Effect of Bacillusbaekryungensis YD13 Supplemented in Diets on Growth Performance and Immune Response of Sea Cucumber(Apostichopus japonicus)
2014-04-17YANFajunTIANXiangliandDONGShuanglin
YAN Fajun,TIAN Xiangli,and DONG Shuanglin
The Key Laboratory of Mariculture of Ministry of Education, Fisheries College, Ocean University of China, Qingdao 266003, P.R.China
© Ocean University of China,Science Press and Spring-Verlag Berlin Heidelberg 2014
1 Introduction
The sea cucumber(Apostichopus japonicus)in family Holothuroidea,order Echinodermata,has been considered as one of the most economically valuable aquaculture species in China(Zhanget al.,2004).In recent years,unfortunately,the rapid expansion and intensification of sea cucumber farming have caused diverse problems including pollution and the outbreak of diseases,for example,viscera ejection and skin ulceration syndrome,resulting in a considerable economic loss(Denget al.,2009).It is urgent to develop effective solutions in order to solve these problems.
The abuse of antibiotics in aquaculture for disease control and growth promotion has led to the evolution of resistant bacteria and raised the concerns of food safety(Boyd and Massaaut,1999; Esiobuet al.,2002).The application of probiotics is highly appreciated by environment-friendly and sustainable aquaculture practices(Balcázaret al.,2006).Probiotics refer to live microbes supplemented in feed,which should be beneficial to animals(Fuller,1989).They may inhibit pathogens and enhance the immune response,enzymatic digestion,feed utilization,and growth performance of animals(Verschuereet al.,2000; Balcázaret al.,2006)as were reported in fish and shrimp(Rengpipatet al.,1998; Rengpipatet al.,2000; Robertsonet al.,2000; Ziaei-Nejadet al.,2006; Nayak,2010; Sunet al.,2010).However,studies on sea cucumber probiotics are scarce.
Bacillussecrete diverse exoenzymes(Moriarty,1998).They have been used as potential probiotics in fish(Sunet al.,2010)and shrimp(Rengpipatet al.,1998; Rengpipatet al.,2000; Ziaei-Nejadet al.,2006).Bacillus baekryungensisYD13 was previously isolated from the sediment of sea cucumber outdoor culturing ponds and showed high activities of protease and amylase and a high capability of degrading protein,starch and COD in sea cucumber feed containing liquid medium(Yanet al.,2012).
The aim of this study was to evaluate the effect of YD13 on the growth performance and immune response of sea cucumber as a feed additive.Five immune relating enzymes,lysozyme(LSZ),acid phosphatase(ACP),alkaline phosphatase(ALP),superoxide dismutase(SOD)and catalase(CAT),have been reported to take part in immune defense and correlate well with immune compe-tence of invertebrates(Canicatti and Roch,1989; Campa-Córdovaet al.,2002; Mazorraet al.,2002; Chenet al.,2005; Wanget al.,2009; Guet al.,2010),which were chosen as indices in this study.
2 Materials and Methods
2.1 Bacterial Strain
YD13 was identified asBacillusbaekryungensisusing 16S rRNA gene sequencing(JN210568).It was a colony yellowish,Gram-positive,rod shape and spore forming bacterium,producing a high content of lipase,casease,catalase,protease and amylase(Yanet al.,2012).
2.2 Safety Test of the Strain
The pathogenic effect of YD13 was examined by injecting sea cucumber intraperitoneally with 0.1 ml saline suspension and bathing sea cucumber in seawater,which contained 0(control),1×104,1×106and 1×108CFU mL−1YD13,respectively,20 individuals each(Austinet al.,1995; Verschuereet al.,2000),with their health and mortality observed for 14 days and recorded daily.
2.3 Preparation of YD13 Supplemented Diet
YD13 was cultured in 2216E at 16 ℃± 1℃.The bacterial cells(about 1011CFU g−1wet weight)were harvested by centrifugation,and then added into the feed alive.Artificial dry feed served as the basic diet(Qingdao Great Seven Biotechnology Co.,Ltd.,China)which contained≥20% crude protein,≥3% crude lipid,≥2% calcium,≤16% crude fiber,and ≤25% ash.YD13 containing diets were prepared daily with a method reported early(Alyet al.,2008).The diets contained 0,1×104,1×106and 1×108CFU g−1of YD13,respectively.
