PRODUCTION, PURIFICATION AND CHARACTERIZATION OF RECOMBINANT CU/ZN SUPEROXIDE DISMUTASE OF BRUCELLA ABORTUS
2014-04-13ShustovManatUnyshivaSarinaEskendirova
Shustov A V, Manat Y, Unyshiva G B, Sarina N I, Eskendirova S Z
(National Center for Biotechnology, Astana, Republic of Kazakhstan)
PRODUCTION, PURIFICATION AND CHARACTERIZATION OF RECOMBINANT CU/ZN SUPEROXIDE DISMUTASE OF BRUCELLA ABORTUS
Shustov A V, Manat Y, Unyshiva G B, Sarina N I, Eskendirova S Z
(National Center for Biotechnology, Astana, Republic of Kazakhstan)
Abstract:Immunoreactive cytosolic Cu/Zn superoxide dismutase (Cu/Zn-SOD) ofBrucella abortuswas expressed and characterized in the present study. The gene encoding Cu/Zn-SOD was synthesized using two-round of PCR procedure. The Cu/Zn-SOD gene was cloned into pET-22b(+) vector for expression. The presence of recombinant Cu/Zn-SOD with a relative molecular weight of 20.8 kDa was demonstrated in the lysates of the induced in SDS-PAGE. The recombinant Cu/Zn-SOD reacted with mouse and bovine antiserum samples in Western blot. The preservation of its immunoreactivity indicates that the recombinant Cu/Zn-SOD might be a good antigen for development of vaccine and diagnostic reagents.
Brucellosis is a major veterinary and human health problem in most parts of the world, which is caused by different species ofBrucellathat are genetically very similar but have slightly different host specificity[1].B. melitensis, B.abortusandB. suisstrains cause abortion and infertility in their natural hosts goats, sheep, cattle and swine, respectively[2]. Human Beings become infected by contact with fluids from infected animals or derived food products like unpasteurized milk and cheese[3].
The superoxide dismutases (SOD) are components of the bacterial defense system against reactive oxygen species. In gram-negative bacteria includingBrucella, the Cu/Zn-dependent superoxide dismutase (Cu/Zn-SOD) along with catalase is found in the periplasmic space. It has been known that Cu/Zn-SOD is an importantBrucellaantigen that has been investigated for development of vaccine and diagnostic reagents for the prevention and control of brucellosis[4,5].
In the present study, we synthesized the gene fragment encoding theBrucellaCu/Zn-SOD, produced a recombinant Cu/Zn-SOD protein, and demonstrated its immune reactivity in mice.
1 Materials and methods
1.1The de novo synthesis of Cu/Zn-SOD geneGenefor bacterial expression of the Cu/Zn-SOD was synthesized in constructive PCR using long oligonucleotides as primers.
