Effect of Dietary Olaquindox on the Growth of Large Yellow Croaker(Pseudosciaena crocea R.)and the Distribution of Its Residues in Fish Tissues
2014-04-17LIHuitaoWANGWeifangMAIKangsenAIQinghuiZHANGChunxiaoandZHANGLu
LI Huitao,WANG Weifang,MAI Kangsen,AI Qinghui,,ZHANG Chunxiao,and ZHANG Lu
1) Key Laboratory of Mariculture of Ministry of Education, Ocean University of China,Qingdao 266003, P.R.China
2) Qingdao Key Laboratory for Marine Fish Breeding and Biotechnology, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, P.R.China
3) Fisheries College, Jimei University, Xiamen 361021, P.R.China
© Ocean University of China,Science Press and Spring-Verlag Berlin Heidelberg 2014
1 Introduction
Olaquindox(OLA),N-(2-hydroxyethyl)-3-methyl-2-quinoxalincarboxamide-1,4-dioxide,one of quinoxaline-N,N-dioxides,has been put under ban.However it was used as a medicinal feed additive early; it promotes the growth of livestock and prevents them from dysentery and bacterial enteritis(Baarset al.,1988; Anon,1989).In 1980s,OLA was used in fish and shrimp farming(Xuet al.,1988; Yeet al.,1992).Unfortunately,it was cognized gradually that OLA is toxic to fish(Caoet al.,2001; Wanget al.,2004; Yanget al.,2005),thus been put under ban by many countries including China.However,its illegal use has never completely disappeared worldwide.
As was documented by the joint FAO/WHO Expert Committee on Food Additives,OLA is almost completely absorbable in the gastrointestinal tract of rat,dog and pig,and histological wide in distribution.The most of OLA is eliminated through urine and less through feces and by expiration.In urine,the original OLA accounts for 70%,while others include approximately 16 metabolites,of them 6 major metabolites have been fully characterized.MQCA is the main persistent residue of OLA in the edible tissues,thus is considered to be a marker of OLA in animals(FAO/WHO,1995; MOA,2002).To date,no study has been carried out on the histological distribution of OLA residue and its metabolites in fish.OLA may induce genotoxicity as was found in human hepatoma G2(HepG2)cells(Zouet al.,2011)and cause phototoxicity to human(WHO,1991; Emmertet al.,2007).We believe that OLA residue and its metabolites in fish tissues may affect human health.
Large yellow croaker(Pseudosciaena crocea)is one of the commercially important marine fish species in China,which has been widely cultured in recent years.Early studies in this fish species focused mainly on its nutrient requirement and feed modification(Duanet al.,2001; Maiet al.,2006; Aiet al.,2007; Liet al.,2007).In this study,the effect of dietary OLA on the growth of large yellow croaker and its histological residue were determined with an emphasis on OLA and its metabolite MQCA.
2 Materials and Methods
2.1 Diet Preparation
Four diets were formulated to contain 0(control,Diet 1),42.5(Diet 2),89.5(Diet 3)and 277.2(Diet 4)mg kg−1OLA with 1% OLA premix(Animal Disease Control Center,Qingdao,China)(Table 1).The protein and lipid content of these diets was about 44% and 11%,respectively,which are considered sufficient to support optimal growth of large yellow croaker(Duanet al.,2001).