2.4 Sea Cucumber and Experimental Design
Outdoor cultured sea cucumber was obtained from ponds located at Jiaonan,Qingdao,China.The animals were acclimated for two weeks in lab,during which they were fed with control diet once a day at a rate of 5% of biomass.The animals were starved for two days,and then 200 individuals selected randomly were divided into 20 aquaria containing 50 L aerating seawater,10 individuals each(4.72–6.06 g,5.44 g ± 0.17 g in average).Four testing diets were given to aquaria,5 each,once a day at 17:00 after water exchanging.The feeding rates were 5% and 6% of the biomass during the first and second months,respectively.Eighty percent of seawater was replaced each day.Water temperature was kept at 16℃ ± 1℃.Dissolved oxygen was maintained at or near saturation with air-stone blowers.Water salinity and acidity were checked daily and kept at 28–30 and pH 7.9–8.2,respectively.
2.5 Immune Assay
After the feeding trial(60 d),all sea cucumbers were starved for 24 h for tissue collection.The coelomic fluids(CLS)from 2 individuals each aquarium were pooled,frozen in liquid nitrogen and stored at −80 ℃ for immune assay.The frozen tissues were thawed at 4℃ ,disrupted ultrasonically at 20 KHz and 4 ℃ for 30s.All enzymatic assays were conducted within 12 h after thawing.
LSZ activity was measured according to Chen and Ji(1992).The assaying condition was the described early(Wanget al.,2008).A LSZ unit was defined as the absorbance change inMicrococcusluteuscell suspension per min.The specific LSZ activity was expressed as LSZ unit per mL CLS.
ACP and ALP assay was carried out with the method described previously(Barrett,1972).The assaying condition was described by Mazorraet al.(2002).An ACP or ALP unit was defined as the amount of enzyme required to produce 1 µmol phenol.The specific ACP/ALP activity was expressed as ACP/ALP unit per 100 mL CLS.
SOD activity was determined with the method of McCord and Fridovich(1969).The assaying condition was the described early(Wanget al.,2008)A SOD unit was defined as the amount of enzyme that inhibits the superoxide-induced oxidation by 50%.The specific SOD activity was expressed as SOD unit per mL CLS.
CAT activity was analyzed following the described(Goth,1991).The Assaying condition was the same as the described early(Wanget al.,2008).A CAT unit is defined as the amount catalyzing 1 µmol H2O2per second.The specific CAT activity was expressed as CAT unit per mL CLS.
Activities of all enzymes described as the above were analyzed with the kits produced by Nanjing Jiancheng,China.
2.6 Vibrio splendidus Challenge
At the end of feeding trial,a challenge test was conducted following the described(Yanet al.,2014).The presence of disease and mortality of sea cucumber byVibrio splendiduswas monitored for 7 d.
2.7 Statistics
After the feeding trial(60 d),sea cucumber in each aquarium was weighed.The specific growth rate(SGR,% d−1)and the feed conversion efficiency(FCE,%)were determined using the following equations:

wheretis the trial period in days(60 in this study),W0is the initial wet weight of sea cucumber(in grams),Wtis the final wet weight of sea cucumber at dayt(in grams).Data were presented as means ± SE.The values were first tested for the homogeneity of variances and then compared with a one-way ANOVA followed by Duncan multiple comparison tests using SPSS 13.0(SPSS Inc.,Richmond,CA,USA)to determine whether there was a significant difference among all treatments.The differ-ence was considered significant onceP< 0.05.
3 Results
3.1 Growth Performance
As showed in the safety test,YD13 did not induce any disease symptoms or mortality of sea cucumber,thus being not pathogenic toA.japonicus.Effect of YD13 on the growth performance ofA.japonicuswas shown in Fig.1.SGR(Fig.1a)and FCE(Fig.1b)of YD134 and YD136 were significantly higher than those of control(P< 0.05); however,such a difference was not found between YD138 and control.
