Effect of Dissolved Oxygen on Swimming Ability and Physiological Response to Swimming Fatigue of Whiteleg Shrimp (Litopenaeus vannamei)
2014-04-17DUANYanZHANGXiumeiLIUXuxuandDhanrajsinghThakur
DUAN Yan,ZHANG Xiumei,LIU Xuxuand Dhanrajsingh N.Thakur
1) The Key Laboratory of Mariculture of Chinese Ministry of Education, Ocean University of China,Qingdao 266003,P.R.China
2) Liaoning Open Laboratory of Applied Marine Biotechnology, Liaoning Ocean and Fisheries Science Research Institute,Dalian 116023, P.R.China
1 Introduction
The moving ability is a fundamental property by which we often define animal life (Reidyet al.,2000).Shrimp possesses the swim ability with the help of ten beating pleopods.The swimming ability strongly affects the performance of an aquatic animal of finding a mate and obtaining food (Drucker,1996),and can be evaluated by the swimming endurance and speed (Yuet al.,2009).The vulnerability to capture of aquatic animals is dependent on their swimming endurance in the trawl mouth (Wingeret al.,2000).The swimming ability index (SAI) can be described as a cumulative index of swimming capacity including cruising and burst speed (Tsukamotoet al.,1975).Few studies have been reported on the swimming capacity of penaeid shrimp to date.Yuet al.(2009) and Zhanget al.(2006) have shown that the SAI ofMarsupenaeus japonicusandLitopenaeus vannameiis 32.43 and 7.28 cm,respectively.The influencing factors of swimming endurance include the speed of trawler,body size of shrimp,and temperature and salinity of seawater(Main and Sangster,1981; Wardle,1993; Wingeret al.,2000; Zhanget al.,2007).
When crustaceans swim,total protein serves as a predominant source of metabolic energy,which can be used to provide a rapid,non-destructive mean of assessing the physiological condition of animals (Mooreet al.,2000;Mugnier and Justou,2004).Glucose is a major component of circulating blood carbohydrate in crustaceans(Radfordet al.,2005).The mobilization of glucose is generally accepted as a mean of providing extra metabolic energy resource (Jentoftet al.,2005).Adaptation and strategies in response to significantly high speeds may enhance anaerobic metabolism.For anaerobic gly-colysis,crustaceans utilize only one basic pathway,i.e.,fermentation of glycogen into lactate (Zouet al.,1996).
In natural environments,animals face various stresses,such as episodes of hypoxia (Claireauxet al.,1995; Weiet al.,2008).Coastal fluctuation of oxygen generally follows a seasonal pattern directly relating to diurnal variation (Paschkeet al.,2010).Dissolved oxygen (DO) is one of the most important environmental factors that affect physiological processes of aquatic animals (Chenget al.,2003).Shrimp often encounter changes in DO concentration that require full-scale physiological regulatory mechanisms.In hypoxic environment,most crustacean species may exhibit bradycardia,reduction in the metabolic rate(Harper and Reiber,1999),lactate accumulation (Hillet al.,1991b; Lallier and Walsh,1992; Hervantet al.,1999),and modification of the hemolymph acid-base balance,hemocyanin binding capacity,and oxyhemocyanin-protein relationship,thereby limiting the amount of energy consumed by other systems (Paschkeet al.,2010).These findings imply that the physiological regulatory mechanisms of crustaceans in response to different DO concentrations are the limiting factors of their physiological status and may affect their swimming performance.
The effect of environmental factors on the swimming capacity of aquatic animals can be used to describe the optimum habitat for shrimp (Yuet al.,2010).Despite several studies regarding the effect of DO on the swimming performance of fish,the majority of those works have focused on the effect of hypoxia (Daviset al.,1963;Lagardèreet al.,1995; Lefrancoiset al.,2007).To date,no study has been reported on the effect of DO on the swimming performance and physiological response of whiteleg shrimp,L.vannamei.L.vannameiis one of the major commercial shrimp species for fisheries and aquaculture production,with a wide distribution from northern Mexico to northern Peru (Perez-Farfante and Kensley,1997).Its life cycle consists of three stages,i.e.,planktonic larval stage in the ocean,postlarva-to-juvenile stage in the estuary,and adult stage back to the marine environment.The present study aimed to determine the effect of DO on the swimming endurance and physiological response of whiteleg shrimp.Results will be useful for assessing the exercise ability and determining the optimum habitat of penaeid shrimps.
2 Materials and Methods
2.1 Experimental Animals
L.vannameiobtained from a local farm (Shazikou,Qingdao,China) were kept in a 2 m3recirculating tanks for 10 d.The shrimp were fed twice a day with pellets during acclimation (Haima,Fuzhou,China),and fasted for 12 h before the initiation of the experiment (Zhanget al.,2006).Water temperature was maintained at 25.0℃ ±0.5℃ with salinity about 31.0 ± 1.0 and DO > 6.0 mg L−1.Shrimp at the intermolting stage were distinguished by the exoskeleton hardness (Chenget al.,2002) and used for the experiment.The body length and wet mass of experimental shrimp are shown in Table 1.
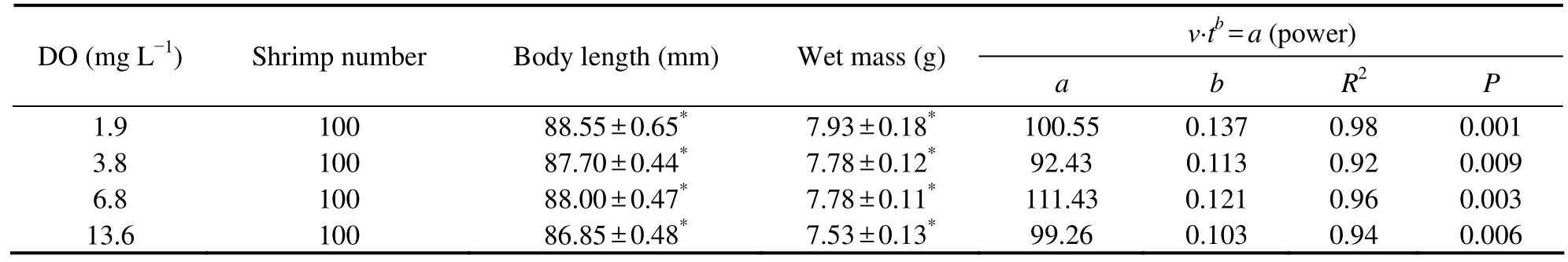
Table 1 Equations and R2 values of the regressions of swimming endurance (t) and swimming speed (ν) of L.vannamei exposed to different dissolved oxygen concentrations (means ± S.E.)
