Repair and regeneration properties of Ginkgo biloba after ischemic brain injury
2014-03-27AparnaRaghavan,ZahoorA.Shah
Repair and regeneration properties of Ginkgo biloba after ischemic brain injury
The irretrievable fate of neurons dominated the neuroscience rhetoric for the first half of this century, a position that was fiercely contested and recently debunked by extensive studies carried out in the field of neuroregeneration research. The turning point came in the year 1928, when Ramon Y. Cajal’s (Lobato, 2008) work suggested that the regenerative capacity of neurons, though limited, could exist beyond their physical being and depended on the environment surrounding them. That the manipulation of the restrictive environment surrounding the neuron could aid the regenerative process was conclusively established by Aguayo and colleagues (Richardson et al., 1980). Since then, various strategies have been employed to target the different phases of regeneration which include: cell-replacement and augmenting endogenous neurogenesis, the use of trophic factors, reversal of the inhibitory cues, and induction of signaling pathways that stimulate axon growth and guidance (Horner and Gage, 2000).
Replacement of damaged tissue with cell-grafts or pluripotent stem cells that could differentiate into neural and glial cell populations has been widely studied (Modo et al., 2002; Jiang et al., 2011). In light of the practical and ethical issues restraining the fi eld of stem cell research, alternative modes of stimulating neurogenesis are highly sought after. We now know that neurogenesis is not only an ongoing process in adults, but it can also be induced by pathological conditions like traumatic brain injury and ischemic stroke (Greenberg, 2007; Yu et al., 2008). This fi eld of research views the nervous system as a plastic entity that can respond to deleterious cues by triggering repair mechanisms (Okano et al., 2007). Neurogenesis is thus a valuable avenue for therapeutic efforts aimed at reducing disability and cognitive decline following ischemic stroke.
Neurogenesis in the adult brain involves the proliferation of precursor cells known as stem cells/neural progenitor cells (NSCs). The repositories for these NSCs are the sub-ventricular zone (SVZ), the sub-granular zone (SGZ) of the dentate gyrus (DG), and to a lesser extent, the posterior periventricular area (PPv) (Gage, 2000; Wiltrout et al., 2007). The NSCs of the SVZ are pluripotent and migrate as a chain of neuroblasts, forming the rostral migratory stream (RMS) that leads to the olfactory bulb, wherein they eventually differentiate to form interneurons (Alvarez-Buylla and Garcia-Verdugo, 2002). This property is retained even in the absence of the olfactory bulb, suggesting that it is not target-oriented (Kirschenbaum et al., 1999) and could thus be routed to serve other purposes. In the case of ischemia, the NSCs are not only known to proliferate but also to defy the RMS and move laterally toward the site of injury (Zhang et al., 2008). The SGZ of the dentate gyrus is the next important site of neurogenesis. Whether its stem cells are truly self-renewing or more lineage-restricted is a matter of contention (Gage et al., 1998), due to which they are referred to as progenitor cells. Both global and focal ischemia induce neurogenesis in the SVZ and the DG, with the focal mode thought to engage neurogenesis in the cortex as well (Wiltrout et al., 2007). The migration of these proliferating NSCs needs to be substantiated with suf fi cient neuronal differentiation and functional integration into the neural network in order to have a signi fi cant impact on the improvement of stroke outcomes. Though ischemia is a potent inducer of proliferation and migration of NSCs, it does not provide an environment conducive to their survival, differentiation and integration (Wiltrout et al., 2007; Niv et al., 2012). However, neurogenesis is a dynamic process and is sensitive to external cues like radiation and fatty diet that shut off the process, whereas exercise and caloric restriction upregulate or enhance it. This is bene fi cial from the therapeutic standpoint and has been utilized in several drug discovery efforts.