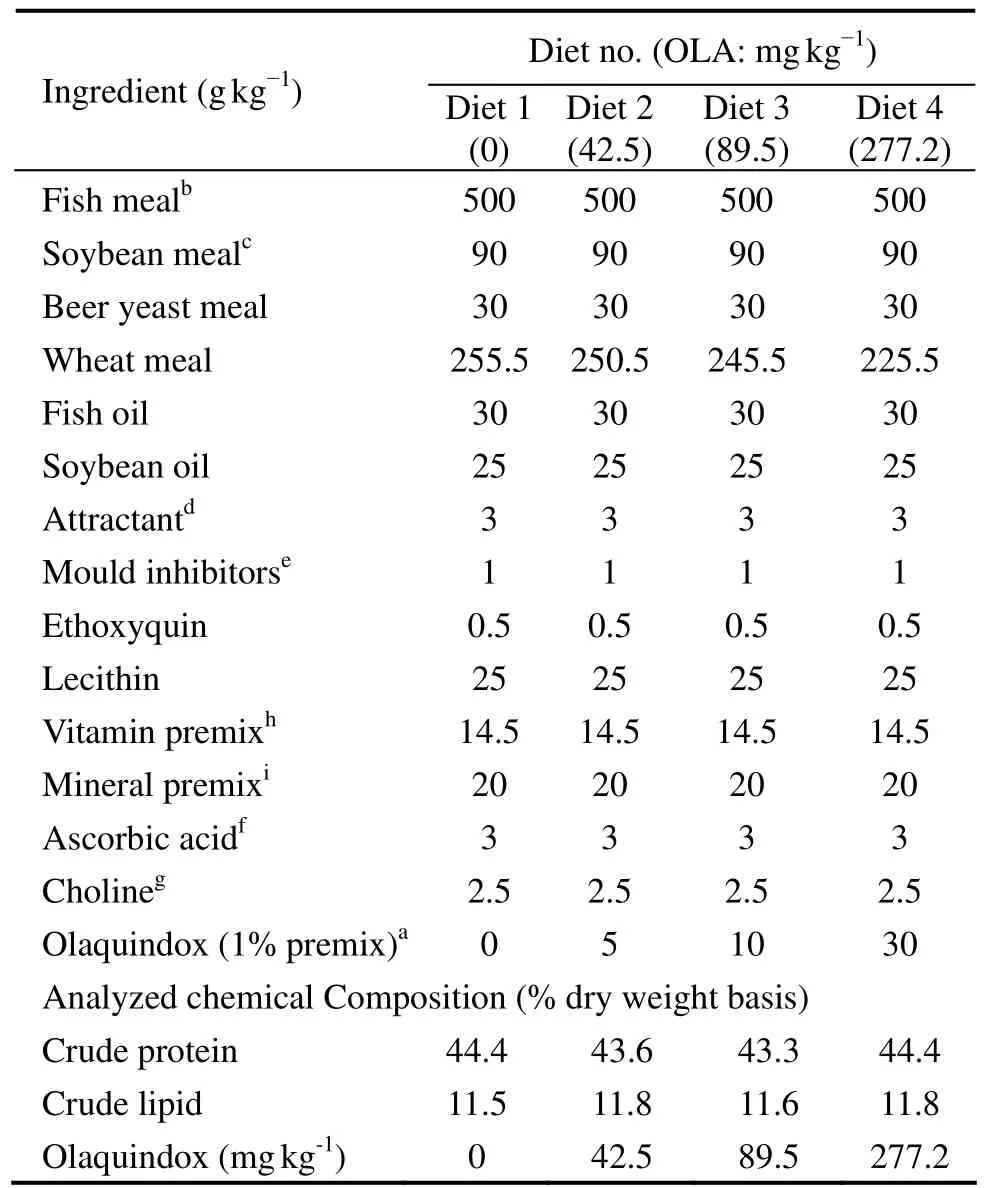
Table 1 Formulation of experimental diets(dry weight basis)
To prepare the diets,the solid ingredients were grounded into fine powder,sieved through a 320-μm mesh and mixed thoroughly with menhaden fish oil.The mixture was made into stiff dough by adding water and then pelleted with an experimental feed mill(F-26 II,South China University of Technology,China).The pellets were dried in a ventilated oven at 60℃ for about 12 h,broken up and sieved.The prepared diets were 1.5 mm× 2.0 mm in size,which were sealed in plastic bags and stored at −15℃.
2.2 Feeding Trial
Large yellow croaker juveniles were obtained from a commercial hatchery and stocked in a floating sea cage(3.0 m × 3.0 m × 3.0 m)in Xihu Bay,Ningbo,China,for 2 weeks.The fish were fed with Diet 1 during the acclimation.
The fish were fasted for 24 h and weighed after being anesthetized with eugenol(1:10000; Shanghai Reagent Corp,China).Fish in similar size(9.75 g ± 0.35 g)were divided into 12 sea cages(1.0 m × 1.0 m × 1.5 m),3 cages each diet and 100 individuals each cage,and fed to satiation twice a day at 05:00 am and 17:00 pm,respectively,for 8 weeks.During feeding trial,water temperature and salinity were maintained at 26.5–32.5℃ and 25.0–28.0 g L−1,respectively,while dissolved oxygen was kept above 7 mg L−1.The natural light rhythm was followed throughout the trial.