2.2 Experimental Design
A total of 480 shrimp were divided equally into 12 rectangular transparent plastic tanks (50 cm × 30 cm × 50 cm)with 4 different DO concentrations (1.9,3.8,6.8,and 13.6 mg L−1).The shrimp were slowly acclimated with increments or decrements of approximately 1–2 mg DO L−1d−1.Water was changed with filtered (1 μm),ultraviolet-sterilized seawater at a daily rate of 5% of the tank volume.Pure oxygen,nitrogen,and air at an appropriate rate were used to obtain the seawater DO at different experimental levels.The actual DO level was measured twice a day at 6:00 and 18:00 using an YSI-55 oxygen detector (YSI Incorporated Yellow Springs,Ohio USA).Additional DO measurements were made according to the weather.The experimental DO concentrations were controlled at 1.9 ±0.3,3.8 ± 0.4,6.8 ± 0.7 and 13.6 ± 2.1 mg L−1.
After 5 d acclimation,100 shrimps were selected each DO treatment for swimming endurance experiment in thermostatically controlled circulating flumes.Each circulating flume was given a known velocity,similar to those described by Yuet al.(2009) (Fig.1).Water conditions in the flume (DO concentration,temperature,and salinity) were approximately the same as those in the tank from which the shrimp were taken.
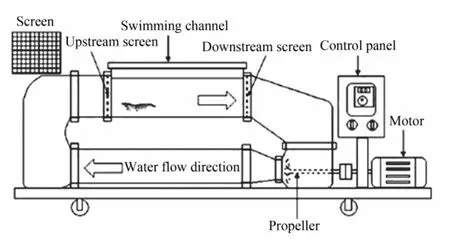
Fig.1 Diagram of the circulating flume (Yu et al.,2009).
The velocities for the test were chosen based on pilot trials (Table 2).Twenty shrimp were used to test the swim endurance at each current velocity.The recorded endurance time of all shrimp was < 9000 s (Zhanget al.,2006).The shrimp were unfed during the experimental period.The SAI,defined as


Table 2 The velocities in swimming flume with different dissolved oxygen concentrations (means ± S.E.)
During each trial,2–4 shrimp were trained for 10 min.The speed was set relatively low at the beginning and gradually increased to the target speed within 1 min.The shrimp were sometimes unable to swim ahead of the flume and the individuals were shifted to the front of the flume using a small net.After three consecutive shifts,the shrimp was considered to be ‘fatigued’ and removed to weigh the body mass.The endurance time was recorded.
2.3 Tissues Collection and Biochemical Assessment
Two-hundred microliter of hemolymph was withdrawn from the ventral sinus using a 1 mL syringe containing an equal volume of anticoagulant solution (450 mmol L−1NaCl,10 mmol L−1KCl,10 mmol L−1Na2-EDTA,10 mmol L−1HEPES,pH 7.3) (Vargas-Alboreset al.,1993).The hemolymph was centrifuged at 200 ×gand 4℃ for 10 min,and the obtained plasma was stored at −34℃ for 24 h.The hepatopancreas and pleopods muscle of shrimp were dissected,and an aliquot (0.1 g) was homogenized in 0.9 mL of isotonic saline solution (450 mmol L−1NaCl,10 mmol L−1KCl) with a mechanical homogenizer.The obtained crude extract was stored at −34℃ for 24 h and then centrifuged at 3000 r min−1and 4℃ for 10 min.The supernatant was used for biochemical analysis.The contents of glycogen,total protein,and lactate were determined using commercial kits (20101009,Nanjing Jiancheng Bioengineering Institute,China).Total protein concentration of the shrimp was determined using the Biuret Protein Assay kit with bovine serum albumin as the standard and was expressed as mg mL−1or mg g−1.The lactate concentration was determined spectrophotometrically using 20 μL of homogenate and was expressed as mmol L−1or mmol (g prot)−1.The glycogen level was quantified by the anthrone-sulfuric acid method.The plasma glucose level was measured with a commercial kit for clinical diagnosis (GOD-PAD,3400196,Zhongsheng Beikong Bio-Technology and Science Inc.,Beijing,China) using 10 μL of plasma samples and was expressed as mmol L−1of animal plasma.The triglyceride level was measured with a commercial kit for clinical diagnosis (GPO-PAP,006304,BHKT Clinical Reagent Co.,Ltd,Beijing,China)using 10 μL of plasma samples and was expressed as mmol L−1of animal plasma.Twenty shrimp with a similar size (pre-exercise samples) were taken from 4 DO treatis proposed to evaluate the swimming capacity ofL.vannamei(Zhanget al.,2006).ments and used as the control.
2.4 Data Analysis
Body mass and body length,as well as the content of tested metabolites in shrimps exposed to different concentrations of DO were compared using one-way analysis of variance (ANOVA),with post hoc examination using Duncan’s tests.The relationship between the swimming endurance and swimming speed as well as that between the SAI and DO concentration were determined using Curve Estimation,and the significance of the regression was tested using ANOVA.Pre- and post-exercise metabolite content in the plasma,muscle and hepatopancreas for each DO concentration were analyzed using one-way ANOVA followed by a Duncan’s multiple range test if necessary.SPSS 13.0 was used for statistical analyses andP< 0.05 was considered statistically significant.
3 Results
3.1 Effect of DO on Swimming Endurance of L.vannamei
As shown in Fig.2,no shrimp swam for the full 9000 s at any of the velocities tested.The swimming endurance of the shrimp decreased as the swimming speed increased at different DO concentrations.The regression can be described by a power model (Table 1).One-way ANOVA analysis showed that the endurance time of the shrimp decreased significantly in all treatments as water speed increased fromv1tov5(P< 0.05) (Fig.2).

