Reversible lesions in the brain parenchyma in Wilson’s disease con fi rmed by magnetic resonance imaging: earlier administration of chelating therapy can reduce the damage to the brain
2014-04-07DukoKoziIgorPetroviMarinaSvetelTatjanaPekmezoviAleksandarRagajiVladimirKosti
Duško B. Kozić, Igor Petrović, Marina Svetel, Tatjana Pekmezović, Aleksandar Ragaji, Vladimir S. Kostić
1 Diagnostic Imaging Center, Institute of Oncology, School of Medicine, University of Novi Sad, Put Doktora Goldmana 4, 21204 Sremska Kamenica, Serbia
2 Institute of Neurology Clinical Centre of Serbia, School of Medicine, University of Belgrade, Dr Subotića 6, 11000, Belgrade, Serbia
3 Institute of Epidemiology, School of Medicine, University of Belgrade, Dr Subotića 6, 11000, Belgrade, Serbia
Reversible lesions in the brain parenchyma in Wilson’s disease con fi rmed by magnetic resonance imaging: earlier administration of chelating therapy can reduce the damage to the brain
Duško B. Kozić1, Igor Petrović2, Marina Svetel2, Tatjana Pekmezović3, Aleksandar Ragaji1, Vladimir S. Kostić2
1 Diagnostic Imaging Center, Institute of Oncology, School of Medicine, University of Novi Sad, Put Doktora Goldmana 4, 21204 Sremska Kamenica, Serbia
2 Institute of Neurology Clinical Centre of Serbia, School of Medicine, University of Belgrade, Dr Subotića 6, 11000, Belgrade, Serbia
3 Institute of Epidemiology, School of Medicine, University of Belgrade, Dr Subotića 6, 11000, Belgrade, Serbia
The aim of this study was to evaluate the resolution of brain lesions in patients with Wilson’s disease during the long-term chelating therapy using magnetic resonance imaging and a possible signi fi cance of the time latency between the initial symptoms of the disease and the introduction of this therapy. Initial magnetic resonance examination was performed in 37 patients with proven neurological form of Wilson’s disease with cerebellar, parkinsonian and dystonic presentation. Magnetic resonance reexamination was done 5.7 ± 1.3 years later in 14 patients. Patients were divided into: group A, where chelating therapy was initiated < 24 months from the fi rst symptoms and group B, where the therapy started ≥ 24 months after the initial symptoms. Symmetry of the lesions was seen in 100% of patients. There was a signi fi cant difference between groups A and B regarding complete resolution of brain stem and putaminal lesions (P= 0.005 andP= 0.024, respectively). If the correct diagnosis and adequate treatment are not established less than 24 months after onset of the symptoms, irreversible lesions in the brain parenchyma could be expected. Signal abnormalities on magnetic resonance imaging might therefore, at least in the early stages, represent reversible myelinolisis or cytotoxic edema associated with copper toxicity.
nerve regeneration; Wilson’s disease; diagnostic imaging; chelating therapy; magnetic resonance imaging; delayed diagnosis; metabolic disorders; copper toxicity; hepatic encephalopathy; pontine myelinolysis; cirrhosis; neural regeneration
Funding:This work was supported by a grant from the Ministry of Science and Technological Development of Serbia, Scientific Project Number 175090.
Kozić DB, Petrović I, Svetel M, Pekmezović T, Ragaji A, Kostić VS. Reversible lesions in the brain parenchyma in Wilson’s disease confirmed by magnetic resonance imaging: earlier administration of chelating therapy can reduce the damage to the brain. Neural Regen Res. 2014;9(21):1912-1916.
Introduction
Wilson’s disease (WD) is a rare and treatable autosomal recessive disease with deficient biliary excretion of copper (Wilson, 1912). The initial symptoms of patients with WD are usually due to either cerebral involvement or liver failure (Ala et al., 2007; Hancu et al., 2011).
