Studies on the Effects on Growth and Antioxidant Responses of Two Marine Microalgal Species to Uniconazole
2014-04-17MEIXueqiaoZHENGKangWANGLingdongandLIYantuan
MEI Xueqiao,ZHENG Kang,WANG Lingdong,and LI Yantuan
Marine Drug and Food Institute, Ocean University of China,Qingdao 266003,P. R.China
© Ocean University of China,Science Press and Springer-Verlag Berlin Heidelberg 2014
1 Introduction
It has been well documented that plant growth regulators are widely used to manipulate plant growth and enhance tolerance of crops to environmental stress(Partet al.,2008; Yucesanet al.,2007).Uniconazole [(E)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-lyl)-1-pen-ten-3-ol],an important member of the triazole family,was developed for use as a plant growth retardant(Wuet al.,2013).It can cause significantly increased yield in soybean(Chenet al.,2000)and enhanced tolerance in plants to stress,such as,waterlogging(Qiuet al.,2005),heat(Zhou and Leul,1999),drought(Zhanget al.,2012)and low temperature injury(Zhou and Leul,1998).It could also improve root growth in winter rape(Zhou and Ye,1996).Uniconazole can enhance stress tolerance in plants possibly because of improved antioxidation defense mechanisms with higher activities of superoxide dismutase(SOD)and peroxidase(POD)enzymes that retard lipid peroxidation and membrane deterioration(Leul and Zhou,1999).Additionally,it has been reported that uniconazole has multiple targets in plant hormone metabolism which plays regulatory roles in many aspects of plant growth and development(Sasakiet al.,2013).As uniconazole is using more and more frequently in agriculture,it may enter into aquatic ecosystem and impose undesirable side effects on biological and functional properties.
Marine phytoplanktons form the basis of the marine food chain and are essential for the marine ecosystem(Meneelyet al.,2013).Marine algae,which have many phytochemicals with various bioactivities,can produce oxygen and organic substance on which most other marine life forms depend to provide food for organisms at higher trophic levels,including fish and invertebrates(Kanget al.,2013).Any disturbance to marine algae,including the release and accumulation of chemical and toxic compounds,is likely to have impact on higher trophic levels(Liet al.,2006).Chemical effects on algae can directly affect the structure and function of marine ecosystem,resulting in oxygen depletion,decreased primary production and water degradation(Campanellaet al.,2000; Wong,2000).
There are many reports concerning the responses of antioxidant system in plants to uniconazole stress(Zhanget al.,2007),but such studies on aquatic organism are very few(Romanowska,2001).Moreover,no information is available on growth and antioxidant re-sponse in marine microalgae to uniconazole.The aim of the present study was to investigate the effects on growth,lipid peroxidation and some antioxidative enzymes activities of two species of marine microalgae,Platymonas helgolandicaandPavlova viridis,in response to different concentrations of uniconazole.Then a new theory basis for the use of uniconazole could be provided.
2 Materials and Methods
2.1 Algae Species and Culture Conditions
The marine microalgaeP.helgolandicaandP.viridiswere provided by the College of Fisheries,Ocean University of China,P.R.China.They are the important food for marine invertebrates,which are commonly found off the seashore of Qingdao.The two species of microalgae have different cell shapes,sizes and surface area to volume ratios.According to previous studies,the two species also show different sensitivities to uniconazole.
Microalgae were maintained in sterilized seawater enriched with f/2 medium.Cultures were grown and maintained in an environmental chamber illuminated with cool white fluorescent tubes at an irradiance of 80−90 μmol photons s−1m−2,a diurnal cycle of 12 h light and 12 h dark and at temperature of 25 ±1℃.
2.2 Experimental Set-up
Experiments were conducted in 500 mL flasks,each containing 250 mL culture media,which had been autoclaved at 121℃ for 20 min.A wide range of concentrations of uniconazole was examined in previous tests to determine the adequate range of uniconazole.The adequate range of uniconazole solution was 0−15 mg L−1,which was prepared by the dilution of a concentrated stock solution.The initial microalgal culture was removed by centrifugation and washed twice with seawater without any nutrition and then the microalgal pellet was resuspended in media of different concentrations of uniconazole.The initial microalgal density was 3×105cells mL−1for treatment.Control cultures without uniconazole were included in each test.Control and treated cultures were grown with the same medium,temperature and photoperiod conditions mentioned above.The tests for each treatment were conducted in triplicate.