Fig.2 Relationship between swimming endurance (t) and swimming speed (v) of L.vannamei exposed to various concentrations of dissolved oxygen.Each value represents the mean ± S.E.(n = 20).Fitted power model is from the Curve Estimation.Values in the same curve with different letters were significantly different (P < 0.05) by ANOVA test.
The SAI ofL.vannameiexposed to different concentrations of DO are shown in Fig.3.The relationship between SAI (cm) and DO (mg L−1) is expressed as follows:SAI = 27.947 DO0.137,R2= 0.9312 (P= 0.035).When the shrimps were maintained well above the air saturation level (about 13.6 mg L−1),the SAI increased from 30.07 cm in hypoxia (about 1.9 mg L−1) to 38.86 cm.

Fig.3 Response curve showing the relationship between swimming ability index (SAI) and dissolved oxygen concentration.
3.2 Physiological Response to Swimming Fatigue
3.2.1 Control
The plasma total protein and lactate content of shrimp exposed to 1.9 mg L−1DO was significantly higher than that of shrimp exposed to higher DO concentrations.The shrimp exposed to 1.9 mg L−1DO contained significantly higher plasma glucose content than those expose to 6.8 and 13.6 mg L−1DO (P< 0.05),with no significant difference among the remaining three treatments (P> 0.05).The plasma triglyceride and pleopods muscle glycogen content of shrimp exposed to 1.9 and 3.8 mg L−1DO were significantly higher than those in other DO treatments.The pleopods muscle lactate content generally decreased with an increasing DO concentration (1.9 mg L−1to 13.6 mg L−1,P< 0.05),with no significant difference between the treatments of 6.8 and 13.6 mg L−1DO (P> 0.05).No statistical difference was observed in the total protein,glycogen,and lactate concentration of the hepatopancreas and pleopods muscle total protein level among the 4 DO treatments (P> 0.05) (Table 3).
3.2.2 Swimming Fatigue
The plasma total protein and hepatopancreas glycogen content were highly depleted in shrimp of the 4 DO treatments due to swimming fatigue (P< 0.05).The decrease of plasma protein and hepatopancreas glycogen content increased and then decreased as the swimming speed rose fromv1tov5at different DO concentrations.In contrast,the plasma lactate accumulated at high levels in shrimp after swimming fatigue (P< 0.05),and the plasma lactate content increased with the velocity in different DO treatments.The effect of swimming fatigue on the plasma triglyceride content showed a significant decrease at the velocities ofv1,v2,andv3with 1.9 mg L−1DO,as well as atv1with 3.8,6.8,and 13.6 mg L−1DO (P< 0.05).Compared to pre-excise samples,the shrimp contained higher plasma glucose contents following swimming fatigue atv2,v3,v4,andv5with 1.9 mg L−1DO,atv3,v4,andv5with 3.8 and 6.8 mg L−1DO,and atv5with 13.6 mg L−1DO.However,the pleopods muscle glycogen content significantly decreased atv1andv2with 3.8 mg L−1DO,atv1with 6.8 mg L−1DO,and atv1,v2,andv4with 13.6 mg L−1DO after swimming fatigue (P< 0.05).In addition,the pleopods muscle lactate content significantly increased atv1,v2,andv3with 1.9 mg L−1DO,atv4andv5with 3.8 mg L−1DO,and atv5with 6.8 mg L−1DO (P< 0.05),whereas no significant change was found in the hepatopancreas lactate and total protein content in the hepatopancreas and pleopods muscle in shrimps of the 4 DO treatments after swimming fatigue at various velocities (P> 0.05)(Table 3).
4 Discussion
4.1 The Effect of DO Concentration on Swimming Endurance of L.vannamei
This study evaluated the capacity of shrimp of tolerating DO in water at two levels of hypoxia.The first (1.9 mg L−1) was established close to the tolerance limit as determined in early experiments (Hall and Van Ham,1998).The second (4 mg L−1) was the lower critical oxygen value commonly recommended for intensive culture practice,which is the minimal oxygen level allowing the shrimp to meet normal energetic requirement without setting up compensatory mechanisms (Seidman and Lawrence,1985; Aquacopet al.,1988; Claireaux and Dutil,1992; Hall and Van Ham,1998; Weiet al.,2008).
The results indicated that the swimming endurance ofL.vannameiwas highly dependent on their swimming speed and the DO concentration in water.The power model relationship proposed in the current study is similar as those found in other studies for shrimp (Zhanget al.,2007,2006; Yuet al.,2009) and several marine species(Bainbridge,1960; Brett,1982; He and Wardle,1988).It is known that the frictional drag increases with the swimming speed (Bone,1975; Schmidt-Nielson,1984;Vogel,1994).Furthermore,the increase in the energetic cost of pleopod beat frequency with swimming speed increase can result in the reduction of swimming endurance.Results from the present study showed that the SAI ofL.vannameiexposed to 6.8 mg L−1DO was 37.97 cm.Yuet al(2009) reported that the SAI ofM.japonicusat 6 mg L−1DO was 32.43 cm,and Zhanget al.(2006) reported that the SAI ofL.vannameiat 6 mg L−1DO was 7.28 cm.The variations in the SAI value could be attributed to the difference in experimental approach,apparatus,species and the range of shrimp body size.
Oxygen can be used to determine the amount of energy directly used in different situations (Rosaset al.,1992).In the present study,the decrease in DO concentration from 13.6 mg L−1to 1.9 mg L−1led to the reduction of the SAI ofL.vannameiby about 22%.This implied that the ability of shrimp to provide sufficient oxygen to their gillsis a limiting factor of their maximum performance (Rosaset al.,1997; Rosaset al.,1998; Rosaset al.,1999).The SAI was markedly impaired at lower DO concentrations(Fig.3) and little improvement was observed in shrimp performance when the DO concentration was well above the air saturation level.The result was consistent with the previously published of other marine species (Daviset al.,1963; Lefrancoiset al.,2007).