If treatment begins on time, especially in the asymptomatic phase of the disease, the occurrence of clinical signs can be prevented, and patients present with normal life expectancy (Walshe, 1993). Complete or partial remission of already developed neurological signs during chelating therapy can be expected in 20% and 60% of patients, respectively (Stremmel et al., 1989; Walche et al., 1993). Potential regression of increased signal intensity (SI) on T2-weighted images in the basal ganglia, brain stem or thalamus may document the effect of chelating therapy in patients with WD on magnetic resonance imaging (MRI).
In this study, we evaluated the resolution of brain lesions on MRI during the long-term chelating therapy, associated with the time latency in establishing the correct diagnosis of WD. To the best of our knowledge, no prior studies evaluating the presence of irreversible changes in the brain parenchyma, associated with late diagnosis of WD and treatment delay, are available in the literature.
Subjects and Methods
Patients
Thirty-seven patients with WD, 24 males and 13 females, underwent initial brain 1.5-T MRI (Table 1). The disease occurs at a wide age range of 12-41 years, with a mean of 28 years. The diagnosis of WD was established by the previously described criteria (Walshe, 1988). The recon fi rmation of WD diagnosis was performed using Leipzig scoring system (Ferenci et al., 2003). Correlation with initial clinical presentation was performed.
Magnetic resonance imaging
The following pulse sequences were used: (a) sagittal flash2D (FL2D) T1-weighted (T1W) matrix = 192 × 256, field of view = 240 × 240 mm2, repetition time (TR) = 266 ms, echo time (TE) = 6 ms, parallel imaging reduction factor (SENSE factor) = 2, slice thickness of 5 mm and slice gap = 0.5 mm; (b) axial turbo spin echo (TSE) - double echo (T2W) matrix = 192 × 256, field of view = 230 × 230 mm2, TR = 3,300 ms, TE = 93 ms, parallel imaging reduction factor (SENSE factor) = 1, slice thickness of 6 mm and slice gap = 0.6 mm.
MR reexamination
Follow-up MRI study was performed in 14 patients, 5.71 ± 1.29 years after initial scanning (median 5.71). They underwent follow-up after the introduction of D-penicillamine treatment at the Department for Movement Disorders of our institution between 1996 and 2005 (Tables 2, 3). The time latency between the appearance of the first symptoms and signs and the initiation of the chelation therapy was recorded for each patient. In the course of the follow-up, all the patients were continuously on optimalized D-penicillamine treatment. This study was approved by the Ethical Committee of our institution. All the patients signed informed consent for their participation in the study.
Patients were divided into two groups: Group A ( fi ve patients who initiated chelating therapy < 24 months [range: 6-18 months] from the fi rst symptoms of WD), and group B (nine patients whose therapy started ≥ 24 months [range 24-60 months] after the initial symptoms of the disease).
Data analysis
Signal abnormalities on the follow-up brain MRI study were de fi ned as: (a) complete resolution; (b) partial resolution; (c) stable status/progression. Although the low SI in the globus pallidus, substantia nigra and nucleus ruber related to paramagnetic deposition is usually prominent, these abnormalities were not considered in this study because such SI may be present in the process of normal aging.
The determination of neuroanatomical localization, symmetry, volume of the lesions and the presence of atrophy were rated by two experienced radiologists (Duško Kozić and Robert Semnic, University of Novi Sad Faculty of Medicine, Novi Sad, Serbia) who were blinded to clinical data. Intra and inter-rater reliability for rating and monitoring brain abnormalities between two readers were determined using two-way mixed effect model (absolute agreement) intra-class correlation coef fi cients (ICC) from available scans. The ICC was considered as poor when it was below 0.4, fair to good between 0.4 and 0.75, and excellent for values above 0.75.
The data are presented as the mean ± SD or as percentages. Differences in categorical variables were assessed by Fisher exact test. The signi fi cance level for the analysis was set atP< 0.05.