Natural products have been investigated for their ability to induce the proliferation, differentiation, migration and, finally, functional integration of neural stem cells in the hope of discovering a successful regenerative therapy for stroke. The polyvalent mode of action of most natural product-based drugs is particularly bene fi cial in a complex pathology like ischemia, wherein multiple destructive mechanisms need to be targeted simultaneously for an effective intervention (Wu et al., 2010). In addition to their diverse molecular targets, natural products also enjoy better absorption pro fi les when compared to purely synthetic leads. Recent advances in bioactivity-guided screening assays and reliable characterization techniques have improved the productivity of natural products tremendously, making them the most likely potential source of drug leads (Harvey, 2008). Many traditional herbs have been tested in experimental stroke models, some showing huge potential. Components of Salviae Miltiorrhizae Radix (Zhong et al., 2007), Cornus officinalis (Yao et al., 2009), Ginkgo biloba (G. biloba, Tchantchou et al., 2007; Nada et al., 2014), and some herbal combinations like NeuroAid (Heurteaux et al., 2010) are known to promote neurogenesis in conditions of ischemic and other pathological stress. Most of these natural products function by augmenting physiological repair mechanisms by increasing the synthesis of growth factors and stress-response elements.
G. biloba is a widely studied herb for the treatment of neurological disorders (Di Renzo, 2000); the neurogenesis-enhancing effects of G. biloba form the focus of this perspective. Its cellular mechanisms of action and the consequential regenerative effects outlined here would provide valuable insight into its therapeutic potential for stroke and various related disorders. The standardized extract of this herb, EGb 761, is prescribed as a dietary supplement and is touted to have neuroprotective and neurorestorative properties that have been consistently reproduced in many animal models of CNS disorders (Kehr et al., 2012; Diamond and Bailey, 2013; Tulsulkar and Shah, 2013; Wang et al., 2013a). Its use as a symptomatic treatment for dementia has been established in many preclinical studies (Tchantchou et al., 2007; Wang et al., 2013b) and clinical trials (Oken et al., 1998; Schneider et al., 2005; Mazza et al., 2006; Weinmann et al., 2010). A number of clinical studies conducted in Europe and the US have demonstrated the potential therapeutic effects of G. biloba in multi-infarct dementia, early or mild cognitive decline, and severe types of senile dementias (Weinmann et al., 2010; Amieva et al., 2013). However, “Ginkgo Evaluation of Memory (GEM),”showed no effect of EGb 761 (240 mg daily dose for 7 years) on delaying or preventing Alzheimer’s-related dementia among the population aged 75 or older (DeKosky et al., 2006).
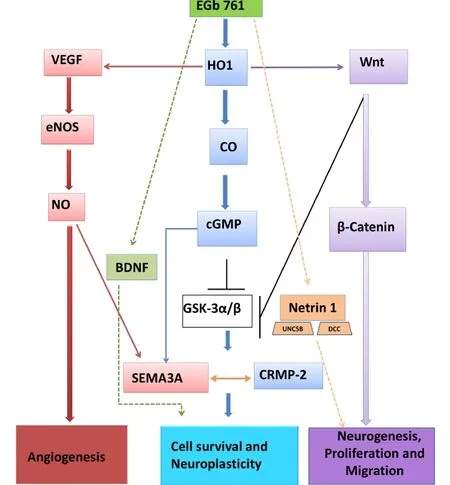
Figure 1 Possible signaling of G. biloba (EGb 761) induced neuroprotection and neurogenesis.We propose that neuroprotective mechanism(s) of EGb 761 are mediated via multiple pathways: 1) EGb 761 activates hemeoxygenase 1 (HO1), which cleaves heme to form biliverdin and carbon monoxide (CO). CO increases intracellular cyclic guanosine monophosphate (cGMP) production, which mediates axon branching by activating chemoattractive semaphorin 3A (SEMA3A) and also by inhibiting glycogen synthase kinase 3 (GSK-3). GSK-3 inhibition increases the activity of collapsing response mediator protein-2 (CRMP-2), leading to cell survival and cell neuroplasticity; 2) EGb 761 activates the vascular endothelial growth factor (VEGF)/endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) pathway that leads to chemoattractant SEMA3A activation and angiogenesis; 3) EGb 761 increases Wnt and activates Wnt/β-catenin pathway and, by inhibiting GSK-3, leads to neurogenesis, proliferation and migration; 4) EGb 761 directly activates brain derived neurotrophic factor (BDNF) and increases cell survival and neuroplasticity; and 5) EGb 761 activates netrin 1 and its receptors deleted in colorectal cancer (DCC) and uncoordinated gene 5B (UNC5B), leading to increased neurogenesis, proliferation and migration. Activation (), inhibition (), mutual activation( ).(Figure modi fi ed from Nada et al. Molecular Neurobiology. 2014;49:945-956.)
