Local inhibition of GABA affects precedence effect in the inferior colliculus
2014-03-24YanjunWangNingyuWangDanWangJunJiaJinfengLiuYanXieXiaohuiWenXiaotingLi
Yanjun Wang, Ningyu Wang, Dan Wang, Jun Jia, Jinfeng Liu, Yan Xie, Xiaohui Wen, Xiaoting Li
Local inhibition of GABA affects precedence effect in the inferior colliculus
Yanjun Wang1, Ningyu Wang1, Dan Wang1, Jun Jia2, Jinfeng Liu1, Yan Xie2, Xiaohui Wen1, Xiaoting Li1
1 Department of Otorhinolaryngology Head and Neck Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
2 Department of Neurophysiology, Capital Medical University, Beijing, China
The precedence effect is a prerequisite for faithful sound localization in a complex auditory environment, and is a physiological phenomenon in which the auditory system selectively suppresses the directional information from echoes. Here we investigated how neurons in the inferior colliculus respond to the paired sounds that produce precedence-effect illusions, and whether their fi ring behavior can be modulated through inhibition with gamma-aminobutyric acid (GABA). We recorded extracellularly from 36 neurons in rat inferior colliculus under three conditions: no injection, injection with saline, and injection with gamma-aminobutyric acid. The paired sounds that produced precedence effects were two identical 4-ms noise bursts, which were delivered contralaterally or ipsilaterally to the recording site. The normalized neural responses were measured as a function of different inter-stimulus delays and half-maximal interstimulus delays were acquired. Neuronal responses to the lagging sounds were weak when the inter-stimulus delay was short, but increased gradually as the delay was lengthened. Saline injection produced no changes in neural responses, but after local gamma-aminobutyric acid application, responses to the lagging stimulus were suppressed. Application of gamma-aminobutyric acid affected the normalized response to lagging sounds, independently of whether they or the paired sounds were contralateral or ipsilateral to the recording site. These observations suggest that local inhibition by gamma-aminobutyric acid in the rat inferior colliculus shapes the neural responses to lagging sounds, and modulates the precedence effect.
nerve regeneration; precedence effect; auditory center; inferior colliculus; gamma-aminobutyric acid; local inhibition; echo suppression; lagging stimulus; NSFC grant; neural regeneration
Funding: This work was supported by the National Natural Science Foundation of China, No. 81271090 and the Beijing Natural Science Foundation, No. 7112055.
Wang YJ, Wang NY, Wang D, Jia J, Liu JF, Xie Y, Wen XH, Li XT. Local inhibition of GABA affects precedence effect in the inferior colliculus. Neural Regen Res. 2014;9(4):420-429.
Introduction
The development and application of artificial cochlea and other assisted listening devices help patients with hearing impairments become a part of the hearing world. However, patients with implanted artificial cochlea often complain that they cannot recognize speech well in natural environments, especially if background of noise is present[1-2]. A similar problem also exists in patients who receive other hearing aids. Researchers think that a poor ability to localize sound in a complex auditory environment is responsible for the weak speech perception observed under these conditions[1,3]. Studies show that sound localization primarily depends on interaural time and intensity differences of sound signals[4-6]. Therefore, in the clinic, hearing aids are used in both ears. Although double hearing aids improve speech perception compared with only a single hearing aid, this is true only in quiet environments, and perception is still quite poor in noisy environments[1-2,4,7-8]. In light of this, sounds in a complex auditory environment are likely localized by means of mechanisms other than the binaural sound-signal difference.
In natural environments, the original sound source produces both a direct wavefront (the leading sound) and many fi ltered and time-delayed re fl ections (the lagging sounds, the re fl ections, or the echoes) generated when the direct wavefront contacts nearby surfaces[9-11]. Because of these lagging sounds, faithful localization of an initial sound source is a particular challenge to the auditory system. Competition between the initial sound and its echoes is thought to be resolved by the auditory precedence effect[11-14], which manifests as improved sound localization in environments with complex sounds. When the onsets of the leading and laggings sounds are suf fi ciently (short inter-stimulus delays/at the echo threshold) the leading stimulus will dominate perception by suppressing perception of the lagging sounds[15-16]. The precedence effect plays a vital role in effectively eliminating echo interference and allowing accurately localization of sound sources in everyday listening environments that would otherwise contain numerous distracting echoes from nearby surfaces[17-18].
