Melatonin reduces traumatic brain injury-induced oxidative stress in the cerebral cortex and blood of rats
2014-03-27NilgenolMustafaNazrolu
Nilgün Şenol, Mustafa Nazıroğlu
1 Department of Neurosurgery, Faculty of Medicine, University of Suleyman Demirel, Isparta, Turkey2 Neuroscience Research Center, University of Suleyman Demirel, Isparta, Turkey
Melatonin reduces traumatic brain injury-induced oxidative stress in the cerebral cortex and blood of rats
Nilgün Şenol1, Mustafa Nazıroğlu2
1 Department of Neurosurgery, Faculty of Medicine, University of Suleyman Demirel, Isparta, Turkey
2 Neuroscience Research Center, University of Suleyman Demirel, Isparta, Turkey
Free radicals induced by traumatic brain injury have deleterious effects on the function and antioxidant vitamin levels of several organ systems including the brain. Melatonin possesses antioxidant effect on the brain by maintaining antioxidant enzyme and vitamin levels. We investigated the effects of melatonin on antioxidant ability in the cerebral cortex and blood of traumatic brain injury rats. Results showed that the cerebral cortex β-carotene, vitamin C, vitamin E, reduced glutathione, and erythrocyte reduced glutathione levels, and plasma vitamin C level were decreased by traumatic brain injury whereas they were increased following melatonin treatment. In conclusion, melatonin seems to have protective effects on traumatic brain injury-induced cerebral cortex and blood toxicity by inhibiting free radical formation and supporting antioxidant vitamin redox system.
nerve regeneration; melatonin; traumatic brain injury; antioxidant; oxidative stress; vitamin E; vitamin C; glutathione; brain; neural regeneration
Şenol N, Nazıroğlu M. Melatonin reduces traumatic brain injury-induced oxidative stress in the cerebral cortex and blood of rats. Neural Regen Res. 2014;9(11):1112-1116.
Introduction
Oxidative stress is defined as an imbalance between high reactive oxygen species and cellular antioxidant defense (Yatin et al., 2000; Nazıroğlu, 2007). In order to scavenge reactive oxygen species, various defense systems exist in the brain. Levels of the antioxidants in the cerebral cortex are considerable low. Therefore, low level of antioxidant and high content of polyunsaturated fatty acids result in limited antioxidant defense in the brain (Cherubini et al., 2008; Nazıroğlu, 2011). Glutathione peroxidase, a selenium containing enzyme, is responsible for the reduction of hydro and organic peroxides in the brain (Nazıroğlu and Yürekli, 2013). Reduced glutathione is a hydroxyl radical and singlet oxygen scavenger that participates in a wide range of cellular functions (Nazıroğlu, 2013; Senol et al., 2014). Vitamin E, alpha-tocopherol, is the most important antioxidant in the lipid phase of cells (Nazıroğlu, 2007). Vitamin C, ascorbic acid, as a free radical scavenger, also transforms vitamin E to its active form (Cherubini et al., 2008; Nazıroğlu, 2011). Vitamin A, retinol, serves as a prohormone for retinoids and is involved in signal transduction at cytoplasmic and membrane sites (Cherubini et al., 2008; Nazıroğlu, 2011).
Traumatic brain injury is one of the most common causes of the mortalities (Cornelius et al., 2013). Secondary events occur after primary events like shearing of nerve cells and blood vessels, cause post-traumatic neurodegenerations with an increase in reactive oxygen species and reactive oxygen species-mediated lipid peroxidation (Cornelius et al., 2013). Three different types of metabolic disturbances occur after traumatic brain injury: in fl ammation, ischemia and calcium ion entry (Campolo et al., 2013). Ischemia/reperfusion negatively in fl uence outcome as oxygen and glucose deprivation reduces cerebral oxidative metabolism. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a major complex that produces reactive oxygen species during the ischemic period (Niesman et al., 2014). Concurrently with these metabolic disturbances, also aTher traumatic brain injury, several factors like extravagated blood products, tissue debris, intracellular components, prostaglandins, reactive oxygen-nitrogen species trigger inflammation (Cornelius et al., 2013). Once blood-brain barrier is disrupted, neutrophils, monocytes, and lymphocytes accumulate in the injured brain area, and microglia are activated by Ca2+entry, all these initiate inflammatory response (Esposito and Cuzzocrea, 2010). Leucocytes release cytokines, proteases, prostaglandins, and free oxygen-nitrogen species (Kayan et al., 2012).