EGb 761 is standardized to contain 24% fl avone glycosides and 6% terpenoids. Most of the neuroprotective effects of EGb 761 stem from its antioxidant effects, although its ability to increase cerebral blood fl ow and modulate neurotransmitter activity are also thought to be contributory (Diamond et al., 2000). Recent studies on the mechanisms of action of this extract have unraveled a host of other effects, many of which are not related to its anti-oxidant effects. This has broadened the scope of EGb 761 beyond the traditional realm of neuroprotection to the restorative and recovery potential for stroke therapy. An important, recently established example is the discovery of the multifaceted actions of EGb 761-mediated upregulation of hemeoxygenase 1 (HO1) (Shah et al., 2011; Nada and Shah, 2012; Nada et al., 2014). HO1 is a stress-inducible anti-oxidant enzyme respon-sible for catabolizing pro-oxidant heme into carbon monoxide, bilirubin and biliverdin. HO1 is crucial for anti-oxidant defense and is, more importantly, a viable target for stroke therapy. HO1 not only breaks down heme, but also most of its byproducts have neuroprotective properties (Ahmad et al., 2006), thus creating a milieu for HO1 upregulation to affect diverse signaling cascades.
We and others have unequivocally proven HO1 to be one of the prime targets of EGb 761-mediated protection, as observed in animal models of stroke and other conditions of oxidative stress (Chen et al., 2001; Zhuang et al., 2002; Saleem et al., 2008; Shah et al., 2011; Nada and Shah, 2012). EGb 761 enhances neurogenesis, and the crosstalk between HO1-induction and enhanced neurogenesis has only been recently discovered (Vanella et al., 2013; Nada et al., 2014). Our group demonstrated that EGb 761-treated mice not only showed an increase in the number of NSCs post-stroke, but also the majority of these NSCs were found in the proximity of the injury site or penumbra area. This was further evidenced by the upregulation of netrin-1 and its receptors, DCC and UNC5B, which mediate axonal attraction and repulsion. It is known that attractive and repulsive guidance cues dictate post-stroke axonal sprouting as well as migration of neuroblasts towards the site of the injury (Carmichael, 2008). Netrin-1 overexpression promotes neuronal migration and aids cell survival (Tang et al., 2008). Thus, EGb 761-mediated overexpression of netrin-1 signi fi es its ability to promote a suitable environment for the migration of neuroblasts. As previously discussed, the migration of newly formed NSCs alone would be less beneficial if not followed by neuronal differentiation and the survival of the NSCs upon reaching the target or injury site. We found that EGb 761 also enhanced the expression of Wnt, the ligand that is responsible for triggering the canonical Wnt pathway or the Wnt/β-catenin pathway, which constitutes one of the primary signaling mechanisms crucial for endogenous neurogenesis. Potentiation of the Wnt cascade is known to improve functional outcomes after stroke by increasing neuronal differentiation and survival of the newly formed neurons (Shruster et al., 2012). EGb 761-treated mice also showed upregulated expression of brain derived growth factor (BDNF) in NSCs, which is a speci fi c marker for neuronal cells and enhances proliferation and differentiation of NSPCs (Nada et al., 2013) (Figure 1).