2.3 Sampling and Content Assaying
2.3.1 Sampling
The fish were fasted for 24 h ahead of harvest.Total number of fish in each cage was counted and their body weights were measured.Twenty fish per cage were randomly collected and stored frozen at −20℃ to determine whole-body proximate composition and the content of OLA and its metabolite.The skin,liver and muscle were sampled under caliginous light carefully and freeze-dried in ALPHA 1-2,a freeze dryer(Martin Christ,Germany)for 24 h,then immediately stored frozen at −20℃.
2.3.2 Analysis of ingredients,diets and fish body composition
Analyses of ingredients,diets and fish body composition were made following the usual procedures(AOAC,1995):samples were dried to a constant weight at 105℃ to determine the dry matter content; crude protein was determined by measuring nitrogen(N × 6.25)using the Kjeldahl method; crude lipid was measured by ether extraction using Soxhlet method; ash by combustion at 550℃ for 16 h.
The content of OLA and MQCA was determined with a liquid chromatography(LC)equipped with ultraviolet(UV)detector set at 260 nm and 320 nm,respectively.
2.3.3 Content assaying
For content determination,the skin,liver and muscle were homogenized with 5 mL saturated ammonium sulfate,and then 10 mL mixed solution of acetonitrile-ethyl acetate(3:2)was added to homogenize for 1–2 min following with a 10-min centrifugation at 4200 r min−1.The supernatant were evaporated to dryness under a stream of nitrogen(Organomation Associates,Berlin,MA,USA)at 50℃water-bath.The residue were dissolved with 2 mL acetonitrile and degreased with 3 mL n-hexane for twice and centrifuged.The stock solution were evaporated to dryness under a stream of nitrogen at a water bath of 50℃and 0.5 mL methanol was added following a 1–2 min shaking mixture and centrifugation,of which 10 µL supernatant was injected into the liquid chromatography(LC)equipped with ultraviolet(UV)detector set at 260 nm to determine the concentration of OLA.The skin,liver and muscle were hydrolyzed by 5 mL 3 mol L−1NaOH at a 95−100℃ water bath for 40–45 min.The alkaline hydrolysate was acidified to pH≤1 with 3 mL HCl and extracted with 15 mL ethyl acetate following with a 10-min centrifugation at 4200 r min−1.The supernatant was washed with 5 mL distilled water by shaking to clarify and rejecting the lower water phase twice.Then 5 mL 0.5 mol L−1citric acid buffer(pH 6.0)was added into the extraction and mixed to get the lower buffer phase.Then,the aqueous extraction was acidified with 3 mL HCl.The acidified aqueous extraction was transferred into a cation exchange column(AG MP-50 resin)for ion-exclusion chromatography.The column was washed with 50 mL 1 mol L−1HCl and 70 mL mixed solution of methanol with water(50 + 50)to collect the eluate,respectively.The eluate was extracted with 25 mL CHCl3triplicate after adding 2 mL HCl and rotary evaporated to dryness at 45–50℃.The residue were dissolved with 2 mL methanol twice and evaporated to dryness under a stream of nitrogen at a water bath of 50℃,then 0.5 mL methanol was added following a 1–2 min shaking mixture and centrifugation,of which 10 µL supernatant was injected into the liquid chromatography(LC)equipped with ultraviolet(UV)detector set at 320 nm to determine the concentration of MQCA.
2.4 Data Processing
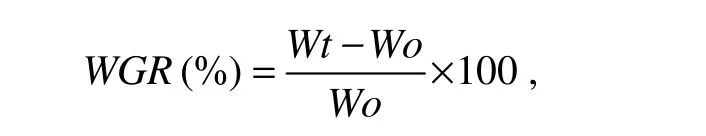
The weight gain rate(WGR)of fish was determined with the equation whereWtandWoare final and initial mean body weight of fish,respectively.Data corresponding to each diet were subjected to one-way ANOVA with SPSS 10.0 for windows.When overall difference was significant(P< 0.05),Tukey’s test was used to compare the means between diets(Zar,1984).
3 Results
3.1 Survival and Growth
Fish fed the diet with 277.2 mg kg−1OLA were slow in feed intaking and nervous to any stimulation(e.g.,mad jumping).Occasionally,hyperemia and hemorrhage were observed at the base of fins and the corner of the mouth and in abdomen.The survival rate ranged from 86.0% to 92.0% was significantly influenced by dietary treatment(Table 2).Fish fed diet with 42.5 and 89.5 mg kg−1OLA survived significantly higher than those fed with control diet and 277.2 mg kg−1OLA diet(P< 0.05); while fish fed diet with 277.2 mg kg−1OLA had a significantly lower survival rate than the control)(P< 0.05).The WGR of fish was significantly influenced by the dietary OLA.Fish fed diets with 42.5 and 89.5 mg kg−1OLA had signifycantly higher WGR(173.0% and 184.0%,respectively)than control(133.3%)(P< 0.05),and the fish fed with control diet and 277.2 mg kg−1OLA diet had similar WGR(Table 2).