Melatonin, a hormone secreted from pineal gland and synthesized from tryptophan or formed as the metabolic end product of serotonin, is a non-enzymatic antioxidant and neuroprotective agent (Espino et al., 2012; Nazıroğlu et al., 2012b). As an antioxidant, melatonin scavenges the free radicals and/or stimulates the enzymes of antioxidant defense system (Reiter et al., 1997). The e fficacy of melatonin for post-traumatic brain injury has been shown in in vivo and in vitro studies (Esposito and Cuzzocrea, 2010; Campolo et al., 2013). Melatonin has been shown to counteract oxidative stress-induced pathophysiologic conditions like cerebral ischemia/reperfusion injury, neuronal excitotoxicity and chronic inflammation (Reiter et al., 1997; Ekmekcioglu, 2006; Celik and Nazıroğlu, 2012; Espino et al., 2012; Nazıroğlu et al., 2012b). We proposed the hypotheses that modulation of oxidative stress in blood and the cerebralcortex by means of treatment with melatonin may cause an increase in antioxidant vitamin level.
Hence, we aimed to evaluate whether there would be a protective e ff ect of melatonin on oxidative stress and enzymatic and non-enzymatic antioxidant levels in traumatic brain injury rats.
Materials and Methods
Animals
Thirty-two 6-month-old male Sprague-Dawley rats, weighing 350 ± 20 g, were used for the experimental procedures. The ambient temperature and relative humidity of the animal room were 21 ± 1°C and 60 ± 7%, respectively. The room was illuminated with arti fi cial light for a 12-hour dark/ light cycle. The animals were allowed free access to standard pelleted food and tap water. All studies were performed with the approval of the ethical committee of Medical Faculty of Suleyman Demirel University (Protocol No. 2013/2-1).
Experimental design
Thirty-two rats were randomly and evenly divided into four groups as follows: Control group (n = 8): Placebo (0.1 mL ethanol + 0.9 mL isotonic saline) was intraperitoneally given to the rats. Melatonin group (n = 8): Only melatonin (10 mg/kg body weight) was intraperitoneally given to rats at 1 hour after brain trauma (Dilek et al., 2010). Melatonin was obtained from Sigma Chemical Co. (Istanbul, Turkey). Traumatic brain injury group (n = 8): a contusional head trauma model was established (Marmarou et al., 1994; Senol et al., 2014). Traumatic brain injury + melatonin group (n = 8): Melatonin (5 mg/kg body weight) was intraperitoneally administrated to rats at 1 hour after brain trauma. At 24 hours after melatonin administration, rats were sacri fi ced, and the cerebral cortex and blood samples were taken. Melatonin was dissolved in ethanol (0.1 mL) and diluted with 0.9 mL isotonic saline (0.9%, v/w) before use.
Induction of traumatic brain injury
Marmarou method (Marmarou et al., 1994; Senol et al., 2014) was used to make a diffuse head trauma. Rats were anesthetized with a cocktail of ketamine hydrochloride (80 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally before induction of traumatic brain injury. The animals were placed in the prone position on the trauma table under anesthesia. After skin incision, a steel disc (10 mm × 3 mm) was placed on the midline between coronal and lambdoid sutures on the animal’s skull, and a 300 g weight was freely dropped through a cylindrical tube, with 19 mm inner diameter, from 2 m height onto the head of the animal.
