.
[26]Vaddi HK,Ho PC,Chan SY.Terpenes in propylene glycol as skin-penetration enhancers:permeation and partition of haloperidol,Fourier transform infrared spectroscopy,and differential scanning calorimetry.J Pharm Sci 2002;91:1639-1651.
[27]Watanabe H,Obata Y,Ishida K,et al.Effect of l-menthol on the thermotropic behavior of ceramide 2/cholesterol mixtures as a model for the intercellular lipids in stratum corneum.Colloids Surf B Biointerfaces 2009;73:116-121.
[28]Obata Y,Hatta I,Ohta N,et al.Combined effects of ethanol and l-menthol on hairless rat stratum corneum investigated by synchrotron X-ray diffraction.J Control Release 2006;115:275-279.
[29]Fujii M,Takeda Y,Yoshida M,et al.Comparison of skin permeation enhancement by 3-l-menthoxypropane-1,2-diol and l-menthol:the permeation of indomethacin and antipyrine through Yucatan micropig skin and changes in infrared spectra and X-ray diffraction patterns of stratum corneum.Int J Pharm 2003;258:217-223.
[30]Negishi J,Takayama K,Higashiyama K,et al.Promoting effect of O-alkylmenthol and O-acylmenthol derivatives on the percutaneous absorption of ketoprofen in rats.STP Pharm Sci 1995;5:156-161.
[31]Nakamura Y,Takayama K,Higashiyama K,et al.Promoting effect of O-ethylmenthol on the percutaneous absorption of ketoprofen.Int J Pharm 1996;145:29-36.
[32]Takahara J,Takayama K,Isowa K,et al.Multi-objective simultaneous optimization based on artif i cial neural network in a ketoprofen hydrogel formula containing O-ethylmenthol as a percutaneous absorption enhancer.Int J Pharm 1997;158:203-210.
[33]Takayama K,Takahara J,Fujikawa M,et al.Formula optimization based on artif i cial neural networks in transdermal drug delivery.J Control Release 1999;62:161-170.
[34]Obata Y,Sato H,Li CJ,et al.Effect of synthesized cyclohexanol derivatives using l-menthol as a lead compound on the percutaneous absorption of ketoprofen. Int J Pharm 2000;198:191-200.
[35]Chao JL,Obata Y,Higashiyama K,et al.Effect of 1-O-ethyl-3-butylcyclohexanol on the skin permeation of drugs with different physicochemical characteristics.Int J Pharm 2003;259:193-198.
[36]Takanashi Y,Higashiyama K,Komiya H,et al.Thiomenthol derivatives as novel percutaneous absorption enhancers. Drug Dev Ind Pharm 1999;25:89-94.
[37]Zhao L,Fang L,Xu Y,et al.Transdermal delivery of penetrants with differing lipophilicities using O-acylmenthol derivatives as penetration enhancers.Eur J Pharm Biopharm 2007;69:199-213.
[38]Zhao L,Fang L,Xu Y,et al.Effect of O-acylmenthol on transdermal delivery of drugs with different lipophilicity.Int J Pharm 2008;352:92-103.
[39]Zhao L,Li Y,Fang L,et al.Transdermal delivery of tolterodine by O-acylmenthol:in vitro/in vivo correlation.Int J Pharm 2009;374:73-81.
[40]Wen Z,Fang L,He Z.Effect of chemical enhancers on percutaneous absorption of daphnetin in isopropyl myristate vehicle across rat skin in vitro.Drug Deliv 2009;16:214-223.
[41]Zhao N,Cun D,Li W,et al.In vitro percutaneous absorption enhancement of granisetron by chemical penetration enhancers.Drug Dev Ind Pharm 2013;39:561-568.
[42]Doleˇzal P,Hraba´lek A,Semecky´V. ε-Aminocaproic acid esters as transdermal penetration enhancing agents.Pharm Res 1993;10:1015-1019.
[43]Hraba´lek A,Dolezal P,Va´vrova´K,et al.Synthesis and enhancing effect of transkarbam 12 on the transdermal delivery of theophylline,clotrimazole,f l obufen,and griseofulvin.Pharm Res 2006;23:912-919.