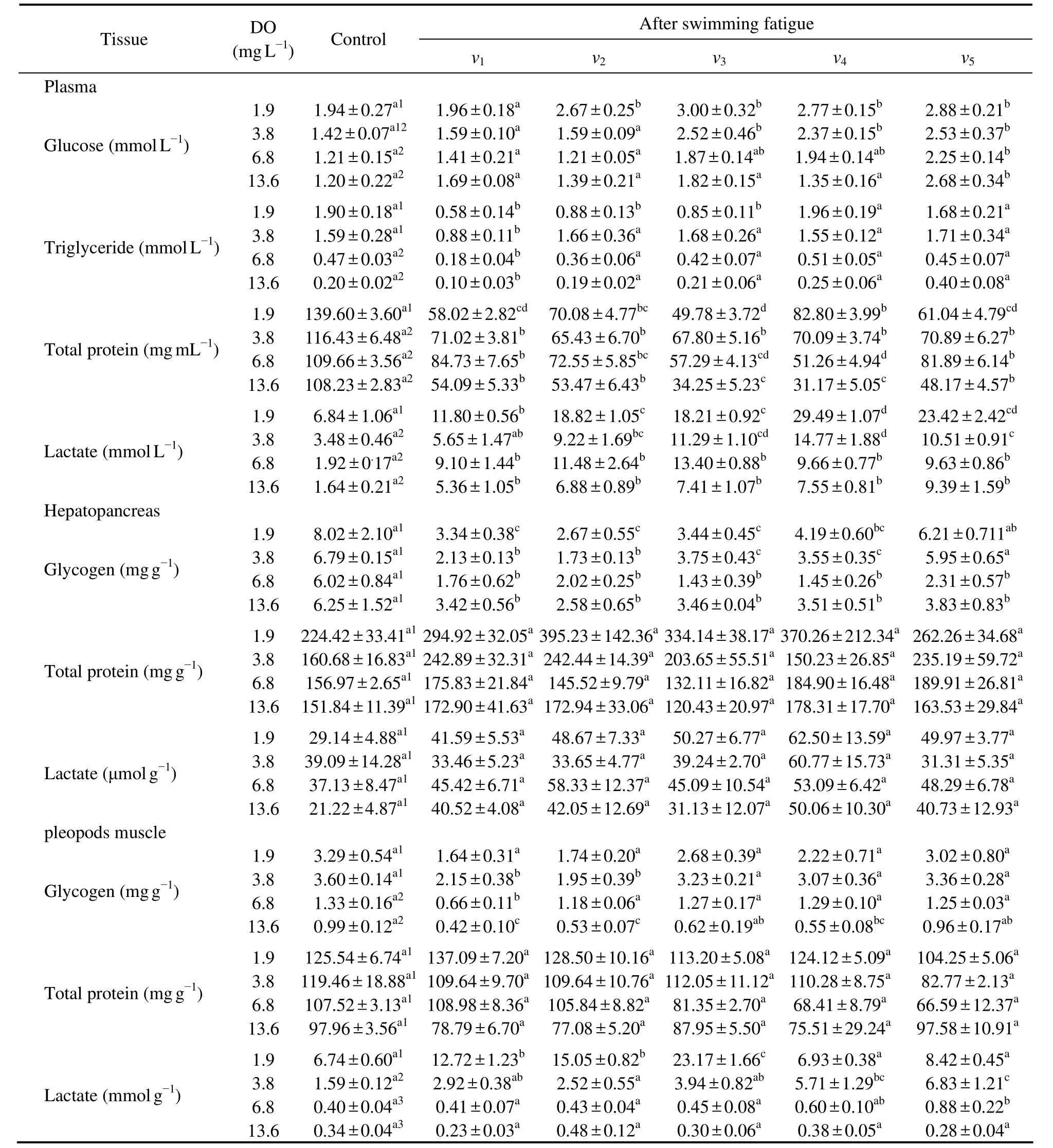
Table 3 Physiological responses in plasma,hepatopancreas,and pleopods muscle of L.vannamei after swimming fatigue at different dissolved oxygen concentrations
Understanding the possible effect of DO on the swimming capacity of aquatic organisms is very useful for environmental quality management.Such an effect is hardly detected in a superficial analysis,but can substantially influence the survival and the success of species and communities (Barbieri,2007).A simplest interpretation of these results is that the metabolic cost of both cardiac and branchial pump tend to restrict the swimming of shrimp(Jones,1971).Moreover,the tachycardia observed inGadus morhuaduring exercise has been found to be more pronounced in hypoxic water (Claireauxet al.,1995).
Alternatively,the reduction in the SAI may be a result of ammonia build-up in the hemolymph and respiratory alkalosis (Claireaux and Dutil,1992; Chenget al.,2003).Harris and Andrews (2005a) have shown thatNephrops norvegicuscauses ammonia build-up in the hemolymph because of a lack of DO (Schmitt and Uglow,1997).The ammonia likely decreased the swimming performance ofSalmo truttaat low DO levels by decreasing the muscle membrane potential or affecting the muscle metabolism(Beaumontet al.,1995).
The increase in oxygen consumption that follows feeding,termed specific dynamic action (SDA) (Whiteleyet al.,2001),would have affected the swimming ability of aquatic organisms.The metabolic profile of SDA in hypoxia is more depressed and prolonged compared with that observed in normoxia (Jordan and Steffensen,2007).However,a study on shore crabCarcinus maenashas reported that the SDA duration was unchanged in hypoxia compared with that in normoxia (Menteet al.,2003).Previous studies on crustacean have shown that the SDA duration is up to several hours to days (Carefoot,1990;Houlihanet al.,1990; Chapelleet al.,1994; Robertsonet al.,2001).The time taken forL.vannameito reestablish the metabolic rate typical of a fasting animal after a meal is unknown.The fasting period prior to the experiment in this study was based on the previously published data for shrimp (Zhanget al.,2006,2007; Yuet al.,2009).
The energetic cost of processing a meal is largely independent on the meal size (Jordan and Steffensen,2007).In our study,the feed ingestion in the 4 DO treatments was different.The effect of SDA on the swimming capacity of shrimp remains unclear and needs to be further investigated.
