The Seasonal Acclimatisation of Locomotion in a Terrestrial Reptile, Plestiodon chinensis (Scincidae)
2014-03-25BaojunSunWenqiTangZhigaoZengandWeiguoDu
Baojun Sun, Wenqi Tang, Zhigao Zengand Weiguo Du*
1Key Laboratory of Animal Ecology and conservation Biology, Institute of Zoology, chinese Academy of Sciences, Beijing 100101, china
2University of chinese Academy of Sciences, Beijing, china
The Seasonal Acclimatisation of Locomotion in a Terrestrial Reptile, Plestiodon chinensis (Scincidae)
Baojun Sun1,2, Wenqi Tang1,2, Zhigao Zeng1and Weiguo Du1,*
1Key Laboratory of Animal Ecology and conservation Biology, Institute of Zoology, chinese Academy of Sciences, Beijing 100101, china
2University of chinese Academy of Sciences, Beijing, china
Studies of the seasonal acclimatisation of behavioural and physiological processes usually focus on aquatic or semi-aquatic ectotherms and focus less effort on terrestrial ectotherms that experience more thermally heterogeneous environments. We conducted comparative studies and thermal acclimation experiments on the locomotion of the chinese skink (Plestiodon chinensis) to test whether seasonal acclimatisation in locomotion exists in these terrestrial ectothermic vertebrates, and whether seasonal acclimatisation is predominantly induced by thermal environments. In natural populations, skinks ran faster during the summer season than during the spring season at high-test temperatures ranging from 27°c to 36°c but not at low-test temperatures ranging from 18°c to 24°c. In contrast, the thermal acclimation experiments showed that the cold-acclimated skinks ran faster than the warm-acclimated skinks at the lowtest temperatures but not at high-test temperatures. Therefore, the seasonal acclimatisation occurs to P. chinensis, and may be induced by temperature as well as other factors like food availability, as indicated by the seasonal variation in the thermal dependence of locomotion, and the discrepancy between seasonal acclimatisation and thermal acclimation on locomotion.
Lizard, temperature, terrestrial ectotherms, thermal acclimation
1. Introduction
Phenotypic plasticity enables an organism to manipulate behavioural and physiological processes in response to environmental variation and thus plays an important role in species’ adaptation to fluctuating environments (Gotthard and Nylin, 1995; Weinig, 2000). Acclimatisation is one form of phenotypic plasticity which means any facultative modification in functional performance in response to changes in an environmental variable in the field, while acclimation is defined as such plasticity induced by experimental treatments in the laboratory (Wilson and Franklin, 2002). In response to a long-term or chronic change of natural environmentssuch as seasonal thermal variation, the organism may attain selective advantages by acclimatisation when facing environmentally restrictive effects on functional performance (Schmidt-Nielsen, 1990; Wilson and Franklin, 2002).
As one of the most important ecological factors, temperature has pervasive effects on virtually every aspect of an organism at levels of organisation from the gene to the whole organism. The thermal acclimation of organisms has thus been the main topics of evolutionary and ecological physiology for the last few decades (e.g., Feder and Hofmann, 1999; Johnston and Temple, 2002; Seebacher and James, 2008). Animals may alter their own functional performance through acclimation or acclimatisation to cope with temperature change (Guderley and St Pierre, 2002; Johnston and Temple, 2002). Given that global climate change is unequivocal and imposes significant effects on many aspects of wild animals and plants from metabolism to population
dynamics (Dillon et al., 2010; Root et al., 2003), an understanding of the acclimatory response of an organism to a changing environment would not only elucidate the processes of adaptation but also provide important implications for the conservation of biodiversity in response to ongoing climate change.