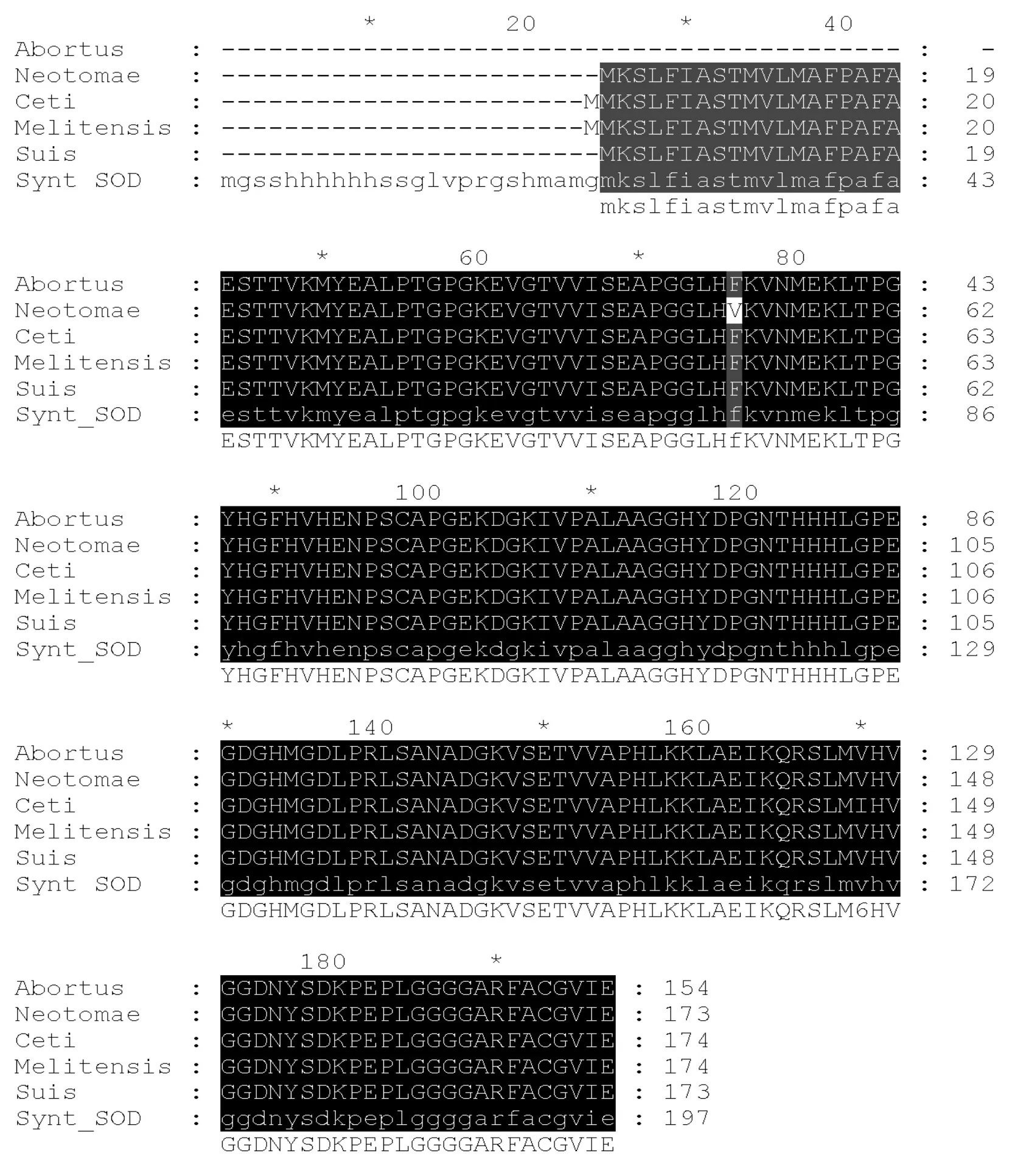
Fig. 1 Comparison of Cu/Zn-SOD from various Brucella species with amino acid sequence of the recombinant protein (Synt_SOD)
At first, amino acid sequences of the Cu/Zn-SOD protein of speciesB. abortus, B. melitensis, B. suis, B. neotomaeandB. cetiwere downloaded from Genbank and compared in a multiple alignment. Fig. 1 demonstrated high evolutionary conservatism of the Cu/Zn-SOD inBrucellagenus. We elected to synthesize one gene encoding the consensus amino acid sequence derived from the alignment. Considering the fact that the Cu/Zn-SOD is a group-specific antigen inBrucella, it is highly probable that the consensus protein covers all of the antigenic diversity of this antigen presented by different regional variants of the microorganism.