2.3 Cell Growth and Dry Weight Assay
In the present study,the cell count ofP.helgolandicaandP.viridisis proportional to the absorbance atA680nm.It supported the study of Kasai(1993).The growth of the microalgae was measured spectrophotometrically(Unic WFZ UV-2800AH)at 680 nm in a cuvette with a 1 cm light path.As our preliminary experiments have quantified,1 OD680nmis equal to about 2.5×106P.helgolandicacells and 4.8×107P.viridiscells.Dry weight was determined by weighing.Microalgal samples cultured for 96 h were centrifuged and washed 3 times with distilled water.After drying 10 h in DHG-9030A constant temperature oven at temperature of 105℃,microalgal samples were weighed on FA2004 electronic balance and the levels were expressed in mg per 106cells.
2.4 Chlorophyll a and Total Carbohydrate Content Assay
Microalgal cells cultured for 96 h were centrifuged at 5000 r min−1for 10 min at 4℃.The cell pellets were transferred to a centrifuge tube,washed twice with distilled water,and then extracted with 10 mL of 96% ethanol for 24 h in darkness at 4℃.After centrifugation at 12 000 r min−1for 15 min,the absorbance of the supernatant at 649 and 665 nm was measured.Photosynthetic pigment(chlorophyll-a)concentration was calculated as described by Van Dijk and Roelof(1988).Total carbohydrate content was determined by phenol-sulfuric acid colorimetric methods using glucose as the standard.
2.5 Lipid Peroxidation Assay
The content of malondialdehyde(MDA)was measured according to the method provided by Heath and Parker(1968).After reaction,the absorbance at 532 nm was read and the value at 600 nm was subtracted.The results were expressed as mmol per 108cells.
2.6 Antioxidative Enzymes Assay
For antioxidant enzymes assays,microalgal cells cultured for 96 h were centrifuged at 5 000 r min−1for 10 min at 4℃.The cell pellets were transferred to a 5 mL centrifuge tube,washed twice with distilled water,then centrifuged again.These microalgal cells were resuspended in pre-cooled sodium phosphate buffer(PSB).After sonication for 5 min in an ice bath,the cell debris was removed by centrifugation at 12 000 r min−1for 20 min at 4℃.The supernatant was considered as enzyme extracts and was stored at 4℃ before any assay.Protein content was determined using bovine serum albumin(BSA)as a standard.The absorbance at 560 nm was determined before and after the photochemical reaction.One unit of SOD activity is defined as the amount of enzyme causing a 50% inhibition of photochemical reduction of nitro blue tetrazolium(NBT)which is expressed as U per mg protein.The change in absorbance at 340 nm was monitored for 150 s.One unit of CAT activity is defined as mg per mg protein.
2.7 Statistical Analysis
The differences between the control and uniconazoletreated samples were analyzed by one-way analysis of variance(ANOVA),taking the level ofP< 0.05 as significance according to Tukey multiple comparison test.The statistical analyses were performed using the software called Statistical Package for Social Sciences(SPSS 13.0 for Windows,SPSS Inc.,USA).The mean values ± SD are reported in the figures.
3 Results
3.1 Effects on Growth and Dry Weight
As shown in Fig.1,uniconazole caused no inhibition on the growth ofP.helgolandicaandP.viridisat low concentrations(≤ 6 mg L−1),while at higher concentrations(≥9 mg L−1),it showed significantly inhibitory effects on growth(P< 0.05).When the concentration of uniconazole was as high as 15 mg L−1,the growth ofP.helgo-landicaandP.viridiswas totally suppressed.
Fig.2 shows the changes of dry weight inP.helgolandicaandP.viridisafter being cultured 96 h with different concentrations of uniconazole.The addition of 0−9 mg L−1uniconazole had no significant effects on dry weight of microalgae.However,a significant decrease in dry weight levels was observed at higher concentrations of uniconazole(≥12 mg L−1).15 mg L−1uniconazole caused significant reductions by 81%(P.helgolandica)and 44%(P.viridis)with respect to controls.

Fig.1 Effects of uniconazole on growth of P.helgolandica and P.viridis.(a)P.helgolandica;(b)P.viridis.Each value is the mean ± SD(bars)of three representative experiments.