Locomotion has decisive effects on foraging, escaping from predators and even reproductive success and is thus tightly related to the Darwinian fitness of animals (e.g., Le Galliard et al., 2004). Locomotion and its determinants have attracted great scientific attention with a number of studies of diverse groups of ectotherms ranging from insects to reptiles (e.g., Huey et al., 1984; Rice and Westneat, 2005; Husak et al., 2006; Strobbe et al., 2009). In several lineages of ectothermic vertebrates, locomotion has been proved to be sensitive to temperature (Angilletta et al., 2002) and shows acclimation or acclimatisation in response to thermal environmental changes (Navas et al., 1999; Wilson et al., 2000). Nonetheless, the evidence for the acclimation or acclimatisation of locomotion mainly comes from aquatic or semi-aquatic ectotherms with little opportunity for behavioural thermoregulation in aquatic homogeneous thermal environments (e.g., Wilson et al., 2000; Johnston and Temple, 2002). In contrast to aquatic ectotherms, most terrestrial ectotherms experience thermally heterogeneous environments (from daily to seasonal) and may be able to maintain ideal body temperatures through behavioural thermoregulation (e.g., Avery, 1982; Huey et al., 1989; Row and Blouin-Demers, 2006). consequently, these animals are thought to have low capabilities of acclimation or acclimatisation in physiological and biochemical processes (Scheiner, 1993; Seebacher and James, 2008).
However, terrestrial ectothermic vertebrates show significant seasonal variation in body temperatures despite rigorous behavioural thermoregulation (e.g., Stevenson, 1985; Ji et al., 1996). In addition, the terrestrial ectothermic vertebrates are also exposed to other seasonally varied factors, such as food conditions, humidity and photoperiod, which may also induce seasonal variation in performance or physiology (e.g., Adolph and Porter, 1993; Lima and Bednekoff, 1999; Madsen and Shine, 2000; Yom-Tov and Geffen, 2006; Sun et al., 2011). Therefore, it would be necessary and interesting to understand how acclimatisation works in terrestrial ectotherms to enhance physiological performance and hence fitness in a seasonally fluctuating environment.
The chinese skink (Plestiodon chinensis) is a mediumsized terrestrial lizard [adult snout-vent length (SVL) is 88 mm–132 mm] mainly observed in southern china and Vietnam (Zhao and Adler, 1993; Zhao et al., 1999). We collected skinks from a wild population in eastern china during the spring and summer to identify the seasonal acclimatisation of locomotion in terrestrial reptiles. Acclimation experiments under thermal environments that mimicked spring and summer were then conducted to test the hypothesis that seasonal acclimatisation is predominantly induced by thermal environments.
2. Materials and Methods
2.1 Seasonal variation in environmental factorsIn 2009, a total of ten temperature loggers (iButton thermochron, DS 1921; Dallas Semiconductor, USA) were set up randomly in both shady and sunny spots in the natural habitat of the skinks in Quzhou of Zhejiang, china to record the thermal environments hourly in spring (April) and summer (July). We also collected data on sunshine hours and precipitation from local Bureau of Meteorology. The sunshine hours are average 7.9 h/ day in July, but only 3.9 h/day in April. The average precipitation is less in July (136 mm) than in April (210 mm). Photoperiod is longer in July (13.9L: 10.1D) than in April (12.8L: 11.2D) on average. In addition, food is more abundant for the skinks in their natural habitat in July than in April (Sun et al., 2011).
2.2 Animal collection and acclimation treatmentsIn April and July of 2010, we collected 11 (6 males and 5 non-gravid females) and 13 (7 males and 6 non-gravid females) adult skinks with intact tails, respectively, to study the seasonal acclimatisation of locomotion. All skinks were measured for SVL (± 0.1 mm), and body mass (± 0.01 g), and then locomotion were determined (see below for the details). Additionally, 22 adult skinks (13 males and 9 non-gravid females) with intact tails were collected in April and used in the acclimation experiment. The skinks were also measured for SVL, body mass before housed individually in plastic cages (300 mm × 200 mm × 185 mm). The cages were lined with paper, halved PVc pipes were provided for shelters, and water and food (Tenebrio molitor larvae) were provided ad libitum. A heating bulb was hung at one end of each cage to provide basking opportunities for the skinks. The two acclimation treatments mimicked the thermal environments of spring and summer based on the 2009 ambient temperature records (see Figure 1 for details). In the cold-acclimated treatment (mimicked spring, 5 males and 4 females), the skinks were kept at a room temperature of 16°c with a basking opportunity of 5 h
per day (from 10:30–15:30). In the warm-acclimated treatment (mimicked summer, 8 males and 5 females), the skinks were kept at a room temperature of 28°c with a basking opportunity of 10 h per day (from 08:00–18:00). The cloacal temperatures of the skinks were measured hourly from 07:00 to 20:00 on the 20thday of the acclimation experiments by inserting a calibrated UNT-T325 electronic thermal meter (± 0.1°c, UNI-Trend Technology, Shanghai, china) at a depth of about 10 mm in cloacae. The acclimation treatments lasted for 30 days, and the locomotion of the skinks were subsequently measured. Independent samples were used in each season, after the experiment, the skinks were released to sites where they were collected. All experimental procedures were approved by the Animal care and Ethics committee at the Institute of Zoology, chinese Academy of Science.