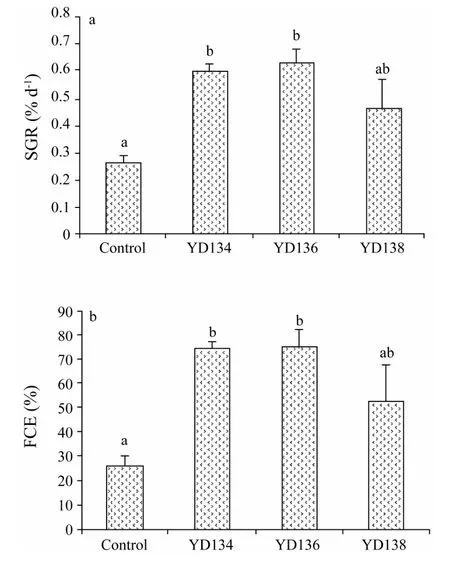
Fig.1 SGR and FCE of A.japonicus fed with 4 testing diets.Values are means ± SE(n =5).Values without a common superscript differ significantly(P < 0.05).
3.2 Immune Responses
LSZ activity was significantly affected by YD13.As showed in Fig.2a),LSZ activity increased significantly in YD134 and YD136 in comparison with control(P< 0.01).Such an activity in YD136 was significantly higher than that in YD134(P< 0.05).However,the activity in YD138 was not higher than that of control(P> 0.05).
ACP activity was significantly influenced by YD13(Fig.2b).ACP activity of all testing probiotic diets was significantly higher than that of control(YD134 and YD136,P< 0.05; YD138,P< 0.01).
ALP activity was significantly influenced by YD13(Fig.2c).ALP activity was significantly enhanced by YD136 and YD138 in comparison with control(P< 0.05).The activity of YD136 was significantly higher than of YD138(P< 0.05).
SOD activity was significantly affected by YD13(Fig.3a).SOD activity of all testing YD13 diets increased significantly in comparison with control(YD134,P< 0.01; YD136 and YD138,P< 0.05).The activity of YD134 was significantly higher than that of YD136 and YD138(P< 0.05).

Fig.2 LSZ,ACP and ALP activity of A.japonicus fed with 4 testing diets.Values are means ± SE(n =5).Values without a common superscript differ significantly(P < 0.05).

Fig.3 SOD and CAT activity of A.japonicus fed with 4 testing diets.Values are means ± SE(n =5).Values without a common superscript differ significantly(P < 0.05).
CAT activity was significantly affected by YD13(Fig.3b).CAT activity of all testing YD13 diets was significantly improved in comparison with control(P< 0.01).The activity of YD136 was significantly greater than of YD138(P< 0.05).
When summarized together,it could be seen that the non-specific immune capacity ofA.japonicuswas enhanced in various degrees with the oral administration of YD13.However,there was some difference in the extents of immune responses when YD13 doses increased from 1×104to 1×108CFU g−1of diet.No enhanced ALP activity was found when the dose of YD13 was low(1×104CFU g−1)(Fig.2c),and a suppression to LSZ,SOD and CAT activity was observed when the dose was high(1×108CFU g−1)(Figs.2a,3).Comparatively,all enzyme activities were improved when YD13 was supplemented in the dose of 1×106CFU g−1.These results indicated that the effect of YD13 toA.japonicuswas dose-dependent to some extent.
3.3 V.splendidus Challenge
The challenge test revealed that YD13 enhanced the immunity and disease resistance againstV.splendidusinfection in sea cucumber(Table 1).The cumulative mortality of sea cucumber in YD136 was 5%,which was significantly lower than those of the control(50%),YD134(15%)and YD138(30%).