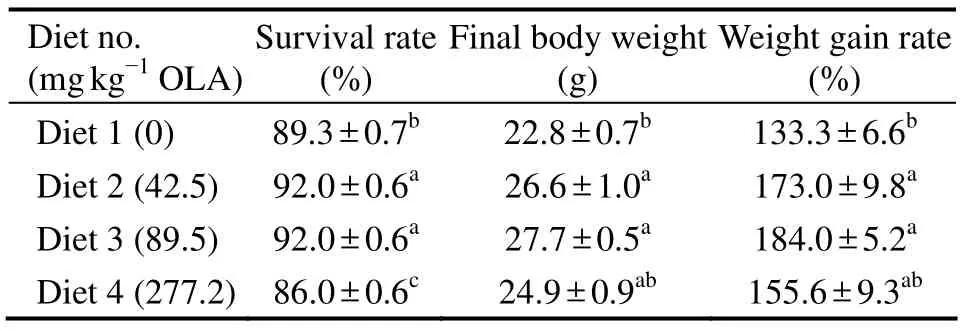
Table 2 Survival and growth performance of large yellow croaker
3.2 Body Composition
The dietary OLA did not significantly influence the composition of fish body.Water(69.2%–70.3%),lipid(8.1%–8.7%)and ash(4.1%–4.3%)content was within a normal range(Table 3).Although body protein content(18.4%)was higher in fish fed diet with 89.5 mg kg−1OLA than that of control(16.5%)(P< 0.05),but such a difference was not significant among other dietary OLA treatments(P> 0.05)(Table 2).
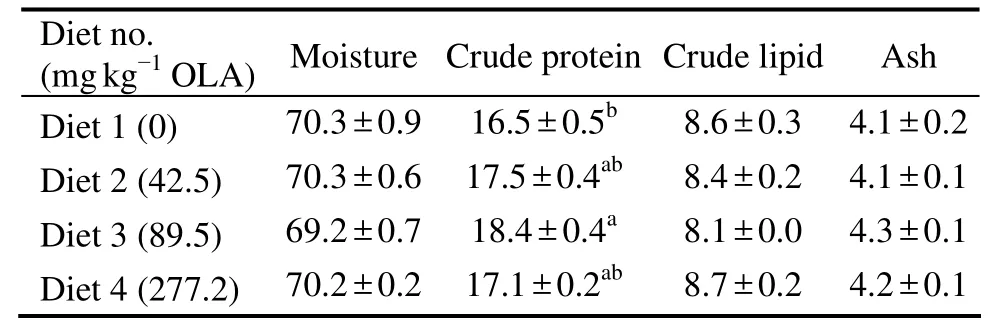
Table 3 Effect of dietary olaquindox on the body composition(% wet weight)of large yellow croaker
3.3 Histological Distribution OLA and MQCA
OLA and MQCA were not detectable in the fish tissues of control group; however,OLA residue and its metabolite MQCA significantly increased in skin,liver and muscle in a dose-dependent pattern(P< 0.05).Fish fed diet with 277.2 mg kg−1OLA had significantly higher OLA and MQCA in their liver(3.44 and 0.39 mg kg−1,respectively),skin(0.46 and 0.09 mg kg−1,respectively)and muscle(0.24 and 0.06 mg kg−1,respectively)than those fed other diets.In average,fish fed diet with 42.5,89.5 and 277.2 mg kg−1OLA had the highest content of OLA and MQCA in their liver followed by skin and muscle(Table 4).