2.3 LocomotionThe locomotion of the skinks was measured at seven temperatures (18°c, 21°c, 24°c, 27°c, 30°c, 33°c, 36°c). Each skink was tested at one temperature per day, and the order of test temperatures was random. The skinks were placed in an incubator (KB240, Binder, Germany) set at one of test temperatures for 2 h prior to each trial. We sampled the cloacal temperatures of several skinks to confirm the consistence between body temperature and test temperature before initiating the measurement of locomotion. The locomotion trials were conducted in a custom-made runway (1800 mm × 150 mm × 200 mm) with transparent glass on the front side to allow the video camera to capture the skink’s locomotion. The skinks were introduced from one end of the runway and ran through 1500 mm (6 intervals of 250 mm) under vigorous stimulation (by tapping the tail of skinks with a painting brush). The locomotion of the skinks was captured using a digital video camera (SONY, DcR-SR220E), and the recorded videos were analysed by Windows Movie Maker (Microsoft corp., USA) for the sprint speed in the fastest 250 mm interval. Each skink was tested three times with a 1 h interval at each temperature; the testing sequence for all individual skinks was the same in each trial. The sprint speed was calculated as the mean of the fastest speed for the three measurements because there was no amongmeasurement difference in the fastest speed (P > 0.15 in all treatments at any temperature). All the locomotion measurements were conducted from 09:00 to 12:00, and after measurements the skinks were transferred back to the cages where food and water were available ad libitum until 20:00.
2.4 Statistical analysisWe analysed the normality of variance distributions with the Kolmogorov-Smirnov test and the homogeneity of variances with Levene’s test. One-way ANOVAs were used to analyse betweentreatment or between-sex difference in the body size of skinks. Repeated measures ANOVAs were utilised to analyse body temperature variations of the skinks in the acclimation treatments over time; while the locomotion variations were analyzed in the same way with test temperature as the repeated measures and treatment as the factor. A Tukey’s post-hoc multiple-comparison was used to distinguish the mean traits values.
3. Results
3.1 Thermal environmentIn the natural habitat where P. chinensis was collected, the mean ambient temperature was 21.1°c (13.5°c–33.0°c) in the spring and 31.9°c (25.0°c–37.3°c) in the summer (Figure 1a). During the acclimation experiments, the mean ambient temperature in the cold-acclimated treatment was 19.1°c (15.3°c–32.0°c) and 30.9°c (26.5°c–36.9°c) in the warm-acclimated treatment (Figure 1b).
3.2 Body size and temperature of lizardsThe body size of skinks did not differ between spring and summer [SVL: 113.8 ± 2.9 mm (n = 11) vs. 111.0 ± 1.1 mm (n = 13), F1,22= 0.980, P = 0.333; body mass: 39.54 ± 3.15 g (n = 11) vs. 32.88 ± 1.45 g (n = 13), F1,22= 4.094, P = 0.056], nor between cold- and warm-acclimated treatments [SVL: 111.8 ± 3.3 mm (n = 9) vs. 112.8 ± 2.8 mm (n = 13), F1,20= 0.071, P = 0.792; body mass: 35.53 ± 2.93 g (n = 9) vs. 36.66 ± 2.87 g (n = 13), F1,20= 2.250, P = 0.149). Body size did not differ between sexes (for SVL and body mass, all P > 0.160).
The body temperatures of P. chinensis in the acclimation treatments corresponded with the thermal environments in treatments. The mean body temperature in the cold-acclimated treatment was 21.6°c (16.8°c–31.2°c) and 32.3°c (25.9°c–37.0°c) in the warm-acclimated treatment (Figure 2). The body temperatures of P. chinensis were significantly higher in the warm-acclimated treatment than in the coldacclimated treatment (F1,30= 2442.9, P < 0.001).