4.2 The Effect of DO on the Physiological Response of L.vannamei
Crustaceans have evolved a variety of compensatory mechanisms to deal with hypoxia (Harper and Reiber,1999).In response to hypoxia,several physiological and biochemical change are enacted byL.vannamei.In the present study,the total protein,glucose,triglyceride,and lactate content were found higher in shrimps exposed DO at low concentration.The increase in energetic substrate might be a compensatory response of the shrimp exposed to hypoxia.Chenget al.(2003) found that both protein and oxyhemocyanin content elevated under hypoxic conditions.Change in the synthesis of hemocyanin is an important adaptive response to hypoxia in crustacea such asN.norvegicus(Hagerman and Uglow,1985; Hagermanet al.,1990),Callinectes sapidus(Defuret al.,1990),Homarus americanus(Senkbeil and Wriston,1981),andC.crangon(Hagerman,1986).It has been shown that hemocyanin constitutes 65.5%–65.8% of the total proteins at various DO levels.Obvious increase in hemolymph proteins under hypoxia is the consequences of hemocyanin increase (Chenget al.,2003).In hypoxia,this was related to an increase in plasma and tissue lactate level,indicating the setting up of anaerobic metabolism(Gäde,1984; Hagermanet al.,1990; Harris and Andrews,2005a; Urbina and Glover,2012).In addition,several studies have shown that hypoxic and other potential stress factors increase the blood glucose level (Kleinholz 1949;Telford 1974; Hall and Van Ham,1998).
Under hyperoxic conditions,both energetic substrate and lactate level are not significantly different from those under normal DO condition (about 6.8 mg L−1).However,a study on hyperoxia has found that hyperoxia enhances the resistance ofPenaeus stylirostristoVibrio alginolyticus(Le Moullacet al.,1998).Liet al.(2006) found that the oxygen consumption was significantly higher in shrimp experiencing hyperoxic conditions,and hyperoxia affected the non-specific immunity factors,leading to the increase in the peroxidase and antibacterial activity.These results indicated that hyperoxia potentially enhanced the resistance and activity of shrimp,despite that no measurement was made on the lipid peroxidation product of the blood of shrimp in order to investigate the effect of hyperoxia on the oxidative stress.
4.3 Physiological Response to Swimming Fatigue
After swimming fatigue,the shrimp showed reduction of plasma total protein and glycogen content in hepatopancreas and pleopods muscle,indicating that the mobilization of protein and glycogen was enhanced in response to swimming (Pascualet al.,2003).Similar results have been found inM.japonicus(Yuet al.,2009) andL.vannamei(Zhanget al.,2006).Furthermore,the metabolic rate of plasma total protein increased inL.vannameiexposed to 6.8 mg L−1and 13.6 mg L−1DO with an increasing swimming speed.We speculated that the increase in the mobilization of protein resulted in the reduction of swimming endurance of shrimp (Yuet al.,2009).Morris and Adamczewska (2002) have reported that the ghost crabsOcypodeexhibit large and rapid elevation in aerobic metabolism with high swimming endurance at low swimming speed.However,we observed that the metabolic rate of plasma total protein was not the highest at the maximum velocity,possibly due to much faster speed and lower endurance of experimental shrimp.
At higher DO concentrations,the swimming endurance of shrimp can be determined by their muscular capability or mobile energy source (Pearson,1990).In the present study,the plasma total protein and glycogen content in hepatopancreas and pleopods muscle significantly decreased in shrimp after swimming fatigue at high DO concentrations,whereas the plasma glucose content increased only at highest velocity.The glucose content in the hemolymph of crustaceans increases under stress conditions (Yoganandhanet al.,2003; Harris and Andrews,2005b).Obvious increase in glucose after swimming fatigue is the consequence of physiological compensation.As glucose fuels anaerobic metabolism,the fact that glucose at high DO level increased only at the highest speed indicated thatL.vannameionly needs to supply ATP generation with anaerobic metabolism at the highest level of speed.This was indicative of a significant response of this species to SAI increase when exposed to high concentrations of DO.
Our results showed that the propulsive force generated during swimming was derived from a combination of anaerobic glycolysis and protein catabolism.Gäde (1984)reported that during swimming,lactate was produced while the content of energetic substrate dropped.In our study,the pleopods muscle lactate concentration was increased significantly,but the increase in the pleopods muscle lactate level at high DO concentrations was less than that at the low DO concentrations.The shrimp released lactate produced in the musculature into the circulation following swimming.The animals were exposed to an environment with low oxygen levels,where they altered the blood flow during hypoxia and redirected blood to tissues that required high levels of oxygen (Hillet al.,1991a; Reiber,1995; Reiber and McMahon,1998; Mc-Mahon,2001).This likely led to the lactate accumulation in muscle and caused the reduction in swimming endurance under lower DO conditions.The muscle lactate content decreased significantly in shrimp exposed to 1.9 mg L−1DO after swimming fatigue at a high velocity.A probable mechanistic link could be the potential anaerobic pathways of energy metabolism (Zouet al.,1996).Alternatively,the lactate could be decomposed into ethanol and carbon dioxide,further dissolved in water or expelled in hypoxic systems (Lin,1999).The reason needs to be further confirmed.
In conclusion,the results showed that swimming endurance ofL.vannameiwas significantly affected by the DO in water and swimming speed.The SAI was a useful index of the effect of DO on the swimming capacity inL.vannamei.In addition,the DO affected the physiological response of shrimp during swimming fatigue.These were of a particular value to the understanding of the locomotive ability of whiteleg shrimp and its physiological change,thereby contributing to the improvement of capture and rearing technique.
Acknowledgements
This study was financially supported by the Public Science and Technology Research Funds Projects of Ocean (200805069),and the Special Fund for Agro-Scientific Research in the Public Interest (201003068).
Aquacop,E.B.,and Soyez,C.,1988.Effects of dissolved oxygen concentration on survival and growth ofPenaeus vannameiandPenaeus stylirostris.Journal of the World Aquaculture Society,19: 13A.
Bainbridge,R.,1960.Swimming and stamina in three fish.The Journal of Experimental Biology,37: 129-153.
Barbieri,E.,2007.Use of metabolism and swimming activity to evaluate the sublethal toxicity of surfactant (LAS-C12) on mugil platanus.Brazilian Archives of Biology and Technology,50: 101-112.
Beaumont,M.W.,Butler,P.J.,and Taylor,E.W.,1995.Plasma ammonia concentration in brown trout in soft acidic water and its relationship to decreased swimming performance.The Journal of Experimental Biology,198: 2213-2220.