Thein silicodesigned sequence for the Cu/Zn-SOD is shown in Fig. 2. This gene was codonoptimized for expression inE.coli. The vector NTI suite was used for reverse translation coupled with codonoptimization for a heterologous species (E.coli K12). The DNAWorks v3.2.2 was used to calculate sequences of primers for thede novosynthesis of DNA fragments. The primers used for thede novogene synthesis are listed in Table 1. These primers were designed for use in PCR with annealing temperature of 62℃ in presence of 50 mmol/L Na+and 2 mmmol/L Mg2+. In the constructive PCR procedure, these primers were divided into two groups and designated as“internal”or“flan king”primers. Each of the internal primers was 100% homologous to the corresponding region in the sequence to be synthesized. Internal primers were interleaved in the sense-antisense-sense-antisense manner. Whole set of internal primers covered the entire length of the DNA fragment to be synthesized except for the very 5′- and 3′- terminal linkers. The terminal linkers with restriction sites for subsequent cloning were included in the flanking primers.

Fig. 2 The gene for Cu/Zn-SOD

Table 1 Primers for synthesis of Cu/Zn-SOD gene
Thede novosynthesis of Cu/Zn-SOD gene was performed as a two-round PCR. Phusion HotStart DNA polymerase (Thermo Scientific) was used to avoid PCR errors. The mixture of 14 internal primers (each at 0.4 pmmol/L final concentration) was subjected to the first round of PCR. The external template was not added to reactions in the first round. Total 30 cycles were done as following∶ denaturation at 95℃ for 1 min, annealing at 60℃ for 1 min and extension at 72℃ for 1 min. The PCR products of the first round were diluted 1∶10 with water and 1 μL template was used in the second round of PCR amplification. For the second round of PCR, a pair of flanking primers (each at 2 pmol/L final concentration) was used. The product of the second round PCR was expected to be 496 bp in length. The PCR products were subject to electrophoresis in 1% agarose gels, cloned into pGEM-T using the pGEM-T Easy Cloning kit (Promega) and sequenced. Double strand automated sequencing was performed for confirmation of the identity of cloned fragment to the designed sequence of the gene.
1.2Protein expressionEscherichia coliBl21(DE3) were transformed with the plasmid pET-22b(+) and grown on solid LB/ampicillin (100 μg/mL) plates at 37℃ overnight. A single colony was selected to grow a 5 mL starter culture overnight at 37℃. The starter culture was inoculated in 500 mL LB/ampicillin (100 μg/mL) and incubated at 37℃ with shaking until the OD600reached 0.6. Expression of recombinant Cu/Zn-SOD was induced by addition of IPTG to a fnal concentration of 1 mmol/L and expression was continued for 12 h at 37℃. Finally cells were harvested and collected by centrifugation. Induced cells, as well as uninduced cells exposed to the same conditions, 12% PAGE, as described by Laemmli.
In order to ascertain the location of the expressed recombinant protein, the bacterial cell suspension was sonicated for 10 min with a pulse interval of 8 s. The sonicated extract was centrifuged at 18 600×gfor 30 min at 4℃. The supernatant and cell pellet, with appropriate controls and molecular mass markers, were analysed by 12% SDS-PAGE. After confirmation of the solubility, the protein was purified by His-tag binding affinity to Ni-NTA agarose. Purification of the cell supernatant was carried out using a HisTrap FF crude with a native purification protocol as specified by the manufacturer. The purified protein was checked by 12% SDS-PAGE followed by Coomassie blue staining, and protein concentration was estimated by the Bradford method.
1.3Animal immunization and Western blotTwo groups of inbred female BALB/c mice (2 mice per group) were used for preparation of antiserum against rCu/Zn-SOD. Different groups of experimental mice were immunized with different concentration of adjuvanted rCu/Zn-SOD 25 μg for group 1 and 50 μg for group 2 of adjuvanted rCu/Zn-SOD. A booster was given on day 12 post the first immunization. Blood samples were collected on day 15 post the booster immunization. Then, serum samples were prepared by centrifugation at 5000×gfor 10 min at 4℃. The final serum preparations were kept at -20℃ till use.