Quite interestingly, our group also found that neurogenesis was signi fi cantly diminished in HO1 knockout mice seven days post-surgery. This fi nding corroborates with our earlier studies that HO1 knockout mice were not protected by ischemic preconditioning (Zeynalov et al., 2009) and suffered from higher infarct volume and severe neurologic deficits after seven days of permanent ischemia (Shah et al., 2011). Since HO1 is an essential target of EGb 761’s action, we were curious to learn whether EGb 761-mediated HO1 induction had a role to play in its neurogenesis-enhancing properties. We have highly plausible explanations to establish the link between the two. For example, it is well known that HO1 is an inducer of vascular endothelial growth factor (VEGF) (Cisowski et al., 2005; Lin et al., 2011), which is not only a promoter of angiogenesis but also has neurogenic properties (Sun et al., 2003). Thus HO1 seems to act on the neurovascular niche that supports the newly formed neurons as well as the vasculature surrounding the network. We have previously demonstrated that EGb 761 upregulates the expression of VEGF concomitant with that of HO1 (Shah et al., 2011). In addition, we have established the link between HO1 and collapsin response mediator protein 2 (CRMP2) in a study examining its neuritogenic potential (Nada and Shah, 2012). CRMP2 is a protein involved in neuronal development and neurite outgrowth, the inactivation of which is known to cause growth cone collapse (Crews et al., 2011; Higurashi et al., 2012). The positive correlation between HO1 and CRMP2 expression further substantiates the involvement of HO1 in neurogenesis. Lastly, the discovery that HO1 acts upstream of the Wnt signaling pathway (Vanella et al., 2013) strongly ties the augmented neurogenesis seen in our study to HO1 upregulation and also highlights how a single protein could perturb multiple signaling mechanisms (Figure 1). To support our preclinical studies, a recent double-blind, placebo-controlled and randomized clinical study for the fi rst time recommended the use of G. biloba in stroke recovery. G. biloba (120 mg daily) treatment for 4 months following an ischemic stroke signi fi cantly reduced NIHSS in stroke patients compared to the placebo group (Oskouei et al., 2013). In order to promote the wide and safe use of G. biloba, further high quality and largescale randomized controlled trials are warranted to test its ef fi cacy in acute ischemic stroke recovery.
Stroke is a leading cause of long-term disability and poses excruciating economic and societal burdens (Go et al., 2014). Therapies aimed at post-stroke recovery may help curb the rising cost of healthcare and are therefore highly sought after. To treat complex neurodegenerative diseases, polypharmacology is a well sought strategy, and natural products, particularly extracts, can offer a treasure of potential drug leads that could someday change the way we think of neuro-regeneration and the use of medicinal plants.
Aparna Raghavan, Zahoor A. Shah
Department of Medicinal and Biological Chemistry, College of Pharmacy and Pharmaceutical Sciences, University of Toledo,
Toledo, OH, USA
Ahmad AS, Zhuang H, Dore S (2006) Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience 141:1703-1708.
Alvarez-Buylla A, Garcia-Verdugo JM (2002) Neurogenesis in adult subventricular zone. J Neurosci 22:629-634.
Amieva H, Meillon C, Helmer C, Barberger-Gateau P, Dartigues JF (2013) Ginkgo biloba extract and long-term cognitive decline: a 20-year follow-up population-based study. PLoS One 8:e52755.
Carmichael ST (2008) Themes and strategies for studying the biology of stroke recovery in the poststroke epoch. Stroke 39:1380-1388.
Chen JX, Zeng H, Chen X, Su CY, Lai CC (2001) Induction of heme oxygenase-1 by Ginkgo biloba extract but not its terpenoids partially mediated its protective effect against lysophosphatidylcholine-induced damage. Pharmacol Res 43:63-69.
Cisowski J, Loboda A, Jozkowicz A, Chen S, Agarwal A, Dulak J (2005) Role of heme oxygenase-1 in hydrogen peroxide-induced VEGF synthesis: effect of HO-1 knockout. Biochem Biophys Res Commun 326:670-676.
Crews L, Ruf R, Patrick C, Dumaop W, Trejo-Morales M, Achim CL, Rockenstein E, Masliah E (2011) Phosphorylation of collapsin response mediator protein-2 disrupts neuronal maturation in a model of adult neurogenesis: Implications for neurodegenerative disorders. Mol Neurodegener 6:67.
DeKosky ST, Fitzpatrick A, Ives DG, Saxton J, Williamson J, Lopez OL, Burke G, Fried L, Kuller LH, Robbins J, Tracy R, Woolard N, Dunn L, Kronmal R, Nahin R, Furberg C, Investigators G (2006) The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials 27:238-253.
Di Renzo G (2000) Ginkgo biloba and the central nervous system. Fitoterapia 71 Suppl 1:S43-47.