Fig.2 Effects of uniconazole on dry weight of P.helgolandica and P.viridis after 96 h.(a)P.helgolandica;(b)P.viridis.Each value is the mean ± SD(bars)of three representative experiments.**:significant difference at P < 0.01; *:significant difference at P < 0.05 with respect to control(one-way ANOVA).
3.2 Effects on Contents of Chlorophyll a and Total Carbohydrate

Fig.3 Effects of uniconazole on chlorophyll a contents of P.helgolanidica (a)and P.viridis (b).Note:compared to control,*P < 0.05,**P < 0.01).
Chlorophyllacontent was measured at 96 h treated with different concentrations of uniconazole(Fig.3).Chlorophyllacontent significantly increased by 17.1%(P< 0.01)inP.helgolandicatreated with 3 mg L−1uniconazole compared with the control.However,12 and 15 mg L−1uniconazole caused a significant reduction of chlorophyll-a content inP.helgolandica(Fig.3a).The addition of 0−9 mg L−1uniconazole had no significant effects on chlorophyll-a content ofP.viridis.12 and 15 mg L−1. uniconazole caused 38.7%(P< 0.05)and 77.2%(P< 0.01)reductions of chlorophyllacontent inP.viridiscompared with control(Fig.3b).
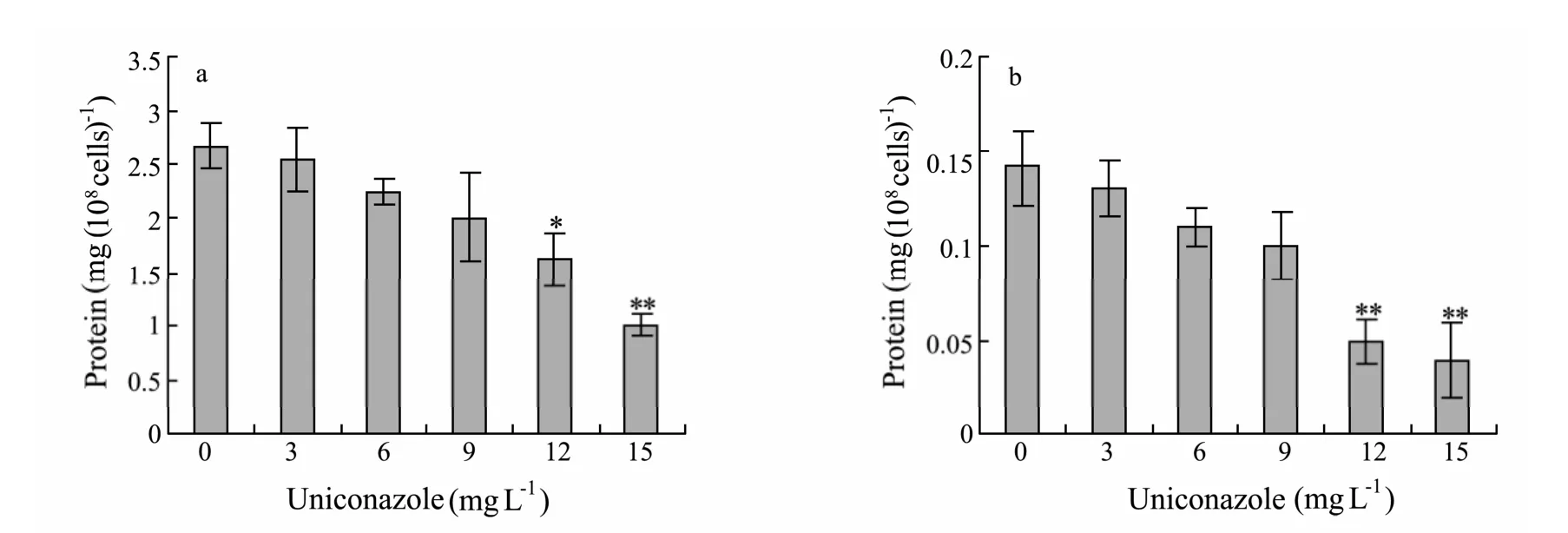
Fig.4 Effects of uniconazole on soluble protein contents of P.helgolandica and P.viridis after 96 h.(a)P.helgolandica;(b) P.viridis.Each value is the mean ± SD(bars)of three representative experiments.**:significant difference at P < 0.01; *:significant difference at P < 0.05 with respect to control(one-way ANOVA).