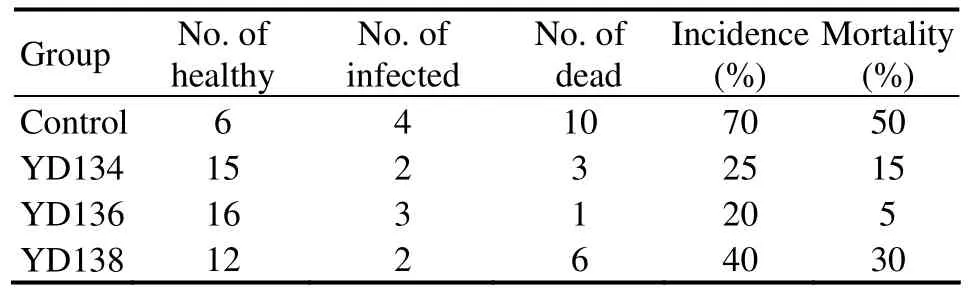
Table 1 Cumulative incidence and mortality during a 7-day V.splendidus challenge of A.japonicus fed with 4 testing diets
4 Discussion
The present study showed that when sea cucumbers were fed with 104,106and 108CFU g−1ofB.baekryungensisYD13 supplementing diets,their SGR and FCE were improved in comparison with control,indicating that YD13 functioned as a probiotic toA.japonicus.This finding was similar to the reported early in many aquatic animals,such as shrimp(Moriarty,1998; Rengpipatet al.,1998; Ziaei-Nejadet al.,2006),fish(Gatesoupe,1991; Wang and Xu,2006)and among others.Probiotics were demonstrated to make a nutritional contribution to oyster(Crassostreagigas)(Douillet and Langdon,1994),or increase digestive enzyme activity of shrimp(Ziaei-Nejadet al.,2006).Such a growth-improving effect has been reported in sea cucumber(Zhaoet al.,2012).Sea cucumber is detritus feeders,ingesting sediment for nutrients including organic matter,bacteria and protozoa and among others(Yanget al.,2006).Bacteria were among the essential constituent food items of sea cucumber in nature(Yingst,1976; Moriarty,1978).Therefore,the promoted growth performance ofA.japonicusmight be nutritional effect of YD13,which might contribute to the digestion of food and/or act as a complementary nutritional food source(Verschuereet al.,2000).However,it is not clear whether the activity of digestive enzymes in intestine ofA.japonicuswas increased by the supplementation of YD13 in diets,and the nutritional function of YD13 was unknown either.Thus,further research is needed to prove the role of YD13 in sea cucumber.
Modulation of immune system is one of the most common benefits of probiotics,and the immune response of animals to probiotics can increase their resistance to pathogens(Rodriguez and Le Moullac,2000; Marielet al.,2004).Like shrimp and many other invertebrates,sea cucumber lacks acquired immunity and its immune system is dependent primarily on non-specific immune response(Smith and Davidson,1994; Söderhall and Cerenius,1998).The critical role of coelomic fluid in its immune defense has been documented(Dybas and Frankboner,1986; Canicatti,1990; Guet al.,2010),which is mainly accomplished through circulating coelomocytes that can recognize foreign materials and react by phagocytosis and many other pathways(Glinski and Jarosz,2000).The ingestion of foreign particles,e.g.,bacterial cells,by sea cucumbers could elicit the function of phagocytic amoebocytes,which in turn results in phagocytosis and induce most of the non-specific immune responses(Smith,1981; Eliseikina and Magarlamov,2002).
LSZ,ACP and ALP are lysosomal hydrolytic enzymes,which participate in the destruction and elimination of foreign agents in invertebrates(Dybas and Frankboner,1986; Canicatti,1990; Xinget al.,2002).It was reported that LSZ activity in coelomic fluid ofA.japonicuswas significantly increased by superfine powder ofAstragalus membranaceussupplemented in diet(Wanget al.,2009).The present study showed similar results of LSZ activity increase in coelomic fluid ofA.japonicusstimulated by YD13.ACP and ALP activity in coelomic fluid were also significantly increased in almost all testing probiotic diets,being similar to the result of Liet al.(2009)who claimed that supplementation ofBacillusOJ in shrimp diet significantly increases the activity of ACP and ALP in serum of shrimp(Litopenaeus vannamei).Thus,all the changes in LSZ,ACP and ALP activity indicated that immune defense responses were greatly elicited inA.japonicusby YD13.