The presence of specific antibodies against rCu/Zn-SOD in mouse serum and positive bovine serum were demonstrated in Western blot. Briefly, purified rCu/Zn-SOD preparations were run in 12% SDS-PAGE gels and transferred onto nitrocellulose membranes. The membranes was then blocked with 1% bovine serum albumin (Sigma, USA) for 2 h at 4℃ and washed five times with PBS (0.01 mol/L, pH 7.2) containing Tween 20 (PBST). Subsequently, the membranes were incubated with serum samples (first membrane with positive bovine serum and second membrane with immunized mice serum) for 2 h at 4℃. The membranes were washed with PBST and incubated with HRP conjugated goat anti-mouse IgG antibody (1∶2 500 dilution) for 1 h at 4℃. Finally, the membranes washed with PBST and the colors developed by adding 4-chloro-l-naphthol in the presence of hydrogen peroxide.
2 Results and discussion
Brucellosis is an emerging disease in many regions of the developing world[6]. Diagnosis of this disease is carried out mostly by isolation and characterization of the bacterium from blood samples[7].However, growth of the bacterium in primary culture is very slow as it takes a minimum of 3-8 days and sometimes as long as 45 days. The slow growth ofBrucellain primary culture delays the diagnosis and treatment. Moreover, the success of the culture technique is not satisfied and depends on many factors such as disease stage, culture medium and availability of viable bacteria in blood samples[8]. Hence, isolation of bacterium is not a feasible diagnostic tool for the confirmation of brucellosis. In the absence of an effective isolation procedure, serological tests, mainly agglutination tests, are relied on for the clinical diagnosis of brucellosis[9]. Several studies have compared the tests available for the diagnosis of brucellosis and confirmed the superiority of ELISAs for detection of chronic and complicated cases[10]. An immunochromatographic test for the rapid detection of antibodies againstB. abortuswas developed. The results suggest that immunochromatographic test is rapid, simple, reliable and suitable for use to detectB. abortusinfection in the field. The Cu/Zn-SOD antigen has a high diagnostic value for use in the development of immunochromatographic test systems for brucellosis[11,12].
In the present study, rCu/Zn-SOD gene was synthesized using a two-round PCR procedure. The resulting PCR products showed a band of the correct length in lanes 1 and 2 (Fig. 3). The rCu/Zn-SOD gene segment obtained in the present study was 496 bp and encoded 164 amino acid residues with a calculated molecular weight of 20.8 kDa. These features were in agreement with the reported molecular mass of Cu/Zn-SOD purified from induced cell extract as determined in SDS-PAGE.
The rCu/Zn-SOD was visualized in the periplasmic extracts analyzed on SDS-PAGE. Because of the presence of the His-tag fusion peptide in expression vector at C-terminal, the recombinant Cu/Zn-SOD had 41 extra amino acids residues including 6×His tag. As shown in Fig. 4, expression of rCu/Zn-SOD was achieved withE. coliBL21(DE3). The recombineant protein showed a molecular weight of about 20.8 kDa.
The rCu/Zn-SOD was purified under native conditions. The recombinant rCu/Zn-SOD that bound to the Ni-NTA agarose was eluted using a pH gradient. The different washes and eluates were analyzed in SDSPAGE and the rCu/Zn-SOD was purified to greater than 90% (Fig. 5A). The immunoreactivity of the rCu/Zn-SOD was confirmed in Western blot. The rCu/Zn-SOD reacted with an anti-His antibody and mouse polyclonal antibody raised against the whole-cell sonicated antigen ofB. abortusand culture-confirmed bovine positive serum samples.
The reactivity of the rCu/Zn-SOD was further confrmed in Western blot using bovine antiserum samples of brucellosis as shown in Fig. 5B.
In conclusion, the gene encodingB. abortusCu/Zn-SOD was cloned and expressed using the pET-22(b+) expression system. The recombinant Cu/Zn-SOD antigen was prepared and confirmed in Western blot withBrucella-positive bovine serum and immunized mice serum. These results suggested that rCu/Zn-SOD ofB. abortusmight be used as a potential antigen for such diagnostic purposes and the protection againstB. abortusinfection.