The effects of uniconazole on total carbohydrate contents of microalgae were the same as on chlorophyllacontent(Fig.4).3 mg L−1uniconazole caused a significant increase(18.3%,P< 0.05)of total carbohydrate content inP.helgolandica.12 and 15 mg L−1uniconazole caused significant reductions of total carbohydrate contents both inP.helgolandica(35.0% and 72.7%)andP.viridis(30.4% and 43.8%).
3.3 Effects on Lipid Peroxidation Products(MDA)
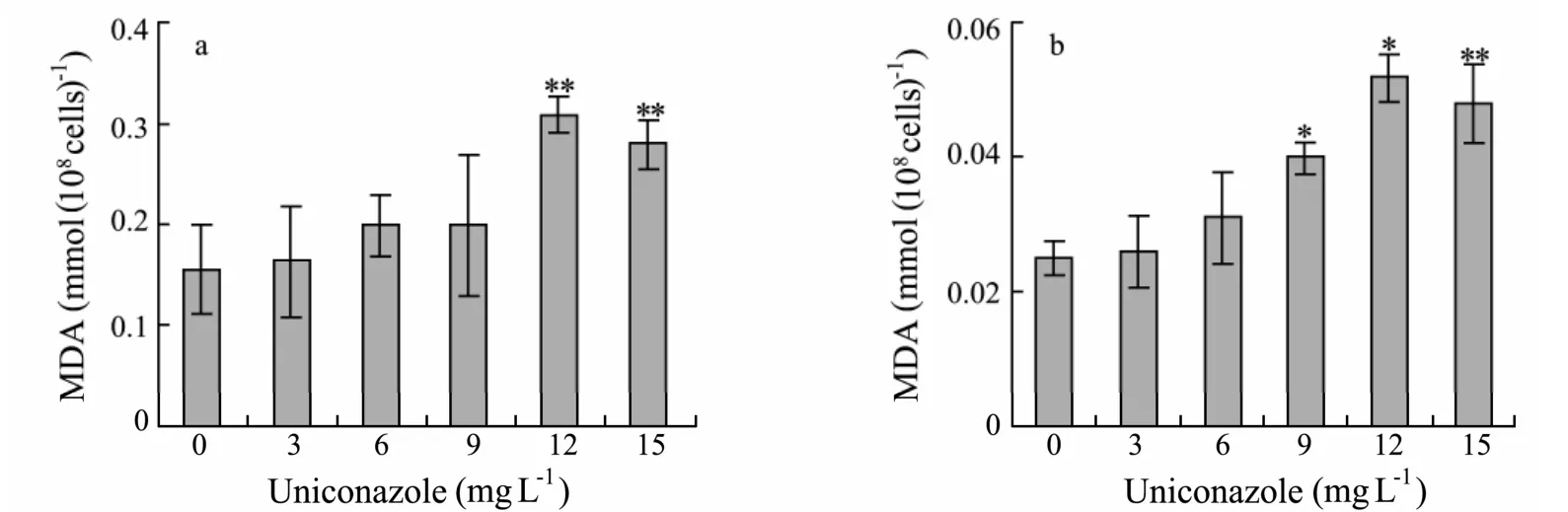
Fig.5 Effects of uniconazole on MDA contents of P.helgolandica and P.viridis after 96 h.(a)P.helgolandica;(b)P.viridis.Each value is the mean ± SD(bars)of three representative experiments.**:significant difference at P < 0.01; *:significant difference at P < 0.05 with respect to control(one-way ANOVA).
MDA content was measured as a lipid peroxidation indicator.Significant increases in MDA levels were observed both inP.helgolandicaandP.viridisat higher concentrations of uniconazole with respect to control(Fig.5).MDAs were significantly increased by 99%(P.helgolandica)and 108%(P.viridis)with respect the control (P< 0.01)at 12 mg L−1uniconazole,respectively.
3.4 Effects on SOD Activity
Total SOD activity in microalgae was measured at 96 h after treating with different concentrations of uniconazole and the results are shown in Fig.6.SOD enzymatic levels increased significantly in 3 mg L−1uniconazole treatment with respect to control (P< 0.05).However,a significant reduction in SOD activity was observed at the concentration of 15 mg L−1.It decreased by 53% inP.helgolandicaand 75% inP.viridisat 15 mg L−1uniconazole with respect to their corresponding controls (P< 0.01).