Results
Image analysis in initial MR study
The lesions were 100% symmetric in the putamina, caudate nuclei, midbrain and pontine tegmentum. Putamen was affected in 78% of patients, but in 100% of those with dystonic clinical presentation (Table 1). Correlation between putaminal affection and dystonic presentation in comparison to cerebellar manifestation of the disease was statistically signi fi cant (P= 0.035). The ICC was excellent.
Image analysis in follow-up MR study
Follow-up MR examination (demographic and clinical characteristics of patients presented in Table 2) revealed complete or partial resolution of the MRI lesions after D-penicillamine treatment in a substantial number of patients with WD in the putamen, caudate nuclei, thalamus, and brainstem (Figure 1). These changes were particularly prominent in mesencephalon and pons and to a lesser degree in basal ganglia. In all three patients with complete resolution, the putaminal lesions were described only on the PDW sequence on initial scanning and they all belong to the group A (patients with introduction of D-penicillamine less than 24 months of the disease onset). In two patients from the group B (patients with delayed onset of such therapy), end-stage neurodegenerative changes were observed in the putaminal periphery (Figure 1A, D). Also, in two patients, focal deposition of paramagnetic substance in putamina and caudate nuclei was found.
Statistical analysis
Although statistical analysis was limited by the small number of patients in different subgroups (Table 3), Fisher’s exact test revealed signi fi cant difference in distribution of patients with complete or partial resolution, as well as those without change of putaminal lesions, between groups A and B (P= 0.024). There was a signi fi cant difference between groups A and B regarding complete resolution of brain stem lesions (P= 0.005).
Discussion
Numerous studies regarding the clinical course of WD disease are available in literature. Compared to previous studies, our results provide new contribution regarding the relationship between complete regression of brain lesions and delay in establishment of correct diagnosis.
Sinha et al. (2007) performed serial follow-up MR scans in 50 patients with WD and revealed an improvement in MRI parameters in 35 patients, with no significant changes in 10, worsening in 4 and a mixture of resolving and evolving changes in 1. However, no correlation between treatment delay and imaging con fi rmation of lesions regression had been performed in that study. Eighteen patients with neurological WD underwent pretreatment and posttreatment brain MRI scans in the study of da Costa Mdo et al. (2009) in order to evaluate the range of abnormalities and the evolution of lesions in WD during different periods, up to 11 years after the beginning of treatment. Neuroimaging pattern of evolution was more favorable for the group of patients that received exclusively D-penicillamin, compared to zinc. Supratentorial white matter is usually spared in WD in the majority of MRI studies. But, in one of more recent studies, involvement of corpus callosum had been reported (Trocello et al., 2011).
Regardless of the type of clinical presentation, most patients with WD will have at least subclinical degree of liver disease. However, the exact factors that induce relatively sharp differentiation of clinical presentation remain un-known (Brewer and Yuzbasiyan-Gurkan, 1992). It is known that severity of clinical course and therapeutic outcome in patients with neurologic form of WD depend significantly on early diagnosis (Prashanth et al. 2004; Brewer, 1995). In the study of Hu et al. (2001), only 30% of 1,011 patients with hepatolenticular degeneration were correctly diagnosed within 3 months after onset and the positive effect of treatment was signi fi cantly better in the group of patients with early diagnosis. In the study of Prashanth et al. (2004), the errors in establishing the diagnosis of WD were found in 62.5% of patients, with a mean (SD) delay of 2 years (0.08 - 30 years). The mean delay in the study of Walshe and Yealland (1993) was 12.8 months, while in the study of Miranda et al. (1995), it was 14 months. Pellecchia et al. (2003) searched 30 patients with WD between years 1970 and 2000 and found that the mean delay to diagnosis was 5.9 ± 5.7 years. These results suggest that WD was significantly less frequently included in differential diagnosis three decades ago, probably due to less reliable determination of copper and ceruloplasmin levels, limited availability of neuroimaging studies and decreased sensitivity for architectural liver changes on ultrasound examination. Aisen et al. (1985) reported no signi fi cant interval change of follow-up MRIs in five patients, probably because their patients had been on D-penicillamine therapy for several years and the follow-up period (4-8 months) was too short for changes to occur. According to Yuzbasiyan-Gurkan et al. (1992), neurological patients started to show clinical improvement 5-6 months after initiation of anticopper therapy and continued to improve over the succeeding 18 months.