[44]Klimentova´J,Hraba´lek A,Va´vrova´K,et al.Synthesis and transdermal penetration-enhancing activity of carbonic and carbamic acid esters-Comparison with transkarbam 12. Bioorg Med Chem Lett 2006;16:1981-1984.
[45]Novotny´M,Klimentova´J,Jan°uˇsova´B,et al.Ammonium carbamates as highly active transdermal permeation enhancers with a dual mechanism of action.J Control Release 2011;150:164-170.
[46]Holas T,Va´vrova´K,Klimentova´J,et al.Synthesis and transdermal permeation-enhancing activity of ketone, amide,and alkane analogs of transkarbam 12.Bioorg Med Chem 2006;14:2896-2903.
[47]Holas T,Va´vrova´K,Sı´ma M,et al.Synthesis and transdermal permeation-enhancing activity of carbonate and carbamate analogs of transkarbam 12.Bioorg Med Chem 2006;14:7671-7680.
[48]Novotny´M,Hraba´lek A,Jan°usova´B,et al.Transkarbams as transdermal permeation enhancers:effects of ester position and ammonium carbamate formation.Bioorg Med Chem Lett 2010;20:2726-2728.
[49]Dell’Amico DB,Calderazzo F,Labella L,et al.Converting carbon dioxide into carbamato derivatives.Chem Rev 2003;103:3857-3897.
[50]Klimentova´J,Kosa´k P,Va´vrova´K,et al.Transkarbams with terminal branching as transdermal permeation enhancers. Bioorg Med Chem Lett 2008;18:1712-1715.
[51]Va´vrova´K,Hraba´lek A,Dolezal P,et al.Biodegradable derivatives of tranexamic acid as transdermal permeation enhancers.J Control Release 2005;104:41-49.
[52]Bu¨yu¨ktimkin S,Bu¨yu¨ktimkin N,Rytting JH.Synthesis and enhancing effect of 2-dodecyl transepidermal(N,N-dimethylamino)propionate(DDAIP)on the delivery of indomethacin,clonidine and hydrocortisone.Pharm Res 1993;10:1632-1637.
[53]Novotny´J,Kovarı´kova´P,Novotny´M,et al.Dimethylamino acid esters as biodegradable and reversible transdermal permeation enhancers:effects of linking chain length, chirality and polyf l uorination.Pharm Res 2009;4:811-821.
[54]Bu¨yu¨ktimkin S,Bu¨yu¨ktimkin N,Rytting JH.Interaction of indomethacin with a new penetration enhancer,dodecyl 2-(NN-dimethylamino)propionate(DDAIP):its effect on transdermal delivery.Int J Pharm 1996;127:245-253.
[55]Fujii M,Bu¨yu¨ktimkin S,Bu¨yu¨ktimkin N,et al.Enhancement of skin permeation of miconazole by phospholipid and dodecyl 2-(N,N-dimethyl amino)propionate(DDAIP).Int J Pharm 2002;234:121-128.
[56]Va´vrova´K,Lorencova´K,Klimentova´J,et al.Transdermal and dermal delivery of adefovir:effects of pH and permeation enhancers.Eur J Pharm Biopharm 2008;69:597-604.
[57]Va´vrova´K,Lorencova´K,Novotny´J,et al.Permeation enhancer dodecyl 6-(dimethylamino)hexanoate increases transdermal and topical delivery of adefovir:inf l uence of pH, ion-pairing and skin species.Eur J Pharm Biopharm 2008;70:901-907.
[58]Hraba´lek A,Dolezal P,Farsa O,et al.Esters of 6-dimethylaminohexanoic acid as skin penetration enhancers. Pharmazie 2000;55:759-761.
[59]Fox LT,Gerber M,Plessis JD,et al.Transdermal drug delivery enhancement by compounds of natural origin.Molecules 2011;16:10507-10540.
[60]Monti D,Tampucci S,Chetoni P,et al.Niaouli oils from different sources:analysis and inf l uence on cutaneous permeation of estradiol in vitro.Drug Deliv 2009;16:237-242.