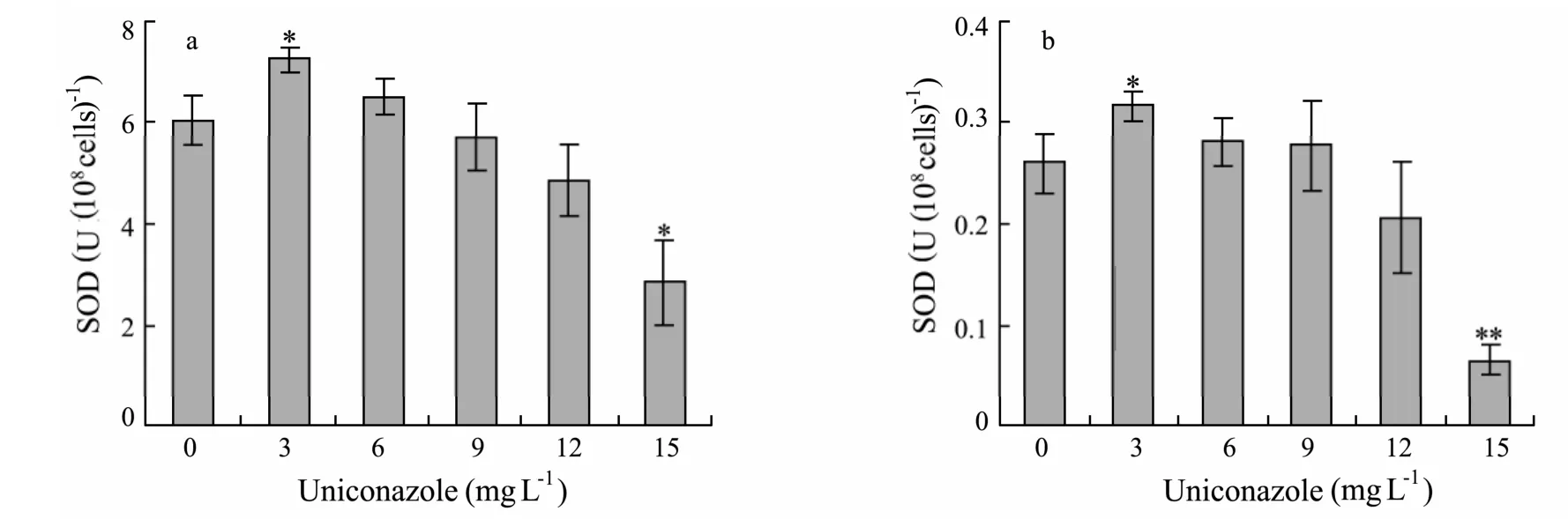
Fig.6 Effects of uniconazole on SOD activity of P.helgolandica and P.viridis after 96 h.(a)P.helgolandica;(b)P.viridis.Each value is the mean ± SD(bars)of three representative experiments.**:significant difference at P < 0.01; *:significant difference at P < 0.05 with respect to control(one-way ANOVA).
3.5 Effects on CAT Activity
As shown in Fig.7a,CAT activity ofP.helgolandicawas increased by 25% (P< 0.01)at 6 mg L−1uniconazole,whereas it decreased by 71% (P< 0.01)below control levels at 15 mg L−1uniconazole.Significant increases in CAT enzymatic levels ofP.viridiswere observed at the range of 3−9 mg L−1uniconazole(Fig.7b).However,a significant reduction in CAT levels(96%,P< 0.01)was observed inP.viridisat 15 mg L−1uniconazole with respect to the control value.