Anesthesia and preparation of the cerebral cortex and blood samples
Following anesthesia with ether, rats were sacrificed and the cerebral cortex and blood samples were taken. Then the cerebral cortex was processed as follows: the cortex was dissected out after the brain was split in the mid-sagittal plane as described in our previous study (Nazıroğlu et al., 2013). Cerebral cortex tissues were washed twice with cold saline solution, placed into glass bottles, labeled and stored at -85°C until processing (maximum 4 weeks). After weighing, half of the cortex was placed on ice, cut into small pieces, using scissors, and homogenized (2 minutes at 3,000 × g) in a 5-fold volume (1:5, w/v) of ice-cold Tris-HCl buffer (50 mmol/L, pH 7.4), by using an ultrasonic homogenizer (Bandelin-2070, BANDELIN electronic, GmbH & Co. KG, Berlin, Germany). All preparation procedures were performed on ice. The blood (2-4 mL) was taken from the heart, using a sterile injector, and contained in anticoagulant (EDTA) coated vacutainer tube for protecting against light. Blood sample was separated into plasma and erythrocytes by centrifugation at 1,500 × g for 10 minutes at 4°C. The erythrocyte samples were washed three times in cold isotonic saline (0.9%, v/ w), then hemolyzed with a 9-fold volume of phosphate buffer (50 mmol/L, pH 7.4). After addition of butylhydroxytoluol (4 µL per mL), the brain homogenate, hemolyzed erythrocytes and plasma samples were stored at -80°C for no more than 4 weeks for measurement of enzymatic activity. The remaining brain tissue homogenate, hemolyzed erythrocytes and plasma samples were used for immediate lipid peroxidation and vitamin assay.
Lipid peroxidation level determinations
Lipid peroxidation levels in the brain tissue homogenate, hemolyzed erythrocytes and plasma samples were assayed for production of lipid peroxidation by monitoring production of thiobarbituric-acid reactive substances as described previously (Placer et al., 1966). The levels of lipid peroxidation in the brain tissue homogenate, hemolyzed erythrocytes and plasma samples were expressed as µmol/ g protein and µmol/L, respectively.
Glutathione, glutathione peroxidase and protein assay
The reduced glutathione content in the cerebral cortex and erythrocytes was measured at 412 nm using the method of Sedlak and Lindsay (1968) as described in own previous studies (Dilek et al., 2010; Nazıroğlu et al., 2012a). Glutathione peroxidase activities of cerebral cortex and erythrocytes were measured spectrophotometrically (Shimadzu UV-1800, Shimadzu Corp., Kyoto, Japan) at 37 °C and 412 nm according to the Lawrence and Burk (Lawrence and Burk, 1976). The protein content in the cerebral cortex and erythrocytes was measured by method of Lowry et al. (1951) with bovine serum albumin as the standard.
Determination of β-carotene, vitamin A, vitamin C and vitamin E in the cerebral cortex
Vitamins A (retinol) and E (α-tocopherol) were determined in the cerebral cortex samples by a modification of the method described by Desai (1984) and Suzuki and Katoh (1990). Approximately 0.25 g brain tissue and 0.25 mL plasma sample was saponified by adding 0.3 mL 60% (w/v in water) KOH and 2 mL 1% (w/v in ethanol) ascorbic acid, followed by heating at 70°C for 30 minutes. After cooling the samples on ice, 2 mL water and 1 mL n-hexane were added to the samples, mixed, and allowed to stand for 10 minutes for phase separation. A 0.5-mL n-hexane extract aliquot was taken, and the vitamin A level was spectrophotometrically(Shimadzu UV-1800, Shimadzu Corp., Kyoto, Japan) measured at 325 nm. Next, reactants were added, and the hexane absorbance value was measured at 535 nm in the spectrophotometer. Calibrations were performed using standard solutions of all-trans retinol and α-tocopherol in hexane. The β-carotene level in the cerebral cortex was determined as described previously (Suzuki and Katoh, 1990). We mixed 2 mL hexane with 0.25 g brain tissue and 0.25 mL plasma. The β-carotene level in the hexane mixture was measured at 453 nm using the spectrophotometer.