Table 1 Frequency of brain lesions detected with magnetic resonance imaging examination in 37 patients with Wilson’s disease
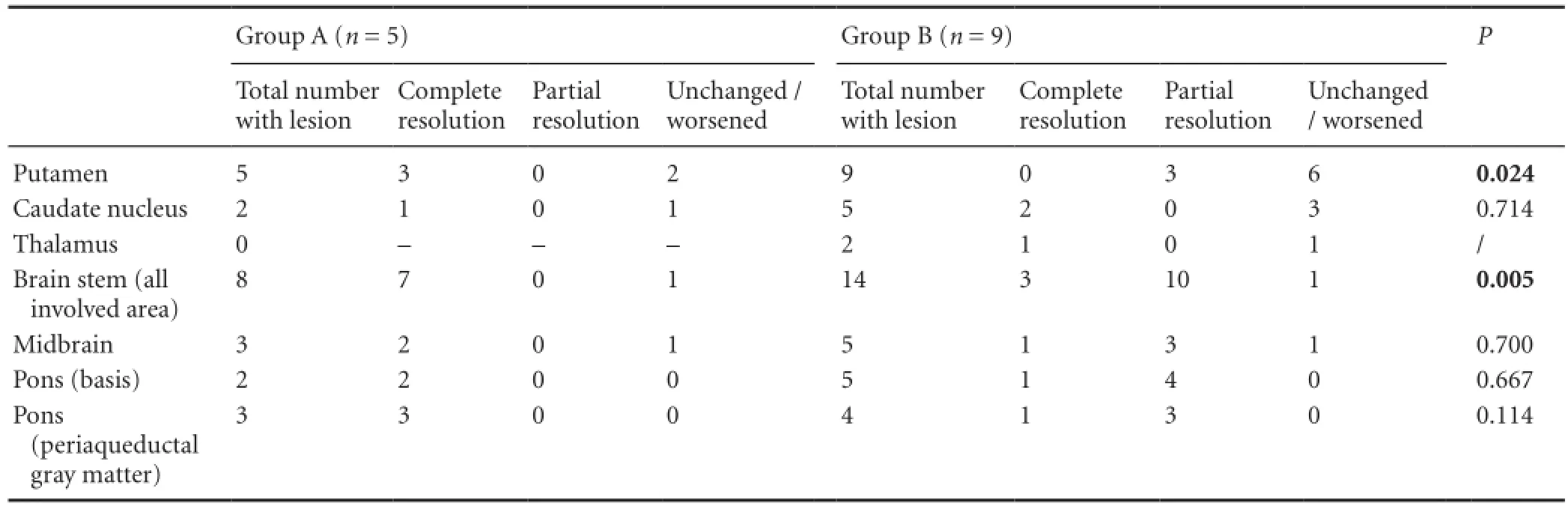
Table 3 Number of patients with observed changes on magnetic resonance imaging (MRI) in speci fi c brain regions between initial and followup MR studies
Our study found complete or partial resolution of MRI lesions in a substantial number of patients with WD during 5.7 ± 1.3 years of D-penicillamine treatment (Table 3). These data are concordant with the studies describing that neuroimaging abnormalities, both on CT and MRI, may improve on D-penicillamine, trientine hydrochloride - trien (Williams and Walshe, 1981; Nazer et al., 1993; Thuomas et al., 1993; Roh et al., 1994; King et al., 1996; Takahashi et al., 1996) or zinc treatment, as a monotherapy or in combination with D-penicillamine (Prayer et al. 1990; Heckmann et al. 1994; Huang and Chu, 1996; Pellecchia et al. 2003). Improvement was also observed in patients after liver transplantation (Stracciari et al. 2000). Besides its effects on the brain tissue, D-penicillamine treatment during 3-year follow-up also improved laparoscopic and histological fi ndings of the liver in one patient with WD (Sakaida et al., 2005). Complete regres-sion of liver nodules in a patient with WD after D-penicillamine treatment has been shown (Kozic et al., 2006).