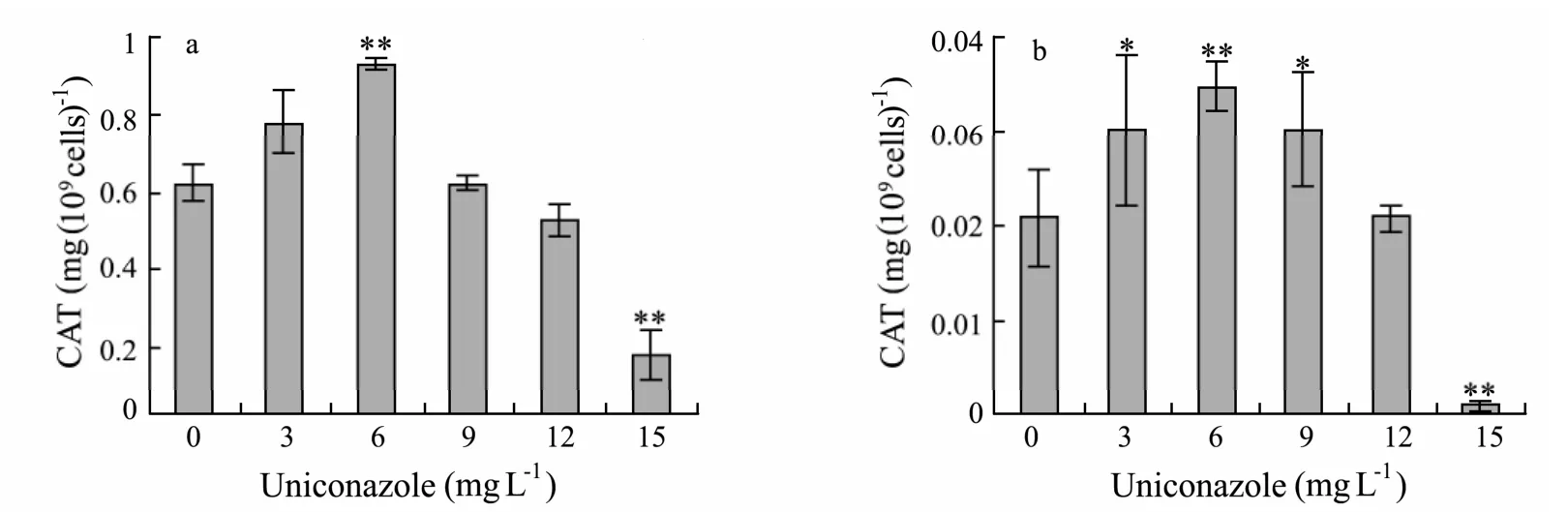
Fig.7 Effects of uniconazole on CAT activity of P.helgolandica and P.viridis after 96 h.(a)P.helgolandica;(b)P.viridis.Each value is the mean ± SD(bars)of three representative experiments.**:significant difference at P < 0.01; *:significant difference at P < 0.05 with respect to control(one-way ANOVA).
4 Discussion
Uniconazole is an active member of triazole family,which is widely used as fungicides and plant growth regulators in agriculture.Uniconazole can inhibit gibberellic acid(GA)biosynthesis,reduce the concentration of endogenous indole-3-acetic acid(IAA),and increase the concentrations of zeatin,abscisic acid(ABA)and ethylene within the plant.It also enhances the photosynthetic rate(Pn)and chlorophyll content(Li and Yang,2004).In the present study,uniconazole significantly increased chlorophyllaand carbohydrate content ofP.helgolandicaat low concentration(3 mg L−1); however,significant decrease in growth,dry weight,chlorophyll-a and carbohydrate contents were observed both inP.helgolandicaandP.viridisat higher concentrations of uniconazole.Chlorophyllais the major pigment participating in photosynthesis.Carbohydrate is the product of photosynthesis.The increase in chlorophyllaand carbohydrate contents suggested that the low concentration of uniconazole could enhance the photosynthetic rate ofP.helgolandica.On the other hand the significant reduction of chlorophyll-a and carbohydrate contents suggested that the photosynthetic system was damaged and photosynthesis efficiency was inhibited inP.helgolandicaandP.viridisin response to higher concentrations of uniconazole.
MDA is the final product of peroxidation of unsaturated fatty acids in phospholipids and responsible for cell membrane damage,which has often been used as an indicator of the level of lipid peroxidation(Scandalios,1993).In the present study,the addition of higher concentrations of uniconazole to the medium caused significant increase in MDA content ofP.helgolandicaandP.viridis.The increase in lipid peroxidation(observed as MDA)measured under the present experimental conditions could be considered as an indicator of increased oxidative damage in microalgae caused by uniconazole.
It is well known that reactive oxygen species(ROS)such as superoxide(O2−),hydroxyl radicals(OH·)and hydrogen peroxide(H2O2)are produced in cells when exposed to environmental stresses.It has been demonstrated that high levels of ROS can lead to severe cellular damage or death.SOD,POD and CAT are essential members in antioxidant system,which can scavenge free radicals and peroxides(Allen,1995).SOD can give rise to H2O2by dismutation of O2−radicals,and then H2O2can be quenched by CAT and POD(Elisabetta and Gioacchino,2004).