Psychoacoustical and behavioral studies of the precedence effect have been well described. Three phenomena (stages) occur in the precedence effect depending on the length ofthe interstimulus delays. (1) The fusion stage: when inter-stimulus delays are shorter, a fused sound is heard[19-20]. The fused sound is located at a position between the leading and the lagging sounds, but closer to the leading one. (2) The localization dominance stage: when inter-stimulus delays are short, only the leading sound is heard, and perception of the lagging sound is suppressed. Sound localization in such situations is dominated by the leading sound location, which is called localization dominance. (3) The discrimination stage: when interstimulus delays are long enough (exceeding the echo threshold), the leading and lagging sounds are perceived as independent sounds, each with its own spatial location[9,21-22]. Localization dominance is a striking perceptual effect that is important for eliminating unnecessary sound images that would otherwise result from the source and its reflections. The ability to unconsciously filter out numerous re fl ective sounds is thus possible if the auditory system groups a sound source and its re fl ections into a single perception through localization dominance. However, this is not to say that the lagging sound is completely ignored, but that the contribution of the leading sound to localization of the fused image is much stronger than that of the lagging one. An additional element that contributes to the precedence effect is the selective suppression of echoes by the auditory system (echo suppression) without eliminating their overall perception[9,23-25].
However, to date the underlying neural mechanisms responsible for mediating echo suppression are not well known. The inferior colliculus is a strategic relay station in the ascending and descending auditory system pathways. The inputs to the inferior colliculus arise from lower nuclei that receive either monaural or binaural sound signals[26-27]. Axonal projections from lower nuclei are purely excitatory, purely inhibitory, or both excitatory and inhibitory[28-29]. For example, inner-vations from the dorsal cochlear nucleus and the medial superior olive are purely excitatory, those from the dorsal nucleus of the lateral lemniscus are purely inhibitory, and those from the lateral superior olive are mixed[30-33]. These multiple convergences result in inferior colliculus cells that have more sharpened and modi fi ed response properties than those found in upstream nuclei.
Because of its strategic position, the inferior colliculus has been widely examined for physiological correlates of the precedence effect. Scholars[23,34-35]proposed several possible sources for the inhibition such as inhibition of the medial superior olive by the medial and lateral nuclei of the trapezoid body, inhibition of the inferior colliculus by the dorsal nucleus of the lateral lemniscus, and intrinsic circuits within the inferior colliculus itself. Litovsky and colleagues[22]suggested that suppression of lagging sounds is controlled by the neural substrates underlying the precedence effect, and that the inferior colliculus may be one such neural substrate, a concept that is consistent with studies of people with lesions to the inferior colliculus.
Gamma-aminobutyric acid (GABA) is a critical inhibitory neurotransmitter. Merchán et al.[36]found many gamma-aminobutyric acid-ergic neurons in the rat inferior colliculus and that gamma-aminobutyric acid-mediated inhibition spread across dendrites and cell bodies of inferior colliculus neurons. Gamma-aminobutyric acid-ergic inputs to the inferior colliculus primarily arise from the dorsal nucleus of lateral lemniscus and the inferior colliculus itself. Gamma-aminobutyric acid inhibition has been proposed to play a major role in modulating sound signals, such as shaping the tuning curves of inferior colliculus neurons, altering response selectivity to complex signals, frequency modulation of directional selectivity, and controlling temporal properties of inferior colliculus cells[31-33,37-39]. Song et al.[40]found that pentobarbital could prolong suppression of the lagging responses in the precedence effect, which presumed that it decreased the rate at which gamma-aminobutyric acid dissociated from its receptor and thus acted as a gamma-aminobutyric acid agonist.
The goal of this study was to investigate how local gamma-aminobutyric acid-ergic inhibition of rat inferior colliculus affects the auditory precedence effect. We directly compared neural responses to lagging sounds before and after microinjections of gamma-aminobutyric acid into the inferior colliculus.
Results
Fifty inferior colliculus neurons were isolated in 22 rats using a search stimulus, of which 36 (72%) exhibited suppression to the lagging sound stimulus at different inter-stimulus delays. First saline, and then gamma-aminobutyric acid were applied iontophoretically to all 36 neurons.
