Diffusional kurtosis imaging: a promising technique for detecting microstructural changes in neural development and regeneration
2014-03-27AmirPaydar
Diffusional kurtosis imaging: a promising technique for detecting microstructural changes in neural development and regeneration
Brain development is one of the most fascinating subjects in the fi eld of biological sciences. Nonetheless, our scienti fi c community still faces challenges in trying to understand the concepts that de fi ne the underlying mechanisms of neural tissue development. After all, it is a very complex subject to grasp and many of the processes that take place during central nervous system maturation are yet to be ascertained. Despite this challenge, we have come to recognize that understanding the natural course of normal brain tissue development on both microscopic and macroscopic scales is the key to deciphering the mechanisms through which these neural networks also heal and regenerate.
Realizing this concept, my good friend and colleague, Dr. Sarah Milla, and I decided to take on a human study to investigate brain maturation using non-invasive imaging techniques in the pediatric population at New York University (NYU) School of Medicine (Paydar et al., 2013). Our research subjects included 59 normal infants with an age spectrum ranging from birth to approximately 5 years of age, when the brain is in its most active stage of development. We implemented a Magnetic Resonance Imaging (MRI) diffusion technique called Diffusional Kurtosis Imaging (DKI) to investigate the microstructural changes that occur in both the white matter (WM) and gray matter (GM) in the developing brain.
Macrostructural changes that take place within the brain during the course of maturation have been well documented by conventional MRI techniques in both normal and pathologic states. However, these conventional techniques are limited in their ability to quantify developmental changes that occur at the microstructural level. Therefore, in vivo characterization and accurate diagnosis of microstructural abnormalities currently remains challenging.
Diffusion imaging, including Diffusion-Weighted Imaging (DWI), has been utilized for evaluation of microstructural changes that are dif fi cult to detect using conventional MRI techniques (Basser and Jones, 2002). In particular, the widely used Diffusion Tensor Imaging (DTI) has been shown to be sensitive to age-related microstructural changes during rodent and human brain development in both physiologic and pathologic states (Takeda et al., 1997; Mukherjee et al., 2002; Deipolyi et al., 2005; Lebel et al., 2008; Cheung et al., 2009; Huang et al., 2009; Treit et al., 2013; Yoshida et al., 2013). As a quantitative measuring tool, DTI has also been implemented in studies investigating the degree of neuronal damage due to both acute and chronic injury (Erjian et al., 2013; Guojie et al., 2014) as well as progressive neurodegeneration (Thomalla et al., 2004; Zhang et al., 2009).
Fractional Anisotropy (FA), the DTI metric that is the primary index of diffusional directionality, can be used to evaluate the anisotropic neuroarchitectural orientation of WM fi ber tracts. FA has demonstrated its sensitivity to certain processes that contribute to tissue organization and increase in anisotropic complexity, particularly myelination, which predominantly occurs in the WM (Beaulieu, 2002; Mukherjee et al., 2002; Lebel et al., 2008). Accordingly, DTI is an excellent tool for investigating the age-related increase in anisotropy that occurs within the network of WM tracks as a result of myelination.
However, DTI is based on a Gaussian approximation of water diffusion, which limits its sensitivity to diffusional and microstructural properties of biological tissues (Veraart et al., 2011). Several years ago, DKI diffusion weighted technique, which exploits diffusional non-Gaussianity, was developed at NYU by our colleagues, Drs. Jens Jensen and Joseph Helpern. This technique takes into account the non-Gaussian diffusional properties of water motion in complex media and is therefore more comprehensive in evaluating brain tissue microstructural complexity (Jensen et al., 2005; Lu et al., 2006; Jensen and Helpern, 2010).
The DKI method is basically a clinically feasible extension of the traditional DTI model, maintaining the ability to estimate all of DTI’s standard diffusion tensor metrics, although with improved accuracy (Veraart et al., 2011). Moreover, DKI provides an additional parameter that quanti fi es non-Gaussian diffusion called diffusional kurtosis, K. By using the K parameter, multiple additional kurtosis metrics, most importantly, mean kurtosis (MK), can be generated.
In our study, we hypothesized that, owing to its potentially higher sensitivity for detection of age-related microstructural changes, MK may provide additional information about brain maturation when compared to that obtainable with the conventional FA metric in both WM and GM. And our results were quite conclusive.