Antioxidant enzymes,SOD and CAT,are key cellular antioxidants in nonspecific immune system,playing important roles in scarvenging reactive oxygen species(ROS)such as O2−and H2O2(Coteuret al.,2002).The ROS are produced mainly by haemocytes during phagocytosis,and they can attack and kill the invaders(Andersonet al.,1992).But excessive ROS are harmful to host cells and can result in cellular injury and death(Chenet al.,2007).The present study showed that SOD activity was significantly enhanced by YD13.The results are similar to the previously reported,that beta-glucan or white spot syndrome virus could enhance the SOD level of shellfish haemolymph(Changet al.,2003; Zhanget al.,2005).CAT activity was also significantly higher than that of control.These results suggested that increased immune response stimulation occurred in the body ofA.japonicus,there eliminating ROS and depressing the oxidative damages during phagocytosis.This could be an indicator of the health ofA.japonicus(Jiet al.,2008).
The enhancement of the growth performance and immune response ofA.japonicusby YD13 was dose-dependent.In this study,significantly better growth was observed in YD134 and YD136; while no significant difference was found between YD138 and control.Furthermore,there was some difference in the extents of immune response for some immune enzymes inA.japonicuswhen YD13 doses increased from 1×104to 1×108CFU g−1of diet.No enhanced ALP activity was found when the dose of YD13 was low(1×104CFU g−1)(Fig.2c),and a suppression to LSZ,SOD and CAT activity was observed when the dose was high(1×108CFU g−1)(Figs.2a,3).These results indicated that the effect of YD13 toA.japonicuswas dose-dependent to some extent.The pathogen challenge test showed that YD13 significantly reduced the mortality of sea cucumbers,especially for YD136.This could be explained by an enhanced immune response ofA.japonicus.Thus,when growth,immunity,and disease resistance were all taken into consideration,1×106CFU g−1supplement of YD13 was recommended toA.japonicusbased on the results of the present study.
In conclusion,the results of this study suggested thatBacillusbaekryungensisYD13 was a potential probiotic when it was supplemented in sea cucumber diet,enhancing the growth performance and immune response of sea cucumber.The beneficial effect induced by YD13 was dose-dependent.The optimum administration dose was 1×106CFU g−1in diet.
Acknowledgements
This work was supported by the National Programs for High Technology Research and Development of China(Grant No.2006AA10Z409); the program for Excellent Youth Foundation of Shandong Province(Grant No.JQ201009); and the program for New Century Excellent Talents in University(Grant No.NCET-08-0503).
Aly,S.M.,Abd-Ei-Rahman,A.M.,John,G.,and Mohamed,M.F.,2008.Characterization of some bacteria isolated fromOreochromis niloticusand their potential use as probiotics.Aquaculture,277(1-2):1-6.
Anderson,R.S.,Paynter,K.T.,and Burreson,E.M.,1992.Increased reactive oxygen intermediate production by hemocytes withdrawn fromCrassostreavirginicainfected withPerkinsus marinus.Biological Bulletin,183:476-481.
Austin,B.,Stuckey,L.F.,Robertson,P.A.W.,Effendi,I.,and Grif fi th,D.R.W.,1995.A probiotic strain ofVibrio alginolyticuseffective in reducing diseases caused byAeromonas salmonicida,Vibrio anguillarumandVibrio ordalii.Journalof Fish Diseases,18:93-96.
Balcázar,J.L.,de Blas,I.,Ruiz-Zarzuela,I.,Cunningham,D.,Vendrell,D.,and Muzquiz,J.L.,2006.The role of probiotics in aquaculture.Veterinary Microbiology,114:173-186.
Barrett,A.J.,1972.Lysosomal enzymes.In:Lysosomes:A Laboratory Handbook.Dingle,J.T.,ed., North-Holland,Amsterdam,46-135.
Boyd,C.E.,and Massaaut,L.,1999.Risks associated with the use of chemicals in pond aquaculture.Aquacultural Engineering,20:113-132.