[61]Amin S,Kohli K,Khar RK,et al.Mechanism of in vitro percutaneous absorption enhancement of carvedilol by penetration enhancers.Pharm Dev Technol 2008;13:533-539.
[62]Nielsen JB.Natural oils affect the human skin integrity and the percutaneous penetration of benzoic acid dosedependently.Basic Clin Pharmacol Toxicol 2006;98:575-581.
[63]Zhang LC,Hu JH,Li L,et al.In vivo and in vitro evaluation of essential oils from Ligusticum chuanxiong HORT on the transdermal delivery of f l urbiprofen in rabbits.Biol Pharm Bull 2006;29:1217-1222.
[64]Khan NR,Khan GM,Wahab A,et al.Formulation,and physical,in vitro and ex vivo evaluation of transdermalibuprofen hydrogels containing turpentine oil as penetration enhancer.Pharmazie 2011;66:849-852.
[65]Pawankumar G,Shiradkar M.Evaluation of synergistic effect of Chlorophytum borivilianum extract on transdermal delivery of pramipexole with its mechanism of action.Adv Appl Sci Res 2012;3:261-267.
[66]Fang JY,Leu YL,Hwang TL,et al.Essential oils from sweet basil(Ocimum basilicum)as novel enhancers to accelerate transdermal drug delivery.Biol Pharm Bull 2004;27:1819-1825.
[67]Fang JY,Leu YL.Development of sesquiterpenes from Alpinia oxyphylla as novel skin permeation enhancers.Eur J Pharm Sci 2003;19:253-262.
[68]Goswami DS,Uppal N,Goyal S,et al.Permeation enhancer for TDDS from natural and synthetic sources:a review.J Biomed Pharm Res 2013;2:19-29.
[69]Barry BW.Lipid-Protein-Partitioning theory of skin penetration enhancement.J Control Release 1991;15:237-248.
[70]Ashton P,Walters KA,Brain KR,et al.Surfactant effects in percutaneous absorption II.Effects on protein and lipid structure of the stratum corneum.Int J Pharm 1992;87:265-269.
[71]Va´vrova´K,Zbytovska´J,Hraba´lek A.Amphiphilic transdermal permeation enhancers:structure-activity relationships.Curr Med Chem 2005;12:2273-2291.
[72]Drakuli′c BJ,Jurani′c IO,Eri′c S,et al.Role of complexes formation between drugs and penetration enhancers in transdermal delivery.Int J Pharm 2008;363:40-49.
[73]Kanikkannan N,Singh M.Skin permeation enhancement effect and skin irritation of saturated fatty alcohols.Int J Pharm 2002;248:219-228.
[74]Hayashi M,Nakamura Y,Higashi K,et al.A quantitative structure-activity relationship study of the skin irritation potential of phenols.Toxicol Vitro 1999;13:915-922.
[75]Smith JS,Macina OT,Sussman NB,et al.A robust structureactivity relationship(SAR)model for esters that cause skin irritation in humans.Toxicol Sci 2000;55:215-222.
[76]Welss T,Basketter DA,Schro¨der KR.In vitro skin irritation: facts and future.State of the art review of mechanisms and models.Toxicol Vitro 2004;18:231-243.
*Corresponding author.Tel./fax:+86 24 23986330.
E-mail address:fangliang2003@yahoo.com(L.Fang).
Peer review under responsibility of Shenyang Pharmaceutical University
Production and hosting by Elsevier
1818-0876/$-see front matter © 2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
http://dx.doi.org/10.1016/j.ajps.2014.01.001
Permeation enhancer
Mechanism
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Current prodrug strategies for improving oral absorption of nucleoside analogues
- Application of sialic acid/polysialic acid in the drug delivery systems
- Mesoporous carbon as a carrier for celecoxib:The improved inhibition effect on MDA-MB-231 cells migration and invasion
- Development and evaluation of lafutidine solid dispersion via hot melt extrusion:Investigating drug-polymer miscibility with advanced characterisation
- Determination of azithromycin in raw materials and pharmaceutical formulations by HPLC coupled with an evaporative light scattering detector
- Rapid and sensitive analysis of cyclobenzaprine by LC-MS/MS:Application to a pharmacokinetic study of cyclobenzaprine in dog