We demonstrated a progressive rise in both FA and MK throughout seven WM regions (splenium and genu of corpus callosum, frontal and parietal WM, anterior and posterior limbs of the internal capsule, and external capsule) that we examined in the fi rst 2 years of life. This fi nding suggested that both DTI and DKI can re fl ect the age-related increase in diffusional anisotropy in WM tracts, predominantly as a function of myelination in the fi rst 2 years. However, our data also showed that MK continues to rise beyond the FA plateau at the 2-year mark in all WM regions, showing its ability to resolve the more delayed microstructural changes that occur in the WM beyond 2 years of age. In other words, compared to DTI, DKI offers additional characterization of the isotropic diffusion barriers that continue to develop in the WM even after myelination and axonal packing have already peaked.
Our study also supported the hypothesis that DKI is sensitive to age-related microstructural changes that occur in the isotropic GM, for which DTI has previously shown to have limited sensitivity (Mukherjee et al., 2002; Cheung et al., 2009). Our results proved that, unlike FA, MK showed a steady rise in signal in the interrogated GM regions (putamen and thalamus) overtime, accounting for speci fi c isotropic GM changes to which FA is not as sensitive. Therefore, when compared to FA, MK can better resolve the progression of GM organization with respect to age by accounting for other isotropic microstructural barriers that form at the cellular level.
Finally, we generated three-dimentional DKI tractography images at various stages of development. These tractography images, as illustrated in Figure 1 courtesy of Paydar and colleagues (2013), qualitatively display DKI’s ability to detect the progressive age-related increase in volume and coherent orientation of central WM tracts throughout development.
In summary, DKI is an innovative diffusion MRI technique that can provide a more comprehensive evaluation of age-related changes in the microstructural complexity of both WM and GM when compared to DTI. Indeed, both DTI and DKI can detect the anisotropic WM changes which occur predominantly during the fi rst 2 years of life as a result of myelination. However, DKI is able to identify other isotropic WM changes that occur beyond the fi rst 2 years. It also provides greater characterization of GM maturation. Accordingly, DKI offers sensitive and comprehensive measures for the quantitative evaluation of age-related microstructural changes in both WM and GM.
So, our investigation has demonstrated the potential utility of a valuable MRI technique for detection of microstructural changes within neural tissues, particularly in the setting of normal braindevelopment. But what is the relevance of this discovery for neural regeneration research? The answer to this question is clear.
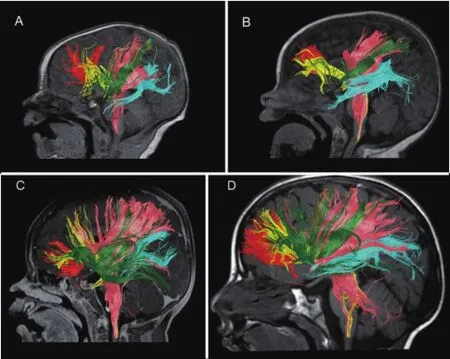
Figure 1 Diffusional Kurtosis Imaging (DKI) tractography at various stages of development, including at birth (A), 6 months (B), 11 months (C), and 2 years 1 month (D) of age.
Speculatively, the diffusion barriers which may form due to the progressive increase in macromolecular reorganization during neural maturation are probably similar to ones that take shape during the course of neural regeneration. These barriers may partly result from many cytoarchitectural changes that take place at the microstructural level during both neural development and regeneration. For example, these changes may include the overall increase in the complexity of intrinsic cellular processes (e.g., proliferation of cell membranes, organelles, and extracellular matrix), axonal pruning and cell packing, myelination and functional reorganization of myelin, as well as addition of basal dendrites and transition of radial glial cells to astrocytic neuropil (Truwit, 2001; Mukherjee et al., 2002; Huppi and Dubois, 2006; Lu et al., 2006; Cheung et al., 2009; Jensen and Helpern, 2010; Veraart et al., 2011; Provenzale et al., 2012; Yoshida et al., 2013).
Therefore, since the increase in tissue complexity that occurs during development may be similar to regeneration, DKI may potentially serve as a valuable measuring tool for detection of cellular processes that alter microstructural complexity of tissues in the setting of neural regeneration. We are optimistic about this great prospect and hope that our neuroscience community will effectively use this non-invasive MRI diffusion technique in both in vivo and in vitro settings for neural regeneration research in the near future.