3.3 LocomotionSprint speed was significantly affected by test temperatures (F6,132= 101.81, P < 0.001), and was generally higher in summer than in spring (F1,22= 5.35, P = 0.030), but did not differ between sexes (F1,22= 1.37, P = 0.256). A significant interaction between test temperatures and season groups indicated that the seasonal variation in sprint speed depended on the test
temperatures (F6,132= 3.90, P = 0.001). Sprint speed was higher for skinks in the summer treatment than for skinks in the spring treatment at high-test temperatures (27°c, 30°c, 33°c, 36°c) (F1,22= 8.00, P = 0.010) but did not differ at low-test temperatures (18°c, 21°c, 24°c) (F1,22= 0.26, P = 0.614) (Figure 3a).
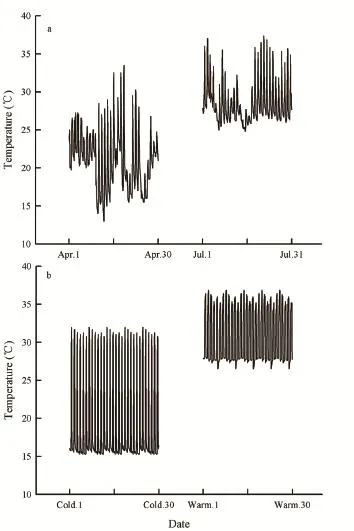
Figure 1 The thermal environment in the natural habitat and thermal acclimation treatments: (a) the thermal environments in spring (April) and summer (July), and (b) the thermal environments in cold- and warm-acclimated treatments. Temperatures were recorded hourly in the natural habitat of the skinks in spring (April) and summer (July). The two acclimation treatments mimicked the thermal environments of spring and summer. The mean ambient temperature was 21.1°c (13.5°c–33.0°c) in the spring and 31.9°c (25.0°c–37.3°c) in the summer. The mean ambient temperature in the cold-acclimated treatment was 19.1°c (15.3°c–32.0°c) and 30.9°c (26.5°c–36.9°c) in the warm-acclimated treatment.
Sprint speed of skinks in the acclimation experiments did not differ between the cold- and warm-acclimated treatments (F1,20= 0.36, P = 0.56), or between sexes (F1,20= 0.03, P = 0.873). However, the sprint speed was significantly affected by test temperatures (F6,120= 68.20, P < 0.001) and the interaction between test temperature and treatment (F6,120= 6.71, P < 0.001). The sprint speed of the skinks in the cold-acclimated treatment was significantly higher than the skinks in the warmacclimated treatment at low-test temperatures (F1,20= 15.25, P = 0.001) but did not show between-treatment differences at high-test temperatures (F1,20= 1.05, P = 0.318) (Figure 3b).
4. Discussion
Similar to many other diurnal lizards, P. chinensis lives in an open and warm environment and rigorously regulates body temperature when surrounding heating sources are available to achieve optimal temperatures for physiological and behavioural functions (Ji et al., 1995; Shu et al., 2010). In spite of the significant role of behavioural thermoregulation in the thermal adaptation of this species, our results suggest that P. chinensis may also use acclimation as an adaptive strategy to respond to environmental variation, as indicated by the seasonal acclimatisation and thermal acclimation of locomotion in this species. Therefore, the acclimation of functional performance which is originally thought to mainly occur in aquatic and semi-aquatic animals (Scheiner, 1993; Wilson et al., 2000; Johnston and Temple, 2002) may also exist in terrestrial species.

Figure 2 The body temperatures of Plestiodon chinensis exposed to different thermal acclimation treatments. The cloacal temperatures of the skinks were measured hourly from 07:00 to 20:00 on the 20th day of the acclimation experiments. The mean body temperature in the cold-acclimated treatment was 21.6°c (16.8°c –31.2°c) and 32.3°c (25.9°c –37.0°c) in the warm-acclimated treatment.