Bone,Q.,1975.Muscular and energetic aspects of fish swimming.In:Swimming and Flyingin Nature.Wu,T.Y.T.,et al.,eds.,Plenum Press of New York,2: 493-528.
Brett,J.R.,1982.The swimming speed of adult pink salmon,Oncorhynchus gorbuscha,at 20℃ and a comparison with sockeye salmon,O.nerka.Canadian Technical Report of Fisheries and Aquatic Sciences,1143: 1-37.
Carefoot,T.H.,1990.Specific dynamic action (SDA) in the supralittoral isopod,Ligia pallasii: identification of the components of the apparent SDA and the effects of dietary amino acid quality and content on SDA.Comparative Biochemistry and Physiology PartA,95 (3): 309-316.
Chapelle,G.,Peck,L.,and Clarke,A.,1994.Effects of feeding and starvation on the metabolic rate of the necrophagous Antarctic amphipodWaldeckia obesa(Chevreux,1905).Journal of Experimental Marine Biology and Ecology,183: 63-76.
Cheng,W.,Liu,C.H.,and Kuo,C.M.,2003.Effects of dissolved oxygen on hemolymph parameters of freshwater giant prawn,Macrobrachium rosenbergii(de Man).Aquaculture,220: 843-856.
Cheng,W.,Liu,C.H.,Yan,D.F.,and Chen,J.C.,2002.Hemolymph oxyhemocyanin,protein,osmolality and electrolyte levels of whiteleg shrimpLitopenaeus vannameiin relation to size and molt stage.Aquaculture,211: 325-339.
Claireaux,G.,and Dutil,J.D.,1992.Physiological response of the atlantic cod (gadus morhua) to hypoxia at various environmental salinities.The Journal of Experimental Biology,163: 97-118.
Claireaux,G.,Webber,D.M.,Kerr,S.R.,and Boutilier,R.G.,1995.Physiology and behaviour of free-swimming Atlantic cod (Gadus morhua) facing fluctuating salinity and oxygenation conditions.The Journal of Experimental Biology,198:61-69.
Davis,G.E.,Foster,J.,Warren,C.E.,and Doudoroff,P.,1963.The influence of oxygen concentration on the swimming performance of juvenile pacific salmon at various temperatures.Transactions of the American Fisheries Society,92 (2): 111-124.
Defur,P.L.,Mangum,C.P.,and Reese,J.E.,1990.Respiratory responses of the blue crabCallinectes sapidusto longterm hypoxia.Biological Bulletin,178: 46-54.
Drucker,E.G.,1996.The use of gait transition speed in comparative studies of fish locomotion.American Zoologist,36:555-566.
Gäde,G.,1984.Effects of oxygen deprivation during anoxia and muscular work on the energy metabolism of the crayfish,Orconectes limosus.Comparative Biochemistry and Physiology A,77: 495-502.
Hagerman,L.,1986.Haemocyanin concentration in the shrimpCrangon crangon(L.) after exposure to moderate hypoxia.Comparative Biochemistry and Physiology A,85: 721-724.
Hagerman,L.,and Uglow,R.F.,1985.Effects of hypoxia on the respiratory and circulatory regulation ofNephrops norvegicus.Marine Biology,87: 273-278.
Hagerman,L.,Søndergaard,T.,Weile,K.,Hosie,D.,and Uglow,R.F.,1990 Aspects of blood physiology and ammonia excretion inNephrops norvegicusunder hypoxia.Comparative Biochemistry and Physiology A,97: 51-55.
Hall,M.R.,and Van Ham,E.H.,1998.The effects of different types of stress on blood glucose in the giant tiger prawnPenaeus monodon.Journal of the World Aquaculture Society,29: 290-299.
Harper,S.Y.,and Reiber,C.,1999.Influence of hypoxia on cardiac functions in the grass shrimp (Palaemonetes pugioHolthuis)Comparative Biochemistry and Physiology A,124:569-573.
Harris,R.R.,and Andrews,M.B.,2005b.Physiological changes in the Norway lobsterNephrops norvegicus(L.) escaping and discarded from commercial trawls on the West Coast of Scotland 1.Body fluid volumes and hemolymph composition after capture and during recovery.Journal of Experimental Marine Biology and Ecology,320: 179-193.
Harris,R.R.,and Andrews,M.B.,2005a.Physiological changes in the Norway lobsterNephrops norvegicus(L.) escaping and discarded from commercial trawls on the West Coast of Scotland II.Disturbances in haemolymph respiratory gases,tissue metabolites and swimming performance after capture and during recovery.Journal of Experimental Marine Biology and Ecology,320: 95-210.
He,P.,and Wardle,C.S.,1988.Endurance at intermediate swimming speeds of Atlantic mackerel,Scomber scombrusL.,herring,Clupea harengusL.,and saithe,Pollachius virensL.Journal of Fish Biology,35: 255-266.
Hervant,F.,Garin,D.,Mathieu,J.,and Freminet,A.,1999.Lactate metabolism and glucose turnover in the subterranean crustaceanNiphargus vireiduring post-hypoxic recovery.The Journal of Experimental Biology,202: 579-592.
Hill,A.D.,Strang,R.H.C.,and Taylor,A.C.,1991a.Radioisotope studies of the energy metabolism of the shore crabCarcinus maenas(L.) during environmental anoxia and recovery.Journal of Experimental Marine Biology and Ecology,150: 51-62.
Hill,A.D.,Taylor,A.C.,and Strang,R.H.C.,1991b.Physiological and metabolic responses of the shore crabCarcinus maenas(L.) during environmental anoxia and subsequent recovery.Journal of Experimental Marine Biology and Ecology,150: 31-50.
Houlihan,D.F.,Waring,C.P.,Mathers,E.,and Gray,C.,1990.Protein synthesis and oxygen consumption of the shore crabCarcinus maenas.Physiological Zoology,63 (4): 749-756.
Jentoft,S.,Aastveit,A.H.,Torjesen,P.A.,and Andersen,Ø.,2005.Effects of stress on growth,cortisol and glucose levels in non-domesticated Eurasian perch(Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss).Comparative Biochemistry and Physiology A,141: 353-58.