Campa-Córdova,A.I.,Hernández-Saavedra,N.Y.,and Ascencio,F.,2002.Superoxide dismutase as modulator of immune function in American white shrimp(Litopenaeus vannamei).Comparative Biochemistry and PhysiologyPart C,133:557-565.
Canicatti,C.,1990.Lysosomal enzyme pattern inHolothuria poliicoelomocytes.Journal of Invertebrate Pathology,56:70-74.
Canicatti,C.,and Roch,P.,1989.Studies onHolothuria polii(Echinodermata)antibacterial proteins.I.Evidence for and activity of a coelomocyte lysozyme.Cellular and Molecular Life Sciences,45(8):756-759.
Chang,C.F.,Su,M.S.,Chen,H.Y.,and Liao,I.C.,2003.Dietary ß-1,3-glucan effectively improves immunity and survival ofPenaeus monodonchallenged with white spot syndrome virus.Fish and Shellfish Immunology,15:297-310.
Chen,C.F.,and Ji,G.L.,1992.Activities and characterization of bacteriolytic substances in serum,skin and intestine mucus of grass carp.Journal of Huazhong Agricultural University,11:276-279.
Chen,J.H.,Mai,K.S.,Ma,H.M.,Wang,X.J.,Deng,D.,Liu,X.W.,Xu,W.,Liufu,Z.G.,Zhang,W.B.,Tan,B.P.,and Ai,Q.H.,2007.Effects of dissolved oxygen on survival and immune responses of scallop(ChlamysfarreriJones et Preston).Fish and Shellfish Immunology,22:272-281.
Chen,H.,Mai,K.S.,Zhang,W.B.,Liufu,Z.G.,Xu,W.,and Tan,B.P.,2005.Effects of dietary pyridoxine on immune responses in abalone,Haliotis discushannaiIno.Fish and Shellfish Immunology,19:241-252.
Coteur,G.,Warnau,M.,Jangoux,M.,and Dubois,P.,2002.Reactive oxygen species(ROS)production by amoebocytes ofAsterias rubens(Echinodermata).Fish and Shellfish Immunology,12:187-200.
Deng,H.,He,C.B.,Zhou,Z.C.,Liu,C.,Tan,K.F.,Wang,N.B.,Jiang,B.,Gao,X.G.,and Liu,W.D.,2009.Isolation and pathogenicity of pathogens from skin ulceration disease and viscera ejection syndrome of the sea cucumberApostichopus japonicus.Aquaculture,287:18-27.
Douillet,P.A.,and Langdon,C.J.,1994.Use of a probiotic for the culture of larvae of the Pacific oyster(Crassostrea gigasThunberg).Aquaculture,119:25-40.
Dybas,L.,and Frankboner,P.V.,1986.Holothurian survival strategies:Mechanisms for the maintenance of a bacteriostatic environment in the coelomic cavity of the sea cucumber,Parastichopuscalifornicus.Developmental and Comparative Immunology,10:311-330.
Eliseikina,M.G.,and Magarlamov,T.Y.,2002.Coelomocyte morphology in the HolothuriansApostichopus japonicus(Aspidochirota:Stichopodidae)andCucumaria japonica(Dendrochirota:Cucumariidae).Russian Journal of Marine Biology,28:197-202.
Esiobu,N.,Armenta,L.,and Ike,J.,2002.Antibiotic resistance in soil and water environments.International Journal of Environmental Health Research,12:133-144.
Fuller,R.,1989.Probiotic in man and animals.Journal of Applied Bacteriology,66:365-378.
Gatesoupe,F.J.,1991.The effect of three strains of lactic bacteria on the production rate of rotifers,Brachionus plicatilis,and their dietary value for larval turbot,Scophthalmus maximus.Aquaculture,96:335-342.
Glinski,Z.,and Jarosz,J.,2000.Immune phenomena in echinoderms.Archivum Immunologiae et Therapiae Experimentalis(Warszawa),48(3):189-193.
Góth,L.,1991.A simple method for determination of serum catalase activity and revision of reference range.Clinica Chimica Acta,196:143-151.