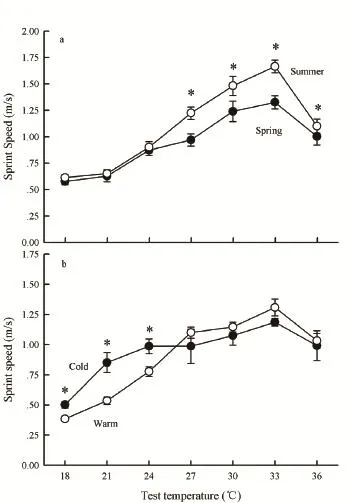
Figure 3 Plestiodon chinensis locomotions at different test temperatures ranging from 18°c to 36°c: (a) individuals from field populations during spring and summer, and (b) individuals from the cold- and warm-acclimated treatments. The asterisks indicate significant differences between acclimation (acclimatisation) groups. Each skink was tested at one temperature per day, and three times within a 1 h interval in each trial. P. chinensis ran faster during summer than during spring at high-test temperatures but not at lowtest temperatures. In contrast, the cold-adapted P. chinensis ran faster than the warm-adapted P. chinensis at low test temperatures but not at the high test temperatures. The sample size for spring was 11, summer was 13, cold-acclimated treatment was 9 and warmacclimated treatment was 13.
Acclimation responses to temperature have been clearly demonstrated in a diversity of species in the laboratory (see review by Angilletta, 2009). Our thermal acclimation experiments indicated that the cold-acclimated P. chinensis ran faster than the warmacclimated P. chinensis at low-test temperatures but not at high-test temperatures. The thermal acclimation of locomotion is largely consistent with previous studies on other ectotherms, such as fish and amphibians (e.g., Fry and Hart, 1948; Wilson et al., 2000; carey and Franklin, 2009). The results partially support the beneficial acclimation hypothesis that an acclimatory response to a particular environment (low temperatures in this case) confers a physiological performance advantage or fitness promotion on an individual organism in that environment over another organism that has no acclimation or is acclimated to another environment (Leroi et al., 1994; Wilson and Franklin, 2002). However, this pattern of thermal acclimation was not observed in seasonal acclimatisation of the natural population. Instead, P. chinensis from the summer population ran faster than P. chinensis from the spring population at high-test temperatures from 27°c to 36°c but not at lowtest temperatures from 18°c to 24°c, which seems to be consistent with the prediction of beneficial acclimation hypothesis (high temperatures in this case).
This discrepancy between seasonal acclimatisation and thermal acclimation suggests that the seasonal acclimatisation in response to other factors masks thermal acclimation in the skink. These environmental factors may be abiotic (e.g., water and sunlight) or biotic (e.g., food and predation). For example, the lower food availability and temperatures (leading to inefficient food assimilation; Ji et al., 1995) observed in spring than in summer may result in the poor condition of the energetic state of P. chinensis in this season. As a result, the decrease in energetic expenditure available for locomotion due to energy limitation may affect the physiological properties of muscle and therefore locomotion (Shu et al., 2010). Therefore, the seasonal acclimatisation in locomotion may be relevant for the effect of body temperature and other environmental factors (e.g. food availability) on energetic metabolism (Johnston et al.,1985; Rall and Woledge, 1990), yet the underlying mechanisms of this physiological process remain unknown.
In summary, our study indicated that the acclimatisation in nature could be a result of combined effects of many environmental factors and is more complex than the acclimation in response to a single environmental factor demonstrated in the laboratory. Disentangling the causes of and identifying the ecological significance of acclimatisation responses in nature would be a significant challenge for eco-physiologists to increase our knowledge of animal physiological adaptations in response to environmental changes. However, the ecological processes and significance of acclimation have received much less attention, while physiologists keep their focus on the biochemical and molecular bases of acclimation (Somero, 2005).
AcknowledgementsWe thank L. SHU, Z. H. LIN for their assistance in lizard collection and D. D. HAN, Y. WANG for their assistance in laboratory. This work was supported by grants from the National Natural Science Foundation of china (30770274) and the “One Hundred Talents Program” of the chinese Academy of Sciences for W. G. DU.