Effect of local gamma-aminobutyric acid application on the precedence effect using a contralaterally leading stimulus
Neuronal responses to the lagging sound were weak when using a short interstimulus delay (e.g., 2 ms), but gradually increased as the interstimulus delay was lengthened. For the 10 ms delay, two separate peaks in fi ring rate were observed, one in response to the leading stimuli and one to the lagging one. The amplitude of the peak induced by the lagging stimulus was lower than that of the leading one. Similar results were also seen using 6 ms and 2 ms inter-stimulus delays. These properties remained unchanged after local microinjections of saline into the inferior colliculus (Figure 1B). However, after local gamma-aminobutyric acid application to the inferior colliculus, responses to the lagging stimuli were obviously weaker. Indeed, with a 2 ms inter-stimulus delay, responses to the lagging stimulus were completely missing (Figure 1C), while those to 6 ms and 10 ms were present, but weaker (Figure 1).
The normalized lagging responses under different interstimulus delays, location of lagging stimulus, and injected drug can be seen in Figure 2A and B. After application of gamma-aminobutyric acid, the normalized response to contralaterally lagging stimuli was lower than that before injection or after saline injection for all delay durations when the leading and lagging sources were both contralateral to the recording site in the rat inferior colliculus (Figure 2A; Fbetween-subject= 339.78, P < 0.01). Similar results were also seen with contralaterally leading stimuli paired with ipsilaterallylagging ones (Figure 2B; Fbetween-subject= 186.63, P < 0.01). No significant changes in response properties were observed after microinjection with saline in either of these conditions (P > 0.05; Figure 2A and B).

Figure 1 Post-stimulus time histograms of 50 responses of a single inferior colliculus neuron to the paired sounds when the leading and lagging sounds were both contralateral.
Effect of local application of gamma-aminobutyric acid on the precedence effect using an ipsilateral leading stimulusFigure 3 shows post-stimulus time histograms from a single neuron using different inter-stimulus delays under the three conditions when the leading stimulus was presented ipsilaterally to the recording location. As with contralateral leading stimuli, gamma-aminobutyric acid administration suppressed the response, while saline injection did not. As when the leading stimuli were contralateral, the effect
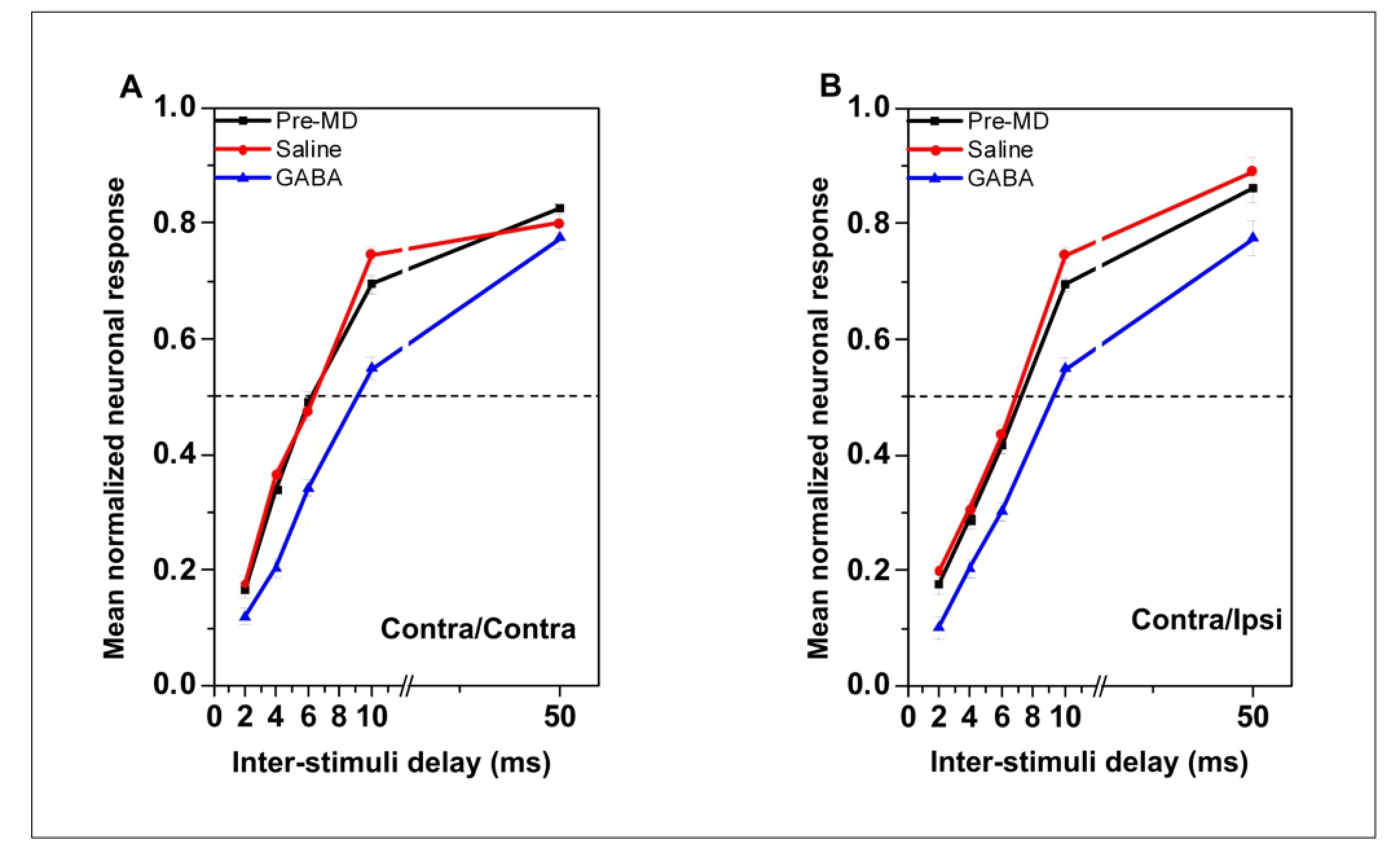