Diamond BJ, Bailey MR (2013) Ginkgo biloba: indications, mechanisms, and safety. Psychiatr Clin North Am 36:73-83.
Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, Richards JA, Schoenberger NE (2000) Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil 81:668-678.
Gage FH (2000) Mammalian neural stem cells. Science 287:1433-1438.
Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J (1998) Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol 36:249-266.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Tow fi ghi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S (2014) Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 129:e28-292.
Greenberg DA (2007) Neurogenesis and stroke. CNS Neurol Disord Drug Targets 6:321-325.
Harvey AL (2008) Natural products in drug discovery. Drug Discov Today 13:894-901.
Heurteaux C, Gandin C, Borsotto M, Widmann C, Brau F, Lhuillier M, Onteniente B, Lazdunski M (2010) Neuroprotective and neuroproliferative activities of NeuroAid (MLC601, MLC901), a Chinese medicine, in vitro and in vivo. Neuropharmacology 58:987-1001.
Higurashi M, Iketani M, Takei K, Yamashita N, Aoki R, Kawahara N, Goshima Y (2012) Localized role of CRMP1 and CRMP2 in neurite outgrowth and growth cone steering. Dev Neurobiol 72:1528-1540.
Horner PJ, Gage FH (2000) Regenerating the damaged central nervous system. Nature 407:963-970.
Jiang M, Lv L, Ji H, Yang X, Zhu W, Cai L, Gu X, Chai C, Huang S, Sun J, Dong Q (2011) Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem 354:67-75.
Kehr J, Yoshitake S, Ijiri S, Koch E, Noldner M, Yoshitake T (2012) Ginkgo biloba leaf extract (EGb 761(R)) and its speci fi c acylated fl avonol constituents increase dopamine and acetylcholine levels in the rat medial prefrontal cortex: possible implications for the cognitive enhancing properties of EGb 761(R). Int Psychogeriatr Suppl 1:S25-34.
Kirschenbaum B, Doetsch F, Lois C, Alvarez-Buylla A (1999) Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci 19:2171-2180.
Lin HH, Lai SC, Chau LY (2011) Heme oxygenase-1/carbon monoxide induces vascular endothelial growth factor expression via p38 kinase-dependent activation of Sp1. J Biol Chem 286:3829-3838.
Lobato RD (2008) Historical vignette of Cajal’s work “Degeneration and regeneration of the nervous system” with a re fl ection of the author. Neurocirugia (Astur) 19:456-468.
Mazza M, Capuano A, Bria P, Mazza S (2006) Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled double-blind study. Eur J Neurol 13:981-985.
Modo M, Stroemer RP, Tang E, Patel S, Hodges H (2002) Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke 33:2270-2278.
Nada SE, Shah ZA (2012) Preconditioning with Ginkgo biloba (EGb 761(R)) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis 46:180-189.
Nada SE, Tulsulkar J, Shah ZA (2014) Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761(R)) after permanent ischemic stroke in mice. Mol Neurobiol 49:945-956.
Niv F, Keiner S, Krishna, Witte OW, Lie DC, Redecker C (2012) Aberrant neurogenesis after stroke: a retroviral cell labeling study. Stroke 43:2468-2475.
Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K (2007) Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem 102:1459-1465.
Oken BS, Storzbach DM, Kaye JA (1998) The ef fi cacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol 55:1409-1415.
Oskouei DS, Rikhtegar R, Hashemilar M, Sadeghi-Bazargani H, Sharifi-Bonab M, Sadeghi-Hokmabadi E, Zarrintan S, Sharifipour E (2013) The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. J Stroke Cerebrovasc Dis 22:e557-563.
Richardson PM, McGuinness UM, Aguayo AJ (1980) Axons from CNS neurons regenerate into PNS grafts. Nature 284:264-265.
Saleem S, Zhuang H, Biswal S, Christen Y, Dore S (2008) Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke 39:3389-3396.
Schneider LS, DeKosky ST, Farlow MR, Tariot PN, Hoerr R, Kieser M (2005) A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Curr Alzheimer Res 2:541-551.