Table 2 Demographic and clinical characteristics of patients with Wilson’s disease
Published MRI studies in WD, including our own, have shown a number of symmetric abnormalities, including: (a) high SI on T2W images of the basal ganglia, brainstem, cerebellar peduncles, and supratentorial white matter (Starosta-Rubinstein et al., 1987; King et al., 1996; van Wassenaer-van Hall et al., 1996; Svetel et al., 2001); (b) low SI on T2W images of the globuspallidus, substantianigra, red nucleus and corpus striatum (van Wassenaer-van Hall et al. 1996; Braffman, 2000); (c) high SI on T1W images of the globuspallidus in patients with portosystemic shunt (van Wassenaer-van Hall et al., 1996; Saatci et al., 1997); and (d) putaminal PDW signal elevation with neither T1W nor long echo T2W abnormalities (Kozic et al., 2003).
The high SI on T2W images of the basal ganglia may represent edema, gliosis, necrosis and cystic degeneration, while high SI of the white matter is most compatible with degeneration and spongy or cystic disintegration (Sener, 1993; van Wassenaer-van Hall et al. 1996). Gliosis, cystic degeneration or disintegration and necrosis represent histologically irreversible end-stage abnormalities of WD (Harper et al., 1992; Kim et al., 2006). In our study, however, not only brain stem lesions, but also putaminal and thalamic lesions, showed frequently total or marked resolution during the long course of chelating therapy, even in patients with longer delay to correct diagnosis. Signal abnormalities on MRI might therefore, at least in the early stages, represent reversible myelinolisis or cytotoxic edema associated with copper toxicity.
The reversibility of MRI changes in this study was particularly impressive in brainstem structures, while the putaminal lesions were more resistant to D-penicillamine, especially in patients with later introduction of the treatment (Table 3, Figure 1). Complete or almost complete resolution of the high SI lesions in the brainstem occurred even in patients with delayed initiation of such therapy (group B). In the follow-up study of 16 patients with neurologically symptomatic WD, Roh et al. (1994) found that high SI lesions in the thalami or the brainstem either disappeared or regressed more extensively and more rapidly than those in the basal ganglia. In our study, partial reversion of T2W/PDW putaminal lesions was detected even in patients from the group B. Sener (2003a, 2004, 1993) found restricted diffusion signal in basal ganglia in the initial stage of WD, followed by inverted restricted diffusion sign in the later course of the disease. Such pattern of the symmetric T2W/PDW signal elevation is indistinguishable from pontine and extrapontinemyelinolysis in different toxic and metabolic disorders (Sener, 2003b, 2004; Kizkin et al., 2004).
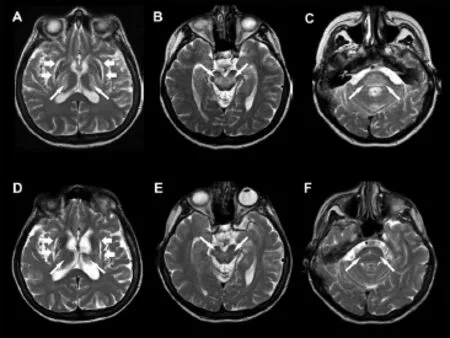
Figure 1 Effect of 24-month-long treatment delay on irreversible changes in the brain parenchyma in a 37-year-old woman with neurologic form of Wilson’s disease.
Recently, Kozic et al. (2012) found that signi fi cant hepatosplenomegaly, macronodular liver cirrhosis and peritoneal effusion were evident in 44% of patients with neurologic form of WD in whom the correct diagnosis was established after 2 or more years, while normal fi nding on MRI was evident in the group of patients where treatment was initiated in the early course of the disease.