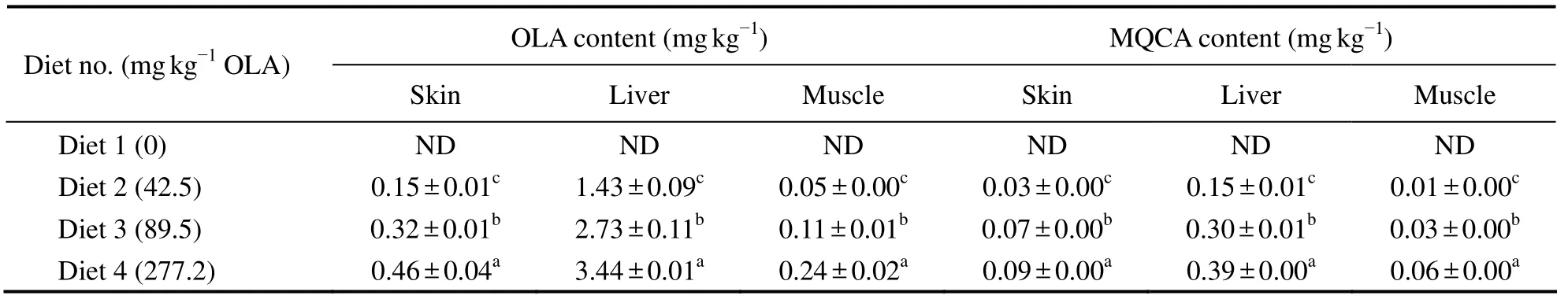
Table 4 Content of OLA and MQCA in different tissues of large yellow croaker
4 Discussion
OLA is a synthetic antimicrobial chemical,which can promote the growth of animals and prevent them from the infection of pathogens.In this study,we found that the survival rate of large yellow croaker was significantly influenced by dietary OLA,indicating that OLA played a role as an antimicrobial agent.However,such a scenario was not appeared when dietary OLA increased to 277.2 mg kg−1,of which the survival rate was lower than that of control.A similar trend was also reported early in grass carp(Yeet al.1992).We believed that the toxicity of OLA(Caoet al.,2001; Wanget al.,2004; Yanget al.,2005),especially when its content is high,caused such a pattern of the influence of OLA on the survival rate of fish.
It is believed that OLA can inhibit the growth of harmful bacteria and influence the metabolism of body nutrients,thus promoting the synthesis of nutrients,especially protein,of animal(Yeet al.,1992; Shenet al.,1995).In this study,the content of protein of the fish fed diet with 42.5 mg kg−1OLA was significantly higher than that of control,which may be explained by these early understandings.Nevertheless,high content of OLA may disrupt such a scenario; the fish fed diet with 277.2 mg kg−1OLA has lower content of protein than control.
The residue of OLA and its metabolites have been documented early(WHO,1991; FAO,1991).As reported early,70% of OLA administrated orally may be eliminated through urine unchanged,and 16 metabolites of OLA have been detected in the urine,of which 6 major metabolites are fully characterized(WHO,1991).Among the metabolites of OLA,MQCA was the last major remaining detectable metabolite in animal,has been designated as the marker substance(FAO/WHO,1995; MOA,2002).In this study,OLA and MQCA were high in content in the tissues of fish fed diet with 42.5,89.5 and 277.2 mg kg−1OLA but not in control,demonstrating that OLA and MQCA accumulated in the tissues of large yellow croaker.The content of OLA in diet significantly affected the histological concentration of its residue and its metabolite MQCA(P< 0.05),and showed an obvious dose-dependent relationship with the residues in tissues.A similar finding was also reported in mirror carp(Yeet al.,2003)and pig(Zhanget al.,2011a).The distribution of OLA and its metabolite MQCA was histologically different.In average,the content of OLA and MQCA was the highest in liver which was followed by that in skin and muscle.This pattern was similar to the report early in carp(Wanget al.,2003; Yeet al.,2003; Liet al.,2012)and tilapia(Chenet al.,2011).The metabolism pathway of OLA in animals may explain such a pattern.First,the liver is the major metabolizing places for OLA(WHO,1991),in which more OLA is transformed than in skin and muscle.Second,liver damage and metabolic disorder caused by OLA may slow the elimination of residues in liver(Zenget al.,1995; Yanget al.,2005).These factors may explain why the content of residual OLA and its metabolite MQCA in liver is the highest.
The toxicity of OLA and their metabolites to fish have been documented intensively,which included cumulative toxicity(Wanget al.,2004; Yanget al.,2005),photosensitive toxicity(De Vrieset al.,1990)and genotoxity(WHO,1991; Caoet al.,2001; Zouet al.,2011; Zhanget al.,2011b; Zhaoet al.,2013).Despite OLA has been put under ban in many countries,its illegal use in aquaculture has never vanished from sight all over the world.In this study,we found that OLA residue and its metabolite MQCA were detectable in diverse tissues of large yellow croaker when it was fed with the diet containing OLA.The OLA residue and its metabolites in farmed large yellow croaker is a potential risk to human health,thus we should keep watch on its use in order to ensure the safety of our food.