Adolph S. c., Porter W. P.1993. Temperature, activity, and lizard
life-histories. Am Nat, 142: 273–295
Angilletta M. J. Jr.2009. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford: Oxford University Press
Angilletta M. J. Jr., Niewiarowski P. H., Navas c. A.2002. The evolution of thermal physiology in ectotherms. J Therm Biol, 27: 249–268
Avery R. A.1982. Field studies of body temperatures and thermoregulation. 93–166. In Gans c., Pough F. H. (Eds.), Biology of the Reptilia, Vol. 12, Physiological Ecology. New York: Academic Press
carey G. R., Franklin c. E.2009. Effect of incubation and rearing temperature on locomotor ability in barramundi, Lates calcarifer Bloch, 1790. Mar Freshwater Res, 60: 203–210
carrier D. R.1990. Activity of the hypaxial muscles during walking in the lizard Iguana iguana. J Exp Biol, 152: 453–470
Dillon M. E., Wang G., Huey R. B.2010. Global metabolic impacts of recent climate warming. Nature, 467: 704–706
Feder M. E., Hofmann G. E.1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Ann Rev Physiol, 61: 243–282
Fry F. E. J., Hart J. S.1948. cruising speed of goldfish in relation to water temperature. J Fish Res Board can. 7: 169–175
Gotthard K., Nylin S.1995. Adaptive plasticity and plasticity as an adaptation-a selective review of plasticity in animal morphology and life-history. Oikos, 74: 3–17
Guderley H., St Pierre J.2002. Going with the flow or life in the fast lane: contrasting mitochondrial responses to thermal change. J Exp Biol, 205: 2237–2249
Higham T. E., Korchari P. G., McBrayer L. D.2011. How muscles define maximum running performance in lizards: an analysis using swing and stance phase muscles. J Exp Biol, 214: 1685–1691
Huey R. B., Bennett A. F., John-Alder H., Nagy K. A.1984. Locomotor capacity and foraging behaviour of Kalahari lacertid lizards. Anim Behav, 32: 41–50
Huey R. B., Peterson c. R., Arnold S. J., Porter W. P.1989. Hot rocks and not-so-hot rocks: retreat-site selection by garter snakes and its thermal consequences. Ecology, 70: 931–944
Husak J. F., Fox S. F., Lovern M. B., Van Den Bussche R. A.2006. Faster lizards sire more offspring: sexual selection on whole animal performance. Evolution, 60: 2122–2130
Ji X., Du W. G., Sun P. Y.1996. Body temperature, thermal tolerance and influence of temperature on sprint speed and food assimilation in adult grass lizards, Takydromus septentrionalis. J Therm Biol, 21: 155–161.
Ji X., Zheng X. Z., Xu Y. G., Sun R. M.1995. Some aspects of thermal biology of the skink Eumeces chinensis. Acta Zool Sin, 41: 268–274 (In chinese)
Johnston I. A., Sidell B. D., Driedzic W. R.1985. Force-velocity characteristics and metabolism of carp muscle fibers following temperature acclimation. J Exp Biol, 119: 239–249
Johnston I. A., Temple G. K.2002. Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotory behaviour. J Exp Biol, 205: 2305–2322
Le Galliard J., clobert J., Ferriere R.2004. Physical performance and darwinian fitness in lizards. Nature, 432: 502–505
Leroi A. M., Bennett A. F., Lenski R. E.1994. Temperature acclimation and competitive fitness: an experimental test of the beneficial acclimation assumption. Proc Natl Acad Sci USA, 91: 1917–1921
Lima S. L., Bednekoff P. A.1999. Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am Nat, 153: 649–659
Madsen T., Shine R.2000. Rain, fish and snakes: climatically driven population dynamics of Arafura filesnakes in tropical Australia. Oecologia, 124: 208–215
Navas c. A., James R. S., Wakeling J. M., Kemp K. M., Johnston I. A.1999. An integrative study of the temperature dependence of whole animal and muscle performance during jumping and swimming in the frog Rana temporaria. J comp Physiol B, 169: 588–596
Rall J. A., Woledge R. c.1990. Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol Regul Integr comp Physiol 259: R197–203
Rice A. N., Westneat M. W.2005. coordination of feeding, locomotor and visual systems in parrottishes (Teleostei: Labridae). J Exp Biol, 208: 3503–3518