Although our study included rather small number of patients with this rare disease, data suggested that reversibility might depend on a time lag between symptoms and signs onset and the initiation of D-penicillamine therapy. The signi fi cance of complete disappearance of high SI lesions in the putamen, mesencephalon and pons was higher among patients who started the treatment less than 24 months from the disease onset.
The main limitation of the study is the fact that only 14/37 patients were available for follow-up brain MR examination. However, the bene fi t of early diagnosis was statistically proven.
In conclusion, we con fi rmed that in patients with WD, some of the brain MRI lesions may be reversible during the long term D-penicillamine therapy. Moreover, our data suggest that likelihood of such resolution may be higher if such treatment starts earlier in the course of the disease. In order to prevent development of irreversible lesions of the brain parenchyma resistant on decoppering treatment, it is highly recommended to consider WD in differential diagnosis in any unexplained liver disease and/or progressive cerebellar, parkinsonian and dystonic symptoms, especially in younger patients. If the correct diagnosis and adequate treatment are not established within 18 months from the onset of symptoms, permanent clinical impairment associated with irreversible lesions within the brain parenchyma could be expected.
Author contributions:Kozić DB designed the study and analyzed experimental data. Petrović I and Svetel M performed the study and participated in data acquisition. Pekmezović T was responsible for data acquisition and data analysis. Ragaji A drafted the manuscript. Kostić VS edited the manuscript. All authors approved the final version of this article.
Con fl icts of interest:None declared.
Aisen AM, Martel W, Gabrielsen TO, Glazer GM, Brewer G, Young AB, Hill G (1985) Wilson disease of the brain: MR imaging. Radiology 157:137-141.
Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML (2007) Wilson’s disease. Lancet 369:397-408.
Braffman BH (2000) The aging brain and neurodegenerative disorders. In: Neuroimaging. Clinical and Physical Principles (Zimmerman RA, Gibby WA, Cormody RF, eds), pp699-729. New York: Springer-Verlag.
Brewer GJ (1995) Practical recommendations and new therapies for Wilson’s disease. Drugs 50:240-249.
Brewer GJ, Yuzbasiyan-Gurkan V (1992) Wilson disease. Medicine 71:139-164.
Compston A (2009) Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver, by S. A. Kinnier Wilson, (From the National Hospital, and the Laboratory of the National Hospital, Queen Square, London) Brain 1912: 34; 295-509. Brain 132:1997-2001.
da Costa Mdo D, Spitz M, Bacheschi LA, Leite CC, Lucato LT, Barbosa ER (2009) Wilson’s disease: two treatment modalities. Correlations to pretreatment and posttreatment brain MRI. Neuroradiology 51:627-633.
Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, Schilsky M, Cox D, Berr F (2003) Diagnosis and phenotypic classi fication of Wilson disease. Liver Int 23:139-142.
Hancu A, Mihai MC, Axelerad AD (2011) Wilson’s disease: a challenging diagnosis. Clinical manifestations and diagnostic procedures in 12 patients. The Medical-Surgical Journal of the Physicians and Naturalists Society from Iasi 115:58-63.
Harper C, Butterworth R. Nutritional de fi ciencies and metabolic disorders (1992). In: Green field’s Neuropathology (Green field JG, Hume Adams J, Duchen LW, eds). 5th ed., pp838-840. London, UK: Edward Arnold.
Heckmann JM, Eastman RW, De Villiers JC, Hewlett R (1994) Wilson’s disease: neurological and magnetic resonance imaging improvement on zinc treatment. J Neurol Neurosurg Psychiatry 57:1273-1274.
Hu J, Lu D, Wang G (2001) Study on the clinical misdiagnosis of hepatolenticular degeneration. Zhonghua Yi Xue Za Zhi 81:642-644.
Huang CC, Chu NS (1996) Wilson’s disease: resolution of MRI lesions following long-term oral zinc therapy. Acta Neurol Scand 93:215-218.