Acknowledgements
This study was supported by the National Key Technologies R & D Program for the 10thand 11thFive-year Plan of China(2001BA505B-06; 2006BAD03B03)and the Program for Changjiang Scholars and Innovative Research Team in University.We thank Liufu Z.G.and Liu X.W.for their assistance in diet preparation,and Wang Z.L.,Chen J.H.,Shentu J.K.,and Tan F.P.for their assistance in laboratory work.
Ai,Q.,Mai,K.,Zhang,L.,Tan,B.,Zhang,W.,Xu,W.,and Li,H.,2007.Effects of dietary β-1,3 glucan on innate immune response of large yellow croaker,Pseudosciaena crocea.Fish and Shellfish Immunology,22:394-402.
Anon,1989.Compendium of data sheets for veterinary products.In:National Office of Animal Health.Datapharm Publications Ltd,London,90pp.
Association of Official Analytical Chemists(AOAC),1995.Official Methods of Analysis of the Association of Official Analytical Chemists.16th edition.Arlington,VA,1-45.
Baars,A.J.,Van der Molen,E.J.,Spierenburg,T.J.,DE Graaf,G.J.,Nabuurs,J.J.A.,and Jager,L.P.,1988.Comparative toxicity of three quinoxaline-di-N-dioxide feed additives in young pigs.Archives Toxicology, 12(Suppl):405-409.
Cao,S.Z.,Zhang,L.,Liang,J.P.,and Liu,Z.P.,2001.Progress of study on the special toxicology of quinoxaline-1,4-dioxide-type antibacterial and growth-promoting agents.Progress in Veterinary Medicine,22:17-20(in Chinese with English abstract).
Chen,Y.,Lin,L.,Zhang,S.,Li,C.,and Li,Z.,2011.Pharmacokinetics of olaquindox and its metabolite in tilapia fish.Progress in Veterinary Medicine,32(10):54-58(in Chinese with English abstract).
De Vries,H.,Bojarski,J.,Donker,A.A.,Bakri,A.,and Beyersbergen van Henegouwen,G.M.,1990.Photochemical reactions of quindoxin,olaquindox,carbadox and cyadox with protein,indicating photoallergic properties.Toxicology,63(1):85-95.
Duan,Q.,Mai,K.,Zhong,H.,Si,L.,and Wang,X.,2001.Studies on the nutrition of the large yellow croaker,Pseudosciaena croceaR.I:Growth response to graded levels of dietary protein and lipid.Aquaculture Research,32(Suppl 1):46-52.
Emmert,B.,Schauder,S.,Palm,H.,Hallier,E.,and Emmert,S.,2007.Disabling work related persistent photosensitivity following photoallergic contact dermatitis from chlorpromazine and olaquindox in a pig breeder.Annals of Agricultural and Environmental Medicine,14:329-333.
FAO/WHO,Joint Expert Committee on Food Additives,1995.Evaluation of certain veterinary drug residues in food.WHO Technical Report Series,851:19.
Food and Agriculture Organization of the United Nations(FAO),1991.Residues of some veterinary drugs in animals and foods.FAO Food and Nutrition Paper.Food and Agriculture Organization,Rome,41/3:85-96.
Li,H.,Mai,K.,Ai,Q.,Zhang,C.,and Zhang,L.,2009.Effects of dietary squid viscera meal on growth and cadmium accumulation in tissues of large yellow croaker,Pseudosciaena croceaR.Frontiers of Agriculture in China,3(1):78-83.
Li,H.,Mai,K.,Ai,Q.,Zhang,L.,Zhang,C.,Zhang,W.,and Liufu,Z.,2007.Apparent digestibility of selected protein ingredients for larger yellow croakerPseudosciaena crocea.Acta Hydrobiologica Sinica,31(3):80-86(in Chinese with English abstract).
Li,S.,Ha,X.,and Mao,H.,2012.Study on the residual rules of olaquindox in carp.Journal of Anhui Agricultural Sciences,40(30):14746-14747(in Chinese with English abstract).