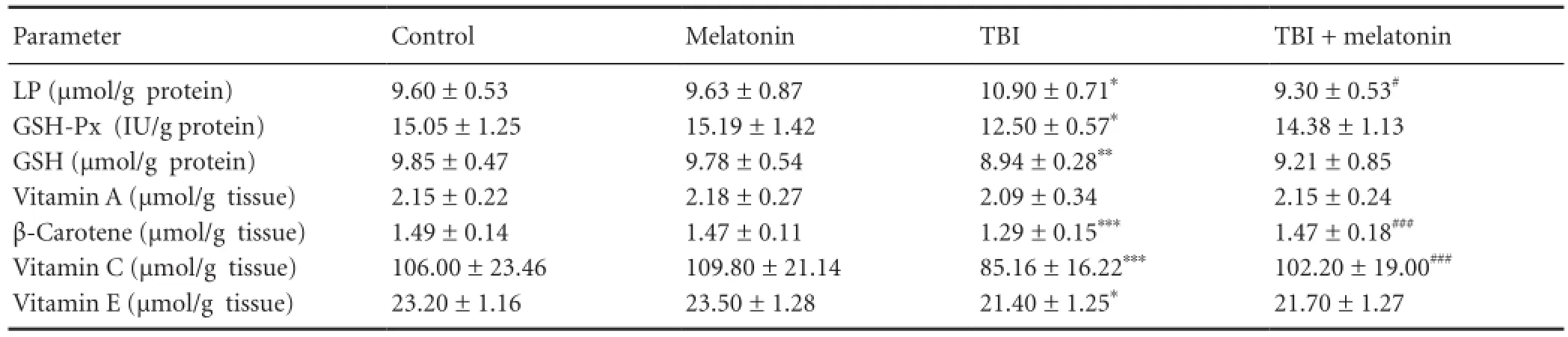
Table 1 The effects of melatonin on glutathione peroxidase (GSH-Px), reduced glutathione (GSH), lipid peroxidation (LP), vitamin A, vitamin C, vitamin E and β-carotene values in the cerebral cortex traumatic brain injury (TBI)-induced rats
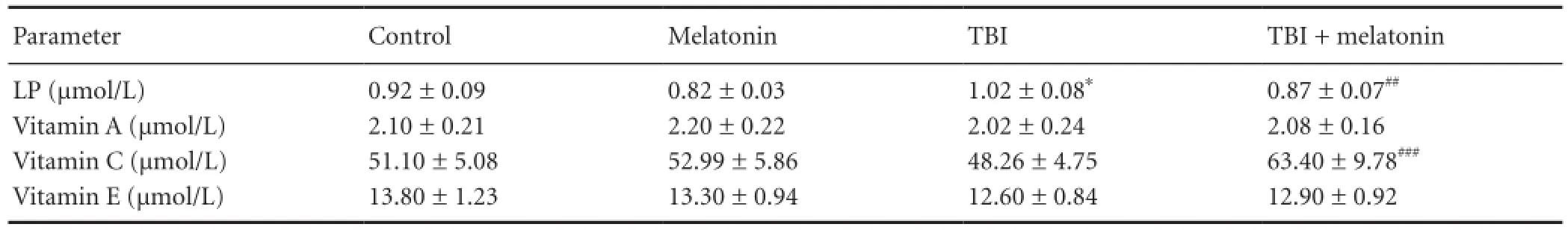
Table 2 The effects of melatonin on plasma lipid peroxidation (LP), vitamin A and vitamin E concentrations in traumatic brain injury (TBI)-induced rats
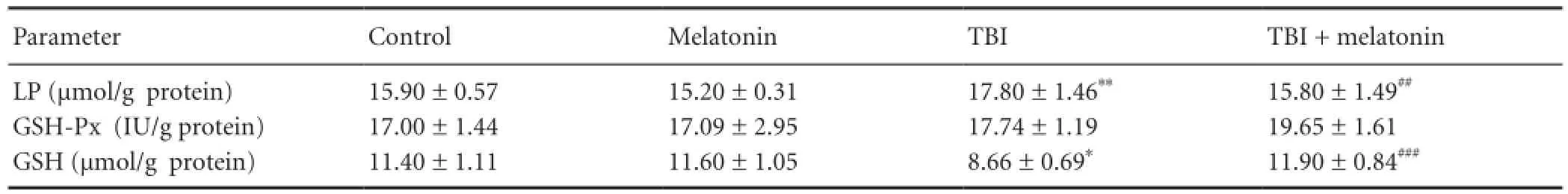
Table 3 The effects of melatonin on erythrocyte glutathione peroxidase (GSH-Px) activity, reduced glutathione (GSH) and lipid peroxidation (LP) levels in traumatic brain injury (TBI)-induced rats
Quanti fi cation of vitamin C (ascorbic acid) in the plasma and cerebral cortex samples was performed according to the method of Jagota and Dani (Jagota and Dani, 1982). The absorbance of the samples was measured spectrophotometrically (Shimadzu UV-1800, Shimadzu Corp., Kyoto, Japan) at 760 nm.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). P-values < 0.05 were regarded as statistically significant. Signi fi cant values were assessed with the Mann-Whitney U test. Data were analyzed using the SPSS statistical program (version 17.0 software, SPSS, Chicago, IL, USA).