Gu,M.,Ma,H.M.,Mai,K.S.,Zhang,W.B.,Ai,Q.H.,Wang,X.J.,and Bai,N.,2010.Immune response of sea cucumberApostichopus japonicuscoelomocytes to several immunostimulantsin vitro.Aquaculture,306:49-56.
Ji,T.T.,Dong,Y.W.,and Dong,S.L.,2008.Growth and physiological responses in the sea cucumber,Apostichopus japonicusSelenka:Aestivation and temperature.Aquaculture,283:180-187.
Li,J.Q.,Tan,B.P.,and Mai,K.S.,2009.Dietary probioticBacillusOJ and isomaltooligosaccharides influence the intestine microbial populations,immune responses and resistance to white spot syndrome virus in shrimp(Litopenaeusvannamei).Aquaculture,291:35-40.
Mariel,G.,Fabiano,T.,and Jenny,R.,2004.Selection of probiotic bacteria and study of their immunostimulatory effect inPenaeus vannamei.Aquaculture,233:1-14.
Mazorra,M.T.,Rubio,J.A.,and Blasco,J.,2002.Acid and alkaline phosphatase activities in the clamScrobicularia plana:Kinetic characteristics and effects of heavy metals.Comparative Biochemistry and Physiology Part B,31:241-249.
McCord,J.M.,and Fridovich,I.,1969.Superoxide dismutase:an enzymatic function for erythrocuprein(hemocuprein).Journal of Biological Chemistry,24:6049-6055.
Moriarty,D.J.W.,1978.Bacterial biomass in coral reef sediments ingested by holothurians.Abstract Third International Echinoderm Conference,Sydney Australia.
Moriarty,D.J.W.,1998.Control of luminousVibriospecies in penaeid aquaculture ponds.Aquaculture,164:351-358.
Nayak,S.K.,2010.Probiotics and immunity:A fish perspective.Fish and Shellfish Immunology,29:2-14.
Rengpipat,S.,Phianphak,W.,Piyatiratitivorakul,S.,and Menasveta,P.,1998.Effects of a probiotic bacterium on black tiger shrimpPenaeus monodonsurvival and growth.Aquaculture,167:301-313.
Rengpipat,S.,Rukpratanporn,S.,Piyatiratitivorakul,S.,and Menasa-veta,P.,2000.Immunity enhancement on black tiger shrimp(Penaeus monodon)by a probiont bacterium(BacillusS11).Aquaculture,191:271-288.
Robertson,P.A.W.,Dowd,C.O.,Burrells,C.,Williams,P.,and Austin,B.,2000.Use ofCarnobacteriumsp.as a probiotic for Atlantic salmon(Salmo salarL.)and rainbow trout(Oncorhynchus mykiss,Walbaum).Aquaculture,185:235-243.
Rodriguez,J.,and Le Moullac,G.,2000.State of the art of immunological tools and health control of penaeid shrimp.Aquaculture,191:109-119.
Smith,L.C.,and Davidson,E.H.,1994.The echinoderm immune system:Characters shared with vertebrate immune systems and characters arising later in deuterostome phylogeny.Annals of the New York Academy of Sciences,712:213-226.
Smith,V.J.,1981.Echinoderms. In:Invertebrate Blood Cells.Vol.2.Ratcliffe,N.A.,and Rowley,A.F.,eds., Academic Press,London,513-562.
Söderhall,K.,and Cerenius,L.,1998.Role of the prophenoloxidase-activating system in invertebrate immunity.Current Opinion in Immunology,10:23-28.
Sun,Y.Z.,Yang,H.L.,Ma,R.L.,and Lin,W.Y.,2010.Probiotic applications of two dominant gutBacillusstrains with antagonistic activity improved the growth performance and immune responses of grouperEpinephelus coioides.Fish and Shellfish Immunology,29:803-809.
Verschuere,L.,Rombaut,G.,Sorgeloos,P.,and Verstraete,W.,2000.Probiotic bacteria as biological control agents in aquaculture.Microbiology and Molecular Biology Reviews,64(4):655-671.