Mai,K.,Zhang,C.,Ai,Q.,Duan,Q.,Xu,W.,Zhang,L.,Liufu,Z.,and Tan,B.,2006.Dietary phosphorus requirement of large yellow croaker,Pseudosciaena croceaR.Aquaculture,251:346-353.
MOA(Ministry of Agriculture of China),2002.Maximum residue limits of veterinary drugs in the foods of animal.Announcement No.235,Annex 2:20.
Shen,W.,Zhang,Z.,and Cai,H.,1995.A preliminary study on the effects of the diets with olaquindox on growth rate of fingerling bluntnose black bream.Cereal and Feed Industry,4:23-24(in Chinese with English abstract).
Wang,K.,Geng,Y.,and Liu,K.,2004.Study on cumulative toxicity of OLA in common carp.Journal of Sichuan Agricultural University,22:183-186(in Chinese with English abstract).
Wang,K.,Geng,Y.,Ye,S.,and Huang,X.,2003.Studies on pathology and tissue concentration of the chronic olaquindox poisoning inCyprinus carpio.Journal of Fisheries of China,27(1):75-82(in Chinese with English abstract).
WHO,1991.Toxicological evaluation of certain veterinary drug residues in food.WHO Food Additive Series 27.World Health Organization,Geneva,227pp.
Xu,J.,Li,A.,Lou,W.,and Lu,D.,1988.The effects of promoter
on the promoting growth ofPenaeus orientalis kishinouye.
Marine Sciencies,5:35-39(in Chinese with English abstract).
Yang,X.L.,Hu,K.,Qiu,J.Q.,and Diao,J.H.,2005.Studies on accumulation and toxic of olaquindox in fishes.Acta Hydrobiologica Sinica,29:13-19(in Chinese with English abstract).
Ye,J.,Chen,Y.,and Shen,Z.,1992.Effect of HMQ supplemental concentration on the growth and survival rate of grass carp.Journal of Zhejiang College of Fisheries,11(1):25-31(in Chinese with English abstract).
Ye,J.,Yang,Y.,Lu,T.,Liu,H.,Zhao,J.,Han,Y.,and Li,H.,
2003.The accumulation and elimination of olaquindox in tissues of mirror carp.Journal of North East Forestry University,31(3):53-56(in Chinese with English abstract).
Zar,J.H.,1984.Production.In:Biostatistical Analysis.Zar,J.H.,ed.,Prentice-Hall,Englewood Cliffs,NJ,293-305.
Zeng,Z.L.,Dong,L.B.,and Chen,Z.L.,1995.Studies on elimination and residues of olaquindox in chicken tissues.Acta Veterinaria et Zootechnica Sinica,26:327-333(in Chinese with English abstract).
Zhang,J.,Zeng,Z.,and Ge,C.,2011a.Analysis on the distribution and correlation of residues of olaquindox in animal plasma and tissue.China Animal Health,5:13-15(in Chinese with English abstract).
Zhang,T.,Tang,S.,Jin,X.,Liu,F.,Zhang,C.,Zhao,W.,Zhang,S.,Sun,C.,and Xiao,X.,2011b.c-Myc influences olaquindox-induced apoptosis in human hepatoma G2 cells.Molecular and Cellular Biochemistry,354:253-261.
Zhao,W.,Tang,S.,Jin,X.,Zhang,C.,Zhang,T.,Wang,C.,Sun,Y.,and Xiao,X.,2013.Olaquindox-induced apoptosis is suppressed through p38 MAPK and ROS-mediated JNK pathways in HepG2 cells.Cell Biology and Toxicology,29:229-238.
Zou,J.,Chen,Q.,Jin,X.,Tang,S.,Chen,K.,Zhang,T.,and Xiao,X.,2011.Olaquindox induces apoptosis through the mitochondrial pathway in HepG2 cells.Toxicology,285:104-113.
杂志排行
Journal of Ocean University of China的其它文章
- A Numerical Study of Tidal Asymmetry:Preferable Asymmetry of Nonlinear Mechanisms in Xiangshan Bay,East China Sea
- Effect of Bacillusbaekryungensis YD13 Supplemented in Diets on Growth Performance and Immune Response of Sea Cucumber(Apostichopus japonicus)
- Evaluation of Three Harvest Control Rules for Bigeye Tuna(Thunnus obesus)Fisheries in the Indian Ocean
- Studies on the Effects on Growth and Antioxidant Responses of Two Marine Microalgal Species to Uniconazole