Results
Lipid peroxidation levels
The cerebral cortex, plasma and erythrocyte lipid peroxidation levels in four groups are shown inTables 1-3. Lipid peroxidation levels in the cerebral cortex, plasma and erythrocytes in the traumatic brain injury group were signi fi cantly (P < 0.05) higher than in the control group. The cerebral cortex (P < 0.05), plasma (P < 0.01) and erythrocyte (P <0.01) lipid peroxidation levels in the traumatic brain injury + melatonin group were signi fi cantly lower than in the traumatic brain injury group, respectively.
Glutathione peroxidase activity and reduced glutathione level
The cerebral cortex glutathione peroxidase activity was signi fi cantly (P < 0.05) lower in the traumatic brain injury group as compared to control and traumatic brain injury + melatonin groups although its activity did not differ between traumatic brain injury and traumatic brain injury + melatonin groups. Hence, the glutathione peroxidase activity was increased by melatonin administration (Table 1). There was no statistical change in the glutathione peroxidase activity in erythrocyte sample among the four groups (Table 3). Re-duced glutathione level in the cerebral cortex (P < 0.01) and erythrocyte samples (P < 0.05) were signi fi cantly decreased in the traumatic brain injury groups than in the control and traumatic brain injury + melatonin groups. The decreased reduced glutathione levels in the cerebral cortex and erythrocyte samples were increased by melatonin administration (Table 1). On the other word, the erythrocyte reduced glutathione level in the traumatic brain injury + melatonin group was significantly (P < 0.001) higher than in the traumatic brain injury group (Table 3).
Antioxidant vitamin levels
The β-carotene, vitamin C, and vitamin E levels in the cerebral cortex and plasma samples of four groups are shown inTables 1and2, respectively. The vitamin A and vitamin E levels in the cerebral cortex and plasma samples were not a ff ected by melatonin and traumatic brain injury. The β-carotene (P < 0.001), vitamin C (P < 0.001) and vitamin E (P <0.05) levels in the cerebral cortex were signi fi cantly lower in the traumatic brain injury group as compared to the control and melatonin groups. Cerebral cortex β-carotene and vitamin C levels and plasma vitamin C level were signi fi cantly increased (P < 0.001) following melatonin administration.
Discussion
Results from this study demonstrated that the cerebral cortex, plasma and erythrocyte lipid peroxidation levels were increased by traumatic brain injury induction although cerebral cortex and erythrocyte reduced glutathione, cerebral cortex glutathione peroxidase, cerebral cortex vitamin C, vitamin E, and β-carotene levels were decreased by the induction of traumatic brain injury. Traumatic brain injury induction in the animals is characterized by an increase in lipid peroxidation and decreases in glutathione peroxidase, β-carotene and vitamin C and vitamin E antioxidant levels. Administration of melatonin caused a decrease in the cerebral cortex, plasma and erythrocyte lipid peroxidation levels although reduced glutathione, β-carotene, vitamin C and vitamin E levels increased. A limited number of in vivo or in vitro studies in tissues except the brain of experimental animals have been reported regarding the effects of melatonin on antioxidant enzymatic system and lipid peroxidation levels (Kerman et al., 2005; Ozdemir et al., 2005; Lee et al., 2009; Fernández-Gajardo et al., 2014). To the best of our knowledge, the current study is the fi rst to compare the medicine with particular reference to its e ff ects on oxidative stress and antioxidant redox system in the blood and cerebral cortex of traumatic brain injury-induced rats.