Fig. 3 Electrophoretic results of PCR products 1,2: PCR products
Fig. 4 SDS–PAGE analysis of rCu/Zn-SOD in pET-22(b+) vector with modifed buffers under denaturing conditions.
Fig. 5 A: SDS-PAGE and Western blot analysis of purif ed rCu/Zn-SOD
References
[1] AI Dahouk S, Tomaso H, Nöckler K,et al. Laboratory based diagnosis of brucellosis - a review of the literature. Part II∶ serological tests for brucellosis[J]. Clin Lab, 2003, 49 (11-12)∶ 577-589.
[2] Henk L, Kadri S M. Brucellosis[J]. Indian Practising Doctor, 2004, 1(3)∶ 60-64.
[3] Smits H L, Kadri S M. Brucellosis in India∶ a deceptive infectious disease[J]. Indian J Med Res, 2005, 122(5)∶375-384.
[4] Chaudhuri P, Singha H, Goswami T K,et al. DNA prime and Protein Boost Immunization with Combined SOD-L7/L12 Antigen Confers Protection to Mice against Brucella abortus 544 Challenge[J]. Adv Anim Vet Sci, 2013, 1 (5)∶ 143-147.
[5] Batra H V, Agarwal G S, Bala K,et al. Comparison of dot-enzyme linked immunosorbent assay (dot-ELISA) kit with other serological tests for the detection of Brucella antibodies in bovines[J]. Ind J Comp Microbiol Immunol Infect Dis, 1998, 19, 104-107.
[6] National Association of State Public Health Veterinarians. Public health implications ofBrucella canisinfections in Humans [Z]//Summary findings and recommendations of theBrucella canisworkgroup, 2012.
[7] Queipo-Ortuño M I, Morata P, Oc ó n P,et al. Rapid diagnosis of human brucellosis by peripheral-blood PCR assay[J]. J Clin Microbiol, 1997, 35 (11)∶ 2927-2930.
[8] Mantur B G, Biradar M S, Bidri R C,et al. Protean clinical manifestations and diagnostic challenges of human brucellosis in adults∶ 16 years' experience in an endemic area[J]. J Med Microbiol, 2006, 55 (7)∶ 897-903.
[9] Ciocchini A E, Rey Serantes D A, Melli L J,et al. Development and validation of a novel diagnostic test for human Brucellosis using a glyco-engineered antigen coupled to magnetic beads[J]. PLOS Negl Trop Dis, 2013, 7(2)∶ e2048.
[10] Farid M, Nadia M A, Mowafy and Khalil I. Significance of Brucella AGGLU-tinin in febrile Egyptian children[J]. Sci Med J Cai Med Synd, 1990, 2 (3)∶ 183-192.
[11] Sotnikov D V. Immunochromatographic serodiagnosys of cattle brucellosis with the use of colloidal[J]. Mod Probl Sci Educ. Published online.
[12] Contreras-Rodriguez A, Seleem M N, Schurig G G,et al. Cloning, expression and characterization of immunogenic aminopeptidase N from Brucella melitensis[J]. FEMS Immunol Med Microbiol, 2006, 48 (2)∶ 252-256.
中图分类号:S852.614
文献标识码:A
文章编号:1674-6422(2014)02-0046-06
流产布鲁氏菌的Cu/Zn超氧化物歧化酶的表达及其特性分析
摘要:本研究体外表达了流产布鲁氏菌的Cu/Zn超氧化物歧化酶(Cu/Zn-SOD)并对表达产物进行了免疫原性分析。扩增编码Cu/Zn-SOD的基因,并将其克隆连接到pET-22b(+)载体中。SDS-PAGE 结果显示诱导细菌的裂解物有重组Cu/Zn-SOD表达,分子量为20.8 kDa。Western blot结果显示重组Cu/Zn-SOD均与鼠、牛的阳性血清反应。由此,说明体外表达的重组Cu/Zn-SOD具有很好的抗原性,为今后疫苗开发及诊断试剂的开发奠定了物质基础。