Kim TJ, Kim IO, Kim WS, Cheon JE, Moon SG, Kwon JW, Seo JK, Yeon KM (2006) MR imaging of the brain in Wilson disease of childhood: fi ndings before and after treatment with clinical correlation. AJNR Am J Neuroradiol 27:1373-1378.
King AD, Walshe JM, Kendall BE, Chinn RJ, Paley MN, Wilkinson ID, Halligan S, Hall-Craggs MA (1996) Cranial MR imaging in Wilson’s disease. AJR Am J Roentgenol 167:1579-1584.
Kizkin S, Sarac K, Ozisik HI, Ozcan C (2004) Central pontine myelinolysis in Wilson’s disease: MR spectroscopy findings. Mag Reson Imaging 22:117-121.
Kozic D, Svetel M, Petrovic B, Dragasevic N, Semnic R, Kostic VS (2003) MR imaging of the brain in patients with hepatic form of Wilson’s disease. Eur J Neurol 10:587-592.
Kozic D, Svetel M, Petrovic I, Sener RN, Kostic VS (2006) Regression of nodular liver lesions in Wilson’s disease. Acta Radiol 47:624-627.
Kozic DB, Semnic R, Petrovic I, Svetel M, Ostojic J, Kostic VS (2012) Are irreversible morphological [corrected] signs of portal hypertension in neurological form of Wilson’s disease associated with treatment delay? A pilot study. Acta Neurol Belg112:261-264.
Miranda M, Brinck P, Roessler JL, Troncoso Sch M, Gonzalez M, Alarcon T, Villagra R (1995) Wilson’s disease: a review apropos of a clinical experience in 16 patients. Rev Med Chil 123:1098-1107.
Nazer H, Brismar J, al-Kawi MZ, Gunasekaran TS, Jorulf KH (1993) Magnetic resonance imaging of the brain in Wilson’s disease. Neuroradiology 35:130-133.
Pellecchia MT, Criscuolo C, Longo K, Campanella G, Filla A, Barone P (2003) Clinical presentation and treatment of Wilson’s disease: a single-centre experience. Eur Neurol 50:48-52.
Prashanth LK, Taly AB, Sinha S, Arunodaya GR, Swamy HS (2004) Wilson’s disease: diagnostic errors and clinical implications. J Neurol Neurosurg Psychiatry 75:907-909.
Prayer L, Wimberger D, Kramer J, Grimm G, Oder W, Imhof H (1990) Cranial MRI in Wilson’s disease. Neuroradiology 32:211-214.
Roh JK, Lee TG, Wie BA, Lee SB, Park SH, Chang KH (1994) Initial and follow-up brain MRI fi ndings and correlation with the clinical course in Wilson’s disease. Neurology 44:1064-1068.
Saatci I, Topcu M, Baltaoglu FF, Kose G, Yalaz K, Renda Y, Besim A (1997) Cranial MR fi ndings in Wilson’s disease. Acta Radiol 38:250-258.
Sakaida I, Kawaguchi K, Kimura T, Tamura F, Okita K (2005) D-Penicillamine improved laparoscopic and histological fi ndings of the liver in a patient with Wilson’s disease: 3-year follow-up after diagnosis of Coombs-negative hemolytic anemia of Wilson’s disease. J Gastroenterol 40:646-651.
Sener RN (1993) Wilson’s disease: MRI demonstration of cavitations in basal ganglia and thalami. Pediatr Radiol 23:157.
Sener RN (2003a) Diffusion MR imaging changes associated with Wilson disease. AJNR Am J Neuroradiol 24:965-967.
Sener RN (2003b) Acute carbon monoxide poisoning: diffusion MR imaging fi ndings. AJNR Am J Neuroradiol 24:1475-1477.
Sener RN (2004) Diffusion magnetic resonance imaging patterns in metabolic and toxic brain disorders. Acta Radiol 45:561-570.