It is well known that the production of lipid peroxidation (malonaldehyde) as the end product of lipid peroxidation is a major indicator of oxidative brain injury. Results of recent studies indicated that lipid peroxidation has been linked to microvascular damage and ischemia/reperfusion injury which, is severe enough, can lead to overproduction of oxidative stress. In addition, inflammation is closely related to the overproduction of reactive oxygen species and plays an important role in various brain injuries. Melatonin, as an antioxidant, is one of the drugs that are studied for traumatic brain injury and it, as a free radical scavenger, protects against free radical formation (Reiter et al., 1997; Espino et al., 2012; Nazıroğlu et al., 2012). It has also been reported that melatonin stimulates various antioxidants such as reduced glutathione and glutathione peroxidase that contribute to the antioxidant defense system, and suppresses lipid peroxidation by preventing traumatic brain injury-mediated neurotoxicity (Martín et al., 2000). Lee et al. (2009) had studied the neuroprotective e ff ect of melatonin on oxidative stress aTher surgical brain injury in rats and they reported its bene fi cial e ff ect on neurological oxidative stress. Seifman et al. (2014) reported that endogenous melatonin decreased oxidative stress in cerebrospinal fluid of patients after severe traumatic brain injury and correlated with oxidative stress and metabolic disarray. To the best of our knowledge, there is no report on the modulatory role of melatonin on antioxidant vitamins in animals and human with traumatic brain injury. We fi rstly detected signi fi cant decreases in lipid peroxidation levels in the cerebral cortex, plasma and erythrocyte samples of traumatic brain injury-induced rats by melatonin treatment although reduced glutathione, glutathione peroxidase, vitamin C, vitamin E and β-carotene levels were increased by melatonin treatment. Thus, melatonin with antioxidant and anti-inflammatory properties are proposed to be useful in brain oxidative damage induced by traumatic brain injury.
Inactivation of reactive oxygen species can be carried out by antioxidant vitamins. Vitamin E, alpha-tocopherol, is the most important antioxidant in the lipid phase of cells. Vitamin E acts to protect cells against the e ff ects of free radicals, which are potentially damaging byproducts of the body’s metabolism (Nazıroğlu and Özgül, 2013). Vitamin C, as well as being a water soluble reactive oxygen species scavenger, also transforms vitamin E to its active form (Bowman, 2012). Levels of the antioxidants in the brain are considerable low. Therefore, low level of antioxidants and high content of PUFA, result in limited antioxidant defense in the cerebral cortex (Nazıroğlu, 2012; Nazıroğlu et al., 2013). Vitamin C, vitamin E and β-carotene levels in the cerebral cortex were decreased in the traumatic brain injury group although their levels in the cerebral cortex were increased in the melatonin-treated groups.The increased levels of the antioxidant vitamins could be due to thier depletion or inhibition as a result of the increased production of free radicals. The increase in the cerebral cortex vitamin C, vitamin E and β-carotene levels in animals during melatonin treatments has been attributed to the inhibition of free radicals and lipid peroxidation (Tuzcu and Baydas, 2006; Dilek et al., 2010; Nazıroğlu et al., 2012). Similarly, Ishaq et al. (2013) reported that traumatic brain injury caused a decrease in vitamins C and E levels in the blood and brain tissue of traumatic brain injury-induced rats.
Reduced glutathione is the predominant antioxidant in the cerebral cortex that is present in millimolar concentrations. Reduced glutathione homoeostasis is implicated in the etiology of a number of brain diseases including traumatic brain injury (Senol et al., 2014). Melatonin is a ubiquitously acting direct reactive oxygen species scavenger and also an indirect antioxidant. It was reported that reduced glutathione level and brain glutathione peroxidase activity, the critical components of reduced glutathione-redox cycle, were signi fi cantly decreased in the experimentally induced traumatic brain injury (Martín et al., 2000; Esposito and Cuzzocrea, 2010;Campolo et al., 2013). Results from the present study showed that traumatic brain injury induced significant decrease in lipid peroxidation in the cerebral cortex and blood samples, while melatonin treatment reversed these e ff ects.