CAT,which directly catalyzes the decomposition of H2O2to H2O and O2,is located mostly in the subcellular respiratory organelles known as peroxisomes(Halliwell and Gutteridge,1999).Thus,decrease in CAT activity would result in H2O2accumulation,which can react with O2−to produce hydroxyl-free radicalsviathe Herbert-Weiss reaction.The hydroxyl-free radicals can directly damage the membrane by attacking unsaturated fatty acids of lipid to induce lipid peroxidation(Okudaet al.,1991).It is found that the level of SOD and CAT activity in our experiments were increased after being treated with lower concentrations of uniconazole(< 9 mg L−1).The increased levels of the SOD and CAT activities indicated that uniconazole-induced stress tolerance in microalgae may be caused by increased antioxidant activity,which in turn reduce oxidative injury to membrane or enzyme activity.However,remarkable reduction in SOD and CAT enzymatic levels were observed as the concentration of uniconazole increased,then a significant increase in MDA content could be the result.The decrease in SOD and CAT activity may lead to accumulation of O2−and H2O2and cause severe damage to cell membrane.
5 Conclusion
In conclusion,the present results revealed that uni-conazole significantly increased chlorophyll-a and carbohydrate contents inP.helgolandicaat low concentratons,and SOD and CAT enzymatic levels in microalgae were also increased.However,remarkable effects were observed on growth and antioxidant mechanism at higher concentrations of uniconazole.The significant inhibition on growth of microalgae(measured by dry weight,chlorophyllaand carbohydrate contents)at higher concentrations of uniconazole may be caused by the reduction in SOD and CAT enzymatic levels and increase in lipid peroxidation(detected through MDA levels).The present results demonstrated that the higher concentrations of uniconazole may increase the oxidative damage to microalgal cell.
Acknowledgements
This project was supported by the National Natural Science Foundation of China(Nos.21071133,51273184 and 81202399),the Program for Science and Technology of Shandong Province(2011GHY11521),and the Natural Science Foundation of Qingdao City(Nos.11-2-4-1-(9)gch),12-1-3-52-(1)-nsh and 12-1-4-16-(7)-jch).
Allen,R.D.,1995.Dissection of oxidative stress tolerance using transgenic plants.Plant Physiology,107:1049-1054.
Campanella,L.,Cubadda,F.,Sammartino,M.P.,and Saoncella,A.,2000.An algal biosensor for the monitoring of water toxicity in estuarine environments.Water Research,25:69-76.
Chen,D.Q.,Li,Y.N.,and Pen,C.L.,2000.The effect of S-3307 on growth characteristics and yield of soybean.Journal of Hubei Agricultural College,20:108-114.
Elisabetta,M.,and Gioacchino,S.,2004.Copper-induced changes of non-protein thiols and antioxidant enzymes in the marine microalgaPhaeodactylum tricornutum.Plant Science,167:289-296.
Halliwell,B.,and Gutteridge,J.M.C.,1999.Free Radicals in Biology and Medicine.3rd edition.Oxford University Press,New York,936pp.
Heath,R.L.,and Packer,L.,1968.Photoperoxidation in isolated chloroplasts I.Kinetics and stoichiometry of fatty acid peroxidation.Archives of Biochemistry and Biophysics,125:189-198.
Kang,M.C.,Cha,S.H.,Wijesinghe,W.A.J.P.,Kang,S.M.,Lee,S.H.,Kim,E.A.,Song,C.B.,and Jeon,Y.J.,2013.Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebra fi sh embryo.Food Chemistry,138:950-955.
Kasai,F.,Takamura,N.,and Hatakeyama,S.,1993.Effects of smetryne on growth of various freshwater algal taxa.Environmental Pollution,79:77-83.
Li,M.,Hu,C.,Zhu,Q.,Chen,L.,Kong,Z.,and Liu,Z.,2006.Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalgaPavlova viridis(Prymnesiophyceae).Chemosphere,62:565-572.
Li,Q.M.,and Yang,W.Y.,2004.Effects of soaking seeds with uniconazole on several photosynthetic characters of maize seedlings.Plant Physiology Communications,40:31-33.