Wang,F.Y.,Yang,H.S.,Gao,F.,and Liu,G.,2008.Effects of acute temperature or salinity stress on the immune response in sea cucumber,Apostichopus japonicus.Comparative Biochemistry and Physiology Part A,151(4):491-498.
Wang,T.,Sun,Y.,Jin,L.,Xu,Y.,Wang,T.,Ren,T.,and Wang,K.,2009.Enhancement of non-specific immune response in sea cucumber(Apostichopusjaponicus)byAstragalus membranaceusand its polysaccharides.Fish and Shellfish Immunology,27:757-762.
Wang,Y.B.,and Xu,Z.R.,2006.Effect of probiotics for common carp(Cyprinus carpio)based on growth performance and digestive enzyme activities.Animal Feed Science and Technology,127:283-292.
Xing,J.,Zhan,W.,and Zhou,L.,2002.Endoenzymes associated with haemocyte types in the scallop(Chlamys farreri).Fish and Shellfish Immunology,13:271-278.
Yan,F.J.,Tian,X.L.,Dong,S.L.,Yang,G.,and Wang,J.,2013.Isolation and selection of normal and low temperatureBacillusdegrading organic pollutants in sea cucumber culturing ponds.Periodical of Ocean University of China,43(6):17-24(in Chinese with English abstract).
Yan,F.J.,Tian,X.L.,Dong,S.L.,Fang,Z.H.,and Yang,G.,2014.Growth performance,immune response,and disease resistance againstVibrio splendidusinfection in juvenile sea cucumberApostichopus japonicusfed a supplementary diet of the potential probioticParacoccus marcusiiDB11.Aquaculture,420-421:105-111.
Yang,H.S.,Zhou,Y.,Zhang,T.,Yuan,X.T.,Li,X.X.,Liu,Y.,and Zhang,F.S.,2006.Metabolic characteristics of sea cucumberApostichopus japonicus(Selenka)during aestivation.Journal of Experimental Marine Biology and Ecology,330:505-510.
Yingst,J.Y.,1976.The utilization of organic matter in shallow marine sediments by an epibenthic deposit-feeding holothurian.Journal of Experimental Marine Biology and Ecology,23:55-69.
Zhang,C.Y.,Wang,Y.G.,Rong,X.J.,Sun,H.L.,and Dong,S.G.,2004.Natural resources,culture and problems of sea cucumber worldwide.Marine Fisheries Research,25:89-93.
Zhang,Z.F.,Shao,M.Y.,and Kang,K.H.,2005.Changes of enzyme activity and hematopoiesis in Chinese prawnFenneropenaeus chinensis(Osbeck)induced by white spot syndrome virus and zymosan A.Aquaculture Research,36:674-681.
Zhao,Y.C.,Zhang,W.B.,Xu,W.,Mai,K.S.,Zhang,Y.J.,and Liufu,Z.G.,2012.Effects of potential probioticBacillus subtilisT13 on growth,immunity and disease resistance againstVibrio splendidusinfection in juvenile sea cucumberApostichopus japonicus.Fish and Shellfish Immunology,32:750-755.
Ziaei-Nejad,S.,Mehran,H.R.,Ghobad,A.T.,Donald,L.L.,Ali-Reza,M.,and Mehdi,S.,2006.The effect ofBacillusspp.bacteria used as probiotics on digestive enzyme activity,survival and growth in the Indian white shrimpFenneropenaeus indicus.Aquaculture,252:516-524.
杂志排行
Journal of Ocean University of China的其它文章
- A Numerical Study of Tidal Asymmetry:Preferable Asymmetry of Nonlinear Mechanisms in Xiangshan Bay,East China Sea
- Evaluation of Three Harvest Control Rules for Bigeye Tuna(Thunnus obesus)Fisheries in the Indian Ocean
- Effect of Dietary Olaquindox on the Growth of Large Yellow Croaker(Pseudosciaena crocea R.)and the Distribution of Its Residues in Fish Tissues
- Studies on the Effects on Growth and Antioxidant Responses of Two Marine Microalgal Species to Uniconazole