In conclusion, melatonin supplementation has protective e ff ect on oxidative stress and antioxidant redox system in the cerebral cortex and blood. Melatonin can regulate reduced glutathione and antioxidant vitamins levels and glutathione peroxidase activity in the cerebral cortex. Hence, use of melatonin in traumatic brain injury may be a potential approach to arresting or inhibiting the oxidative stress caused by excitotoxic agents.
Acknowledgments:Abstract of the study will be submitted in 5th International Congress on Cell Membranes and Oxidative Stress: Focus on Calcium Signaling and TRP Channels, 9-12 September 2014, Isparta Turkey (http://www.cmos.org. tr/2014/).
Author contributions:Şenol N and Nazıroğlu M proposed the present hypothesis and were responsible for writing the report. Şenol N was responsible for the induction of traumatic brain injury. Nazıroğlu M performed statistical analyses. Both of these two authors approved the final manuscript.
Con fl icts of interest:None declared.
Bowman GL (2012) Ascorbic acid, cognitive function, and Alzheimer’s disease: a current review and future direction. Biofactors 38:114-122.
Campolo M, Ahmad A, Crupi R, Impellizzeri D, Morabito R, Esposito E, Cuzzocrea S (2013) Combination therapy with melatonin and dexamethasone in a mouse model of traumatic brain injury. J Endocrinol 217:291-301.
Celik O, Nazıroğlu M (2012) Melatonin modulates apoptosis and TRPM2 channels in transfected cells activated by oxidative stress. Physiol Behav 107:458-465.
Cherubini A, Ruggiero C, Morand C, Lattanzio F, Dell’aquila G, Zuliani G, Di Iorio A, Andres-Lacueva C (2008) Dietary antioxidants as potential pharmacological agents for ischemic stroke. Curr Med Chem 15:1236-1248.
Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, Pennisi G, Radak Z, Calabrese EJ, Cuzzocrea S (2013) Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal 19:836-853.
Desai ID (1984) Vitamin E analysis methods for animal tissues. Methods Enzymol 105:138-147.
Dilek M, Naziroğlu M, Baha Oral H, Suat Ovey I, Küçükayaz M, Mungan MT, Kara HY, Sütçü R (2010) Melatonin modulates hippocampus NMDA receptors, blood and brain oxidative stress levels in ovariectomized rats. J Membr Biol 233:135-142.
Ekmekcioglu C (2006) Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharm 60:97-108.
Espino J, Pariente JA, Rodríguez AB (2012) Oxidative stress and immunosenescence: therapeutic effects of melatonin. Oxid Med Cell Longev 2012:670294.
Esposito E, Cuzzocrea S (2010) Antiin fl ammatory activity of melatonin in central nervous system. Curr Neuropharmacol 8:228-242.
Fernández-Gajardo R, Matamala JM, Carrasco R, Gutiérrez R, Melo R, Rodrigo R (2014) Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs 28:229-248.
Ishaq GM, Saidu Y, Bilbis LS, Muhammad SA, Jinjir N, Shehu BB (2013) E ff ects of α-tocopherol and ascorbic acid in the severity and management of traumatic brain injury in albino rats. J Neurosci Rural Pract 4:292-297.
Jagota SK, Dani HM (1982) A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem 127:178-182.
Kayan M, Nazıroğlu M, Ovey IS, Aykur M, Uğuz AC, Yürekli VA (2012) Non-ionic contrast media induces oxidative stress and apoptosis through Ca²+in fl ux in human neutrophils. J Membr Biol 245:833-840.
Kerman M, Cirak B, Ozguner MF, Dagtekin A, Sutcu R, Altuntas I, Delibas N (2005) Does melatonin protect or treat brain damage from traumatic oxidative stress? Exp Brain Res 163:406-410.
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-de fi cient rat liver. Biochem Biophys Res Commun 71:952-958.
Lee S, Jadhav V, Ayer RE, Rojas H, Hyong A, Lekic T, Tang J, Zhang JH (2009) Dual effects of melatonin on oxidative stress after surgical brain injury in rats. J Pineal Res 46:43-48.
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265-275.
Marmarou A, Foda MA, Brink WV, Campbell J, Kita H, Demetriadou K (1994) A new model of di ff use brain injury in rats. J Neurosurg 80:291-300.
Martín M, Macías M, Escames G, León J, Acuña-Castroviejo D (2000) Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J 14:1677-1679.