Amir Paydar
Center for Biomedical Imaging, Department of Radiology, New York
University School of Medicine, 660 First Ave, 4thFloor, New York, NY, USA
Basser PJ, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design and data analysis-a technical review. NMR Biomed 15:456-467.
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system-a technical review. NMR Biomed 15:435-455.
Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX (2009) Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage 45:386-392.
Deipolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, Jin H, Lu Y, Miller SP, Ferriero DM, Vigneron DB, Barkovich AJ (2005) Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. Neuroimage 27:579-586.
Lin EJ, Long HQ, Li GS, Lei WL (2013) Does diffusion tensor data re fl ect pathological changes in the spinal cord with chronic injury? Neural Regen Res 8:3382-3390.
Jing GJ, Yao XT, Li YY, Xie YT, Li WA, Liu KJ, Jing YC, Li BS, Lv YF, Ma BX (2014) Mild hypothermia for treatment of diffuse axonal injury: a quantitative analysis of diffusion tensor imaging. Neural Regen Res 9:190-197.
Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller MI, Mori S (2009) Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci 29:4263-4273.
Huppi PS, Dubois J (2006) Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med 11:489-497.
Jensen JH, Helpern JA (2010) MRI quanti fi cation of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23:698-710.
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quanti fi cation of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432-1440. Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008) Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044-1055.
Lu H, Jensen JH, Ramani A, Helpern JA (2006) Three-dimensional characterization of non-Gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed 19:236-247.
Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC (2002) Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. Am J Neuroradiol 23:1445-1456.
Paydar A, Fieremans E, Nwankwo JI, Lazar M, Sheth HD, Adisetiyo V, Helpern JA, Jensen JH, Milla SS (2013) Diffusional kurtosis imaging of the developing brain. Am J Neuroradiol 35:808-814.
Provenzale JM, Isaacson J, Chen S (2012) Progression of corpus callosum diffusion-tensor imaging values during a period of signal changes consistent with myelination. AJR Am J Roentgenol 198:1403-1408.
Takeda K, Nomura Y, Sakuma H, Tagami T, Okuda Y, Nakagawa T (1997) MR assessment of normal brain development in neonates and infants: comparative study of T1- and diffusion-weighted images. J Comput Assist Tomogr 21:1-7.
Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J (2004) Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage 22:1767-1774.
Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, and Beaulieu C (2013) Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J Neurosci 33:10098-10109.
Truwit CL (2001) Myelination of the Developing Human Brain. In: Nelson, CA, Luciana M, eds. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press 35-44.
Veraart J, Poot DH, Van Hecke W, Blockx I, Van der Linden A, Verhoye M, Sijbers J (2011) More accurate estimation of diffusion tensor parameters using diffusion kurtosis imaging. Magn Reson Med 65:138-145.
Yoshida S, Oishi K, Faria AV, Mori S (2013) Diffusion tensor imaging of normal brain development. Pediatr Radiol 43:15-27.
Zhang J, Jones M, DeBoy CA, Reich DS, Farrell JA, Hoffman PN, Grif fi n JW, Sheikh KA, Miller MI, Mori S, Calabresi PA (2009) Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy. J Neurosci 29:3160-3171.
Amir Paydar, M.D., Center for Biomedical
10.4103/1673-5374.135309 http://www.nrronline.org/ Paydar A. Diffusional kurtosis imaging: a promising technique for detecting microstructural changes in neural development and regeneration. Neural Regen Res. 2014;9(11):1108-1109.
Imaging, Department of Radiology, New York University School of Medicine, 660 First Ave, 4thFloor, New York, NY 10016, USA, amirpaydar@gmail.com;amir.paydar@nyumc.org.
Presentation: American Society of Neuroradiology (ASNR) 50th Annual meeting; New York, NY, USA (Apr 2012).
Accepted: 2014-05-28
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture at the Taixi (KI3) acupoint activates cerebral neurons in elderly patients with mild cognitive impairment
- High matrix metalloproteinase-9 expression induces angiogenesis and basement membrane degradation in stroke-prone spontaneously hypertensive rats after cerebral infarction
- A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosis
- Somatosensory stimulation suppresses the excitability of pyramidal cells in the hippocampal CA1 region in rats
- Heavy ion and X-ray irradiation alter the cytoskeleton and cytomechanics of cortical neurons
- Potential targets for protecting against hippocampal cell apoptosis after transient cerebral ischemiareperfusion injury in aged rats