Shah ZA, Nada SE, Dore S (2011) Heme oxygenase 1, bene fi cial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience 180:248-255.
Shruster A, Ben-Zur T, Melamed E, Offen D (2012) Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS One 7:e40843.
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA (2003) VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111:1843-1851.
Tang X, Jang SW, Okada M, Chan CB, Feng Y, Liu Y, Luo SW, Hong Y, Rama N, Xiong WC, Mehlen P, Ye K (2008) Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat Cell Biol 10:698-706.
Tchantchou F, Xu Y, Wu Y, Christen Y, Luo Y (2007) EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J 21:2400-2408.
Tulsulkar J, Shah ZA (2013) Ginkgo biloba prevents transient global ischemia-induced delayed hippocampal neuronal death through antioxidant and anti-in fl ammatory mechanism. Neurochem Int 62:189-197.
Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Jr., Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG (2013) Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther 4:28.
Wang JW, Chen W, Wang YL (2013a) A ginkgo biloba extract promotes proliferation of endogenous neural stem cells in vascular dementia rats. Neural Regen Res 8:1655-1662.
Wang N, Chen X, Geng D, Huang H, Zhou H (2013b) Ginkgo biloba leaf extract improves the cognitive abilities of rats with D-galactose induced dementia. J Biomed Res 27:29-36.
Weinmann S, Roll S, Schwarzbach C, Vauth C, Willich SN (2010) Effects of Ginkgo biloba in dementia: systematic review and meta-analysis. BMC geriatrics 10:14.
Wiltrout C, Lang B, Yan Y, Dempsey RJ, Vemuganti R (2007) Repairing brain after stroke: a review on post-ischemic neurogenesis. Neurochem Int 50:1028-1041.
Wu PF, Zhang Z, Wang F, Chen JG (2010) Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol Sin 31:1523-1531.
Yao RQ, Zhang L, Wang W, Li L (2009) Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res Bull 79:69-76.
Yu TS, Zhang G, Liebl DJ, Kernie SG (2008) Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci 28:12901-12912.
Zeynalov E, Shah ZA, Li RC, Dore S (2009) Heme oxygenase 1 is associated with ischemic preconditioning-induced protection against brain ischemia. Neurobiol Dis 35:264-269.
Zhang RL, Zhang ZG, Chopp M (2008) Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology 55:345-352.
Zhong J, Tang MK, Zhang Y, Xu QP, Zhang JT (2007) Effect of salvianolic acid B on neural cells damage and neurogenesis after brain ischemia-reperfusion in rats. Yao xue xue bao 42:716-721.
Zhuang H, Pin S, Christen Y, Dore S (2002) Induction of heme oxygenase 1 by Ginkgo biloba in neuronal cultures and potential implications in ischemia. Cell Mol Biol 48:647-653.
Zahoor A. Shah, Ph.D., Department of Medicinal and Biological Chemistry, University of Toledo, 3000 Arlington Avenue, Toledo, OH 43614, USA, zahoor.shah@utoledo.edu.
10.4103/1673-5374.135308 http://www.nrronline.org/
Acknowledgments: This work was supported by a grant from the National Institutes of Health—National Center for Complementary and Alternative Medicine (R00AT004197) and Start-up Funds from The University of Toledo to Shah ZA.
Con fl icts of interest: None declared.
Accepted: 2014-05-16
Raghavan A, Shah ZA. Repair and regeneration properties of Ginkgo biloba after ischemic brain injury. Neural Regen Res. 2014;9(11):1104-1107.
杂志排行
中国神经再生研究(英文版)的其它文章
- Acupuncture at the Taixi (KI3) acupoint activates cerebral neurons in elderly patients with mild cognitive impairment
- High matrix metalloproteinase-9 expression induces angiogenesis and basement membrane degradation in stroke-prone spontaneously hypertensive rats after cerebral infarction
- A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosis
- Somatosensory stimulation suppresses the excitability of pyramidal cells in the hippocampal CA1 region in rats
- Heavy ion and X-ray irradiation alter the cytoskeleton and cytomechanics of cortical neurons
- Potential targets for protecting against hippocampal cell apoptosis after transient cerebral ischemiareperfusion injury in aged rats