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990-2001.
Nazıroğlu M (2011) TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res 36:355-366.
Nazıroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca (2+) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res 32:134-141.
Nazıroğlu M, Çelik Ö, Özgül C, Çiğ B, Doğan S, Bal R, Gümral N, Rodríguez AB, Pariente JA (2012a) Melatonin modulates wireless (2.45 GHz)-induced oxidative injury through TRPM2 and voltage gated Ca(2+) channels in brain and dorsal root ganglion in rat. Physiol Behav 105:683-692.
Nazıroğlu M, Tokat S, Demirci S (2012b) Role of melatonin on electromagnetic radiation-induced oxidative stress and Ca2+ signaling molecular pathways in breast cancer. J Recept Signal Transduct Res 32: 290-297.
Nazıroğlu M, Kozlu S, Yorgancıgil E, Uğuz AC, Karakuş K (2013) Rose oil (from Rosa × damascena Mill.) vapor attenuates depression-induced oxidative toxicity in rat brain. J Nat Med 67:152-158.
Nazıroğlu M, Özgül C (2013) Vitamin E modulates oxidative stress and protein kinase C activator (PMA)-induced TRPM2 channel gate in dorsal root ganglion of rats. J Bioenerg Biomembr 45:541-549.
Nazıroğlu M, Yürekli VA (2013) E ff ects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol 33:589-599.
Niesman IR, Schilling JM, Shapiro LA, Kellerhals SE, Bonds JA, Kleschevnikov AM, Cui W, Voong A, Krajewski S, Ali SS, Roth DM, Patel HH, Patel PM, Head BP (2014) Traumatic brain injury enhances neuroin fl ammation and lesion volume in caveolin de fi cient mice. J Neuroin fl ammation 11:39.
Ozdemir D, Uysal N, Gonenc S, Acikgoz O, Sonmez A, Topcu A, Ozdemir N, Duman M, Semin I, Ozkan H (2005) E ff ect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol Res 54:631-637.
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Anal Biochem 16:359-364.
Reiter RJ, Guerrero JM, Escames G, Pappolla MA, Acuña-Castroviejo D (1997) Prophylactic actions of melatonin in oxidative neurotoxicity. Ann N Y Acad Sci 825:70-78.
Sedlak J, Lindsay RH (1968) Estimation of total, protein bound and non-protein sulThydryl groups in tissue with Ellmann’s reagent. Anal Biochem 25:192-205.
Senol N, Nazıroğlu M, Yürüker V (2014) N-acetylcysteine and selenium modulate oxidative stress, antioxidant vitamin and cytokine values in traumatic brain injury-induced rats. Neurochem Res 39:685-692.
Suzuki J, Katoh N (1990) A simple and cheap method for measuring vitamin A in cattle using only a spectrophotometer. Jpn J Vet Sci 52:1282-1284.
Tuzcu M, Baydas G (2006) E ff ect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur J Pharmacol 537:106-110.
Yatin SM, Varadarajan S, Butterfield DA (2000) Vitamin E prevents Alzheimer’s amyloid beta-peptide (1-42)-induced neuronal protein oxidation and reactive oxygen species production. J Alzheimers Dis 2:123-131.
Copyedited by Li CH, Song LP, Zhao M
10.4103/1673-5374.135312
Mustafa Naziroğlu, Director of
Neuroscience Research Center, University of Suleyman Demirel, Isparta, Turkey,
mustafanaziroglu@sdu.edu.tr.
http://www.nrronline.org/
Accepted: 2014-06-03
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture at the Taixi (KI3) acupoint activates cerebral neurons in elderly patients with mild cognitive impairment
- High matrix metalloproteinase-9 expression induces angiogenesis and basement membrane degradation in stroke-prone spontaneously hypertensive rats after cerebral infarction
- A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosis
- Somatosensory stimulation suppresses the excitability of pyramidal cells in the hippocampal CA1 region in rats
- Heavy ion and X-ray irradiation alter the cytoskeleton and cytomechanics of cortical neurons
- Potential targets for protecting against hippocampal cell apoptosis after transient cerebral ischemiareperfusion injury in aged rats