Meneely,J.P.,Campbell,K.,Greef,C.,Lochhead,M.J.,and Elliott,C.T.,2013.Development and validation of an ultrasensitive fl uorescence planar waveguide biosensor for the detection of paralytic shell fi sh toxins in marine algae.Biosensors and Bioelectronics,41:691-697.
Okuda,T.,Matsuda,Y.,and Yamanaka,A.,1991.Abrupt increases in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment.Plant Physiology,97:1265-1267.
Part,L.,Botti,C.,and Fichet,T.,2008.Effect of plant growth regulators on floral differentiation and seed production in jojoba(Simmondsia chinensis(Link)Schneider).Industrial Crops and Products,27(1):44-49.
Qiu,J.,Wang,R.M.,Yan,J.Z.,and Hu,J.,2005.Seed film coating with uniconazole improves rape seedling growth in relation to physiological changes under waterlogging stress.Plant Growth Regulation,47:75-81.
Romanowska-Duda,Z.,Tarczynska,M.,and Zalewski,M.,2001.The control of cyanobacterial blooms by plant growth retardants(ancymidol,paclobutrazol,uniconazole).Water Science and Technology:Water Supply,1:247-250.
Sasaki,E.,Ogura,T.,Takei,K.,Kojima,M.,Kitahata,N.,Sakakibara,H.,Asami,T.,and Shimada,Y.,2013.Uniconazole,a cytochrome P450 inhibitor,inhibits trans-zeatin biosynthesis inArabidopsis.Phytochemistry,87:30-38.
Scandalios,J.G.,1993.Oxygen stress and superoxide dismutases.Plant Physioloy,101:7-12.
Van Dijk,H.F.G.,and Roelofs,J.G.M.,1988.Effects of excessive ammonium deposition on the nutritional status and condition of pine needles.Physiologia Plantarum,73:494-501.
Wong,P.K.,2000.Effects of 2,4-D,glyphosate and paraquat on growth,photosynthesis and chlorophyllasynthesis ofScenedesmus quadricaudaBerb614.Chemosphere,41:177-182.
Wu,C.W.,Sun,J.Q.,Zhang,A.P.,and Liu,W.P.,2013.Dissipation and enantioselective degradation of plant growth retardants paclobutrazol and uniconazole in open field,greenhouse,and laboratory soils.Environmental Science and Technology,47:843-849.
Yucesan,B.,Turker,A.U.,and Gurel,E.,2007.TDZ-induced high frequency plant regeneration through multiple shoot formation in witloof chicory(Cichorium intybusL.).Plant Cell,Tissue and Organ Culture,99(3):243-250.
Zhang,J.,Cao,X.L.,Yong,T.W.,and Yang,W.Y.,2012.Seed treatment with uniconazole powder induced drought tolerance of soybean in relation to changes in photosynthesis and chlorophyll fluorescence.Research on Crops,13(1):147-154.
Zhang,M.C.,Duan,L.S.,Tian,X.L.,He,Z.P.,Li,J.M.,Wang,B.M.,and Li,Z.H.,2007.Uniconazole-induced tolerance of soybean to water deficit stress in relation to changes in photosynthesis,hormones and antioxidant system.Plant Physioloy,164(6):709-717.
Zhou,W.J.,and Leul,M.,1998.Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance,enzyme activities and lipid peroxidation in winter rape.Plant Growth Regulation,26:41-47.
Zhou,W.J.,and Leul,M.,1999.Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels,enzyme activities and lipid peroxidation.Plant Growth Regulation,27:99-104.
Zhou,W.J.,and Ye,Q.F.,1996.Physiological and yield effects of uniconazole on winter rape(Brassica napusL.).Plant Growth Regulation,15:69-73.
杂志排行
Journal of Ocean University of China的其它文章
- A Numerical Study of Tidal Asymmetry:Preferable Asymmetry of Nonlinear Mechanisms in Xiangshan Bay,East China Sea
- Effect of Bacillusbaekryungensis YD13 Supplemented in Diets on Growth Performance and Immune Response of Sea Cucumber(Apostichopus japonicus)
- Evaluation of Three Harvest Control Rules for Bigeye Tuna(Thunnus obesus)Fisheries in the Indian Ocean
- Effect of Dietary Olaquindox on the Growth of Large Yellow Croaker(Pseudosciaena crocea R.)and the Distribution of Its Residues in Fish Tissues