Ritter D. A.1995. Epaxial muscle function during locomotion in a lizard (Varanus salvator) and the proposal of a key innovation in the vertebrate axial musculoskeletal system. J Exp Biol, 198: 2477–2490
Ritter D. A.1996. Axial muscle function during lizard locomotion. J Exp Biol, 199: 2499–2510
Root T. L., Price J. T., Hall K. R., Schneider S. H., Rosenzweig c., Pounds J. A.2003. Fingerprints of global warming on wild animals and plants. Nature, 421: 57–60
Row J. R., Blouin–Demers G.2006.Thermal quality influences effectiveness of thermoregulation, habitat use, and behaviour in milk snakes. Oecologia, 148: 1–11
Scheiner S. M.1993. Genetics and evolution of phenotypic plasticity. Ann Rev Ecol Syst, 24: 35–68
Schmidt-Nielsen K.1990. Animal physiology: adaptation and environment (4thed). cambridge, UK: cambridge University Press
Seebacher F., James R. S.2008. Plasticity of muscle function in a thermoregulating ectotherm (crocodylus porosus): biomechanics and metabolism. Am J Physiol Regul Integr comp Physiol, 294: R1024–R1032
Shu L., Sun B. J., Du W. G.2010. Effects of temperature and food availability on selected body temperature and locomotor performance of Plestiodon (Eumeces) chinensis. Anim Biol, 60: 337–347
Somero G. N.2005. Linking biogeography to physiology: Evolutionary and acclimatory adjustments of thermal limits. Front Zool, Doi: 10.1186/1742-9994-2-1
Stevenson R. D.1985. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am Nat, 126: 362–386
Strobbe F., McPeek M. A., De Block M., De Meester L., Stoks R.2009. Survival selection on escape performance and its underlying phenotypic traits: a case of many-to-one mapping. J Evol Biol, 22: 1172–1182
Sun B. J., Du W. G., Shu L., chen Y., Wang Y.2011. The influence of thermal environment and food availability on testosterone and gonadal recrudescence in male chinese skinks [Plestiodon (Eumeces) chinensis]. Gen comp Endocr, 170:
449–454
Weinig c.2000. Plasticity versus canalization: population differences in the timing of shade-avoidance responses. Evolution, 54: 441–451
Wilson R. S., Franklin c. E.2002. Testing the beneficial acclimation hypothesis. Trends Ecol Evol, 17: 66–70
Wilson R. S., James R. S., Johnston I. A.2000. Thermal acclimation of locomotor performance in tadpoles and adults of the aquatic frog Xenopus laevis. J comp Physiol B, 170: 117– 124
Yom-Tov Y., Geffen E. 2006.Geographic variation in body size: the effects of ambient temperature and precipitation. Oecologia, 148: 213–218
Zhao E. M., Adler M.1993. Herpetology of china. Oxford, Ohio: Society for the Study of Amphibians and Reptiles
Zhao E. M., Zhao K. T., Zhou K. Y. 1999.Fauna Sinica, Reptilia Vol. 2. Beijing: Science Press
*corresponding author: Prof. Weiguo DU, from the Institute of Zoology, chinese Academy of Sciences, Beijing, china, with his research focusing on ecological adaptation of reptile.
Email: duweiguo@ioz.ac.cn
Received: 21 May 2014 Accepted: 20 August 2014
杂志排行
Asian Herpetological Research的其它文章
- First Record of the Gekkonid Genus cnemaspis Strauch, 1887 from Gunung Mulu National Park, Northern Sarawak, East Malaysia may Represent an Undescribed Species
- Microbiological and Histological Examinations of Endangered Neurergus kaiseri Tissues Displaying Red-leg Syndrome
- Phylogenetic Patterns of the Southeast Asian Tree Frog chiromantis hansenae in Thailand
- New Karyological and Morphometric Data on Poorly Known Bufo surdus and Bufo luristanicus in comparison with Data of Diploid Green Toads of the Bufo viridis complex from South of Iran
- Distribution and Environmental Suitability of the Smallscaled Rock Agama, Paralaudakia microlepis (Sauria: Agamidae) in the Iranian Plateau
- Description of a New Species of the Genus Brachytarsophrys Tian and Hu, 1983 (Amphibia: Anura: Megophryidae) from Southern china Based on Molecular and Morphological Data