Sinha S, Taly AB, Prashanth LK, Ravishankar S, Arunodaya GR, Vasudev MK (2007) Sequential MRI changes in Wilson’s disease with de-coppering therapy: a study of 50 patients. Br J Radiol 80:744-749.
Starosta-Rubinstein S, Young AB, Kluin K, Hill G, Aisen AM, Gabrielsen T, Brewer GJ (1987) Clinical assessment of 31 patients with Wilson’s disease. Correlations with structural changes on magnetic resonance imaging. Arch Neurol 44:365-370.
Stracciari A, Tempestini A, Borghi A, Guarino M (2000) Effect of liver transplantation on neurological manifestations in Wilson disease. Arch Neurol 57:384-386.
Stremmel W, Niederau C, Strohmeyer G (1989) Diagnostik und Therapie stoffwechselbcdingter Lebererkrankungen. Muench Med Wochenschr 131:257-261.
Svetel M, Kozic D, Stefanova E, Semnic R, Dragasevic N, Kostic VS (2001) Dystonia in Wilson’s disease. Mov Disord 16:719-723.
Takahashi W, Yoshii F, Shinohara Y (1996) Reversible magnetic resonance imaging lesions in Wilson’s disease: clinical-anatomical correlation. J Neuroimaging 6:246-248.
Thuomas KA, Aquilonius SM, Bergstrom K, Westermark K (1993) Magnetic resonance imaging of the brain in Wilson’s disease. Neuroradiology 35:134-141.
Trocello JM, Guichard JP, Leyendecker A, Pernon M, Chaine P, El Balkhi S, Poupon J, Chappuis P, Woimant F (2011) Corpus callosum abnormalities in Wilson’s disease. J Neurol Neurosurg Psychiatry 82:1119-1121.
van Wassenaer-van Hall HN, van den Heuvel AG, Algra A, Hoogenraad TU, Mali WP (1996) Wilson disease: fi ndings at MR imaging and CT of the brain with clinical correlation. Radiology 198:531-536.
Walshe JM (1988) Diagnosis and treatment of presymptomatic Wilson’s disease. Lancet 2:435-437.
Walshe JM, Yealland M (1992) Wilson’s disease: the problem of delayed diagnosis. J Neurol Neurosurg Psychiatry 55:692-696.
Walshe JM, Yealland M (1993) Chelation treatment of neurological Wilson’s disease. Q J Med 86:197-204.
Williams FJ, Walshe JM (1981) Wilson’s disease. An analysis of the cranial computerized tomographic appearances found in 60 patients and the changes in response to treatment with chelating agents. Brain 104:735-752.
Yuzbasiyan-Gurkan V, Grider A, Nostrant T, Cousins RJ, Brewer GJ (1992) Treatment of Wilson’s disease with zinc: X. Intestinal metallothionein induction. J Lab Clin Med 120:380-386.
Copyedited by Sener RN, Shankar SK, Dusek P, Shin HW, Li CH, Song LP, Zhao M
Duško B. Kozić, University of Novi Sad, School of Medicine, Institute of Oncology, Diagnostic Imaging Center, Put Doktora Goldmana 4, 21204 Sremska Kamenica, Serbia, dusko.b.kozic@gmail.com.
10.4103/1673-5374.145360
http://www.nrronline.org/
Accepted: 2014-10-12
杂志排行
中国神经再生研究(英文版)的其它文章
- Hot spots and future directions of research on the neuroprotective effects of nimodipine
- Recovery of cerebellar peduncle injury in a patient with a cerebellar tumor: validation by diffusion tensor tractography
- Amyloid precursor-like protein 2 C-terminal fragments upregulate S100A9 gene and protein expression in BV2 cells
- Inhibition of Sirtuin 2 exerts neuroprotection in aging rats with increased neonatal iron intake
- Adult neurogenesis in the four-striped mice (Rhabdomys pumilio)
- The occurrence of diffuse axonal injury in the brain: associated with the accumulation and clearance of myelin debris