Jones,D.R.,1971.The effect of hypoxia and anaemia on the swimming performance of rainbow trout (Salmo gairdneri).The Journal of Experimental Biology,55: 541-551.
Jordan,A.D.,and Steffensen,J.F.,2007.Effects of ration size and hypoxia on specific dynamic action in the cod.Physiological and Biochemical Zoology, 80 (2): 178-185.
Kleinholz,L.H.,1949.Studies in the regulation of blood-sugar concentration in crustaceans.I.Normal values and experimental hyperglycemia inLihinia emarginuta.Biological Bulletin,96: 218-227.
Lagardère,J.P.,Anras,M.L.B.,Breton,H.,and Claret,J.B.C.,1995.The effects of illumination,temperature and oxygen concentration on swimming activity of turbotPsetta maxima(Linné 1758).Fisheries Research,24: 165-171.
Lallier,F.H.,and Walsh,P.J.,1992.Metabolism of isolated hepatopancreas cells from the blue-crab (Callinectes sapidus)under simulated postexercise and hypoxic conditions.Physiological Zoology,65: 712-723.
Le Moullac,G.,Soyez,C.,Saulnier,D.,Ansquer,D.,Avarre,J.C.,and Levy,P.,1998.Effect of hypoxia stress on the immune response and the resistance to vibriosis of the shrimpPenaeus stylirostris.Fish and Shellfish Immunology,8: 621-629.
Lefrancois,C.,Amat,J.N.,Domenici,P.,Kostecki,C.,and Ferrari,R.,2007.The effect of oxygen and temperature on the energetics of swimming inMugil cephalus.Comparative Biochemistry and Physiology A,146: S75-86.
Li,Y.Q.,Li,J.,and Wang,Q.Y.,2006.The effects of dissolved oxygen concentration and stocking density on growth and non-specific immunity factors in Chinese shrimp,Fenneropenaeus chinensis.Aquaculture, 256: 608-616.
Lin,H.R.,1999.The Physiology of Fishes.Guangdong Higher Education Press of Guangdong China,Guangzhou,57pp.
Main,J.,and Sangster,G.I.,1981.A study of the fish capture process in a bottom trawl by direct observations from a towed underwater vehicle.Scottish Fisheries Research Report,23:1-23.
McMahon,B.R.,2001.Respiratory and circulatory compensation to hypoxia in crustaceans.Respiratory Physiology,128:349-36.
Mente,E.,Legeay,A.,Houlihan,D.M.,and Massabuau,J.C.,2003.Influence of oxygen partial pressures on protein synthesis in feeding crabs.American Journal of Physiology, 284:500-510.
Moore,L.E.,Smith,D.M.,and Loneragan,N.R.,2000.Blood refractive index and whole-body lipid content as indicators of nutritional condition for penaeid prawns (Decapoda: Penaeidae).Journal of Experimental Marine Biology and Ecology,244: 131-143.
Morris,S.,and Adamczewska,A.M.,2002.Utilisation of glycogen,ATP and arginine phosphate in exercise and recovery in terrestrial red crabs,Gecarcoidea natalis.Comparative Biochemistry and Physiology A,133: 813-825.
Mugnier,C.,and Justou,C.,2004.Combined effect of external ammonia andmolt stage on the blue shrimpLitopenaeus stylirostrisphysiological response.Journal of Experimental Marine Biology and Ecology,309: 35-46.
Paschke,K.,Cumillaf,J.P.,Loyola,S.,Gebauer,P.,Urbina,M.,Chimal,M.E.,Pascual,C.,and Rosas,C.,2010.Effect of dissolved oxygen level on respiratory metabolism,nutritional physiology,and immune condition of southern king crabLithodes santolla(Molina,1782) (Decapoda,Lithodidae).Marine Biology,157: 7-18.
Pascual,C.,Sánchez,A.,Sánchez,A.,Vargas-Albores,F.,LeMoullac,G.,and Rosas,C.,2003.Haemolymph metabolic variables and immune response inLitopenaeus setiferusadult males: The effect of an extreme temperature.Aquaculture,218: 637-650.
Pearson,M.P.,Spriet,L.L.,and Stevens,E.D.,1990.Effect of sprint training on swim performance and white muscle metabolism during exercise and recovery in rainbow trout(Salmo gairdneri).The Journal of Experimental Biology,149:45-60.
Perez-Farfante,I.,and Kensley,B.,1997.Penaeoid and Sergestoid Shrimps and Prawns of the World: Keys and Diagnoses for the Families and Genera.Memoires du Museum National D’Historie Nuturelle,Paris,233pp.
Radford,C.A.,Marsden,I.D.,Davison,W.,and Taylor,H.H.,2005.Haemolymph glucose concentrations of juvenile rock lobsters,Jasus edwardsii,feeding on different carbohydrate diets.Comparative Biochemistry and Physiology A,140: 241-249.
Reiber,C.L,and McMahon,B.R.,1998.Progressive hypoxia effects on the crustacean cardiovascular system: A comparison of the freshwater crayfish (Procambarus clarkii) and the lobster (Homarus americanus).Journal of Comparative Physiology B,168: 168-176.
Reiber,C.L.,1995.Physiological adaptations in crayfish.
American Zoologist,35: 1-11.
Reidy,S.P.,Kerr,S.R.,and Nelson,J.A.,2000.Aerobic and anaerobic swimming performance of individual atlantic cod.The Journal of Experimental Biology,203: 347-357.
Robertson,R.F.,El-haj,A.J.,Clarke,A.,and Taylor,E.W.,2001.The effect of temperature on oxygen consumption,ammonia excretion and protein synthesis rates following a meal in the Antarctic isopodGlyptonotus antarcticus.Polar Biology,24: 677-686.
Rosas,C.,Sánchez,A.,Díaz-Iglesia,E.,Brito,R.,Martinez,E.,and Soto,L.A.,1997.Critical dissolved oxygen level toPenaeus setiferusandP.schmitti postlarvae(PL10–18) exposed to salinity changes.Aquaculture,152: 259-272.
Rosas,C.,Sánchez,A.,Escobar,E.,Soto,L.,and Bolongaro-Crevenna,A.,1992.Daily variations of oxygen consumption and glucose hemolymph level related to morphophysilogical and ecological adaptations of crustacea.Comparative Biochemistry and Physiology A,101: 323-328.
Rosas,C.,Martinez,E.,Gaxiola,G.,Brito,R.,Díaz-Iglesia,E.,and Soto,L.A.,1999.The effect of dissolved oxygen and salinity on oxygen consumption,ammonia excretion and osmotic pressure ofPenaeus setiferus(Linnaeus) juveniles.Journal of Experimental Marine Biology and Ecology,234:41-57.
Rosas,C.,Martinez,E.,Gaxiola,G.,Brito,R.,Díaz-Iglesia,E.,and Soto,L.A.,1998.Effect of dissolved oxygen on the energy balance and survival ofPenaeus setiferusjuveniles.Marine Ecology Progress Series,174: 67-75.
Schmidt-Nielsen,K.,1984.Scaling: Why is animal size so important?Cambridge University Press of Cambridge,241pp.
Schmitt,A.S.C.,and Uglow,R.,1997.Haemolymph constituent levels and ammonia efflux rates ofNephrops norvegicusduring emersion.Marine Biology,127: 403-410.
Seidman,E.R.,and Lawrence,A.L.,1985.Growth,feed digestibility,and proximate body composition of juvenilePenaeus vannameiandPenaeus monodongrown at different dissolved oxygen levels.Journal of the World Aquaculture Society,16: 333-346.
Senkbeil,E.G.,and Wriston,J.C.J.,1981.Hemocyanin synthesis in the American lobsterHomarus americanus.Comparative Biochemistry and Physiology B,68: 163-171.
Telford,M.,1974.The effects of stress on blood sugar composition of the lobster,Homarus americanus.Canadian Journal of Zoology,46: 819-826.
Tsukamoto,K.,Kajihara,T.,and Nishiwaki,M.,1975.Swimming ability of fish.Bulletin of the Japanese Society of Scientific Fisheries,4: 167-174.
Urbina,M.A.,and Glover,C.N.,2012.Should I stay or should I go?: Physiological,metabolic and biochemical consequences of voluntary emersion upon aquatic hypoxia in the scaleless fishGalaxias maculatus.Journal of Comparative Physiology B,182 (8): 1057-1067.
Vargas-Albores,F.,Guzmán,M.A.,and Ochoa,J.L.,1993.An anticoagulant solution for haemolymph collection and prophenoloxidase studies of penaeid shrimp (Penaeus californiensis).Comparative Biochemistry and Physiology A,106:299-303.
Vogel,S.,1994.Life in Moving Fluids: The Physical Biology of Flow.Princeton University Press,Princeton,N.J.,467pp.
Wardle,C.S.,1993.Fish behavior and fishing gear.In:Behaviour of Teleost Fishes.Pitcher,T.J.,ed.,Chapman and Hall of London,U.K.,609-643.
Wei,L.Z.,Zhang,X.M.,Li,J.,and Huang,G.Q.,2008.Compensatory growth of Chinese shrimp,Fenneropenaeus chinensisfollowing hypoxic exposure.Aquaculture International,16: 455-470.
Whiteley,N.M.,Robertson,R.F.,Meagor,J.,EI Haj,A.J.,and Taylor,E.W.,2001.Protein synthesis and specific dynamic action in crustaceans: Effects of temperature.Comparative Biochemistry and Physiology Part A,128: 595-606.
Winger,P.D.,He,P.G.,and Walsh,S.J.,2000.Factors affecting the swimming endurance and catchability of Atlantic cod(Gadus morhua).Canadian Journal of Fisheries and Aquatic Sciences,57: 1200-1207.
Yoganandhan,K.,Thirupathi,S.,and Sahul Hameed,A.S.,2003.Biochemical,physiological and hematological changes in white spot syndrome virus-infected shrimp,Penaeus indicus.Aquaculture,221: 1-11.
Yu,X.M.,Zhang,X.M.,Zhang,P.D.,and Yu,C.G.,2009.Critical swimming speed,tail-flip speed and physiological response to exercise fatigue in kuruma shrimp,Marsupenaeus japonicus.Comparative Biochemistry and Physiology A,153:120-124.
Yu,X.M.,Zhang,X.M.,Duan,Y.,Zhang,P.D.,and Miao,Z.Q.,2010.Effects of temperature,salinity,body length,and starvation on the critical swimming speed of whiteleg shrimp,Litopenaeus vannamei.Comparative Biochemistry and Physiology A,157: 392-397.
Zhang,P.D.,Zhang,X.M.,Li,J.,and Huang,G.Q.,2006.Swimming ability and physiological response to swimming fatigue in whiteleg shrimp,Litopenaeus vannamei.Comparative Biochemistry and Physiology A,145: 26-32.
Zhang,P.D.,Zhang,X.M.,Li,J.,and Huang,G.Q.,2007.The effects of temperature and salinity on the swimming ability of whiteleg shrimp,Litopenaeus vannamei.Comparative Biochemistry and Physiology A,147: 64-69.
Zou,E.,Du,N.,and Lai,W.,1996.The effects of severe hypoxia on lactate and glucose concentrations in the blood of the Chinese freshwater crabEriocheir sinensis(Crustacea:Decapoda).Comparative Biochemistry and Physiology A,2:105-109.
杂志排行
Journal of Ocean University of China的其它文章
- Estimating the Budgets of Nutrients for Phytoplankton Bloom in the Central Yellow Sea Using a Modified Lower Tropic Ecosystem Model
- Phytoplankton Assemblage Structure Shaped by Key Environmental Variables in the Pearl River Estuary,South China
- Purification and Characterization of 2-Haloacid Dehalogenase from Marine Bacterium Paracoccus sp.DEH99,Isolated from Marine Sponge Hymeniacidon perlevis
- Molecular Phylogeny of Parapenaeopsis Alcock,1901(Decapoda: Penaeidae) Based on Chinese Materials and 16S rDNA and COI Sequence
- Cytogenetic Mechanism for the Aneuploidy and Mosaicism Found in Tetraploid Pacific Oyster Crassostrea gigas (Thunberg)
- Effect of Hydraulic Loading Rate on the Efficiency of Effluent Treatment in a Recirculating Puffer Aquaculture System Coupled with Constructed Wetlands