Figure 2 Normalized responses to the lagging sound under the three recording conditions when the leading sound was contralateral to the recording site.
of gamma-aminobutyric acid on responses to the lagging stimuli was the same regardless of whether they were presented contralaterally or ipsilaterally to the recording site (Figure 4A and B; Fbetween-subject= 219.57, P < 0.01; Fbetween-subject= 256.79, P < 0.01). Note that while the curve showing the normalized response moved to the right after microinjecting gamma-aminobutyric acid, a similar effect was not observed after microinjecting saline (P > 0.05; Figure 4A and B).
Effect of local gamma-aminobutyric acid inhibition on the half-maximal inter-stimulus delay of the lagging stimulusThe half-maximal inter-stimulus delay is de fi ned as the inter-stimulus delay that results in a 50% responserate to the lagging sound stimulus[9-10]. Figure 5 shows the half-maximal inter-stimulus delay of the recorded neurons before and after drug application under the four paired-source con fi gurations. After gamma-aminobutyric acid microinjection, the half-maximal inter-stimulus delays increased under all conditions (Fc/c= 215.15; Fc/I= 97.13; Fi/c= 152.66; Fi/I= 150.86, P < 0.01; Figure 5), while there was no change after saline injection (P > 0.05; Figure 5).
Discussion
This study examined the effect of local gamma-aminobutyric acid inhibition on neural responses to lagging auditory stimuli in the inferior colliculus of rats. Before injection, responses to the lagging sound stimulus mirrored general changes in perception described by the precedence effect. After gamma-aminobutyric acid microinjection into the inferior colliculus, responses to the lagging stimuli were suppressed, supporting the idea that inhibitory input into the inferior colliculus mediates/underlies the precedence effect.
Gamma-aminobutyric acid inhibition and auditory perception
Gamma-aminobutyric acid is the main inhibitory neurotransmitter in the mammalian central nervous system. It acts by binding to specific receptors to be more negative (hyperpolarization) than normal, and thus making it more difficult for incoming action potentials to depolarize the cell. Three classes of gamma-aminobutyric acid receptor are known in mammals: gamma-aminobutyric acid A, gamma-aminobutyric acid B, and gamma-aminobutyric acid C[41]. The gamma-aminobutyric acid A receptor is composed of fi ve glycoprotein subunits located in the plasma membrane. When gamma-aminobutyric acid A receptors are activated, chloride conductance across the cell membrane increases, which results in hyperpolarization[42].
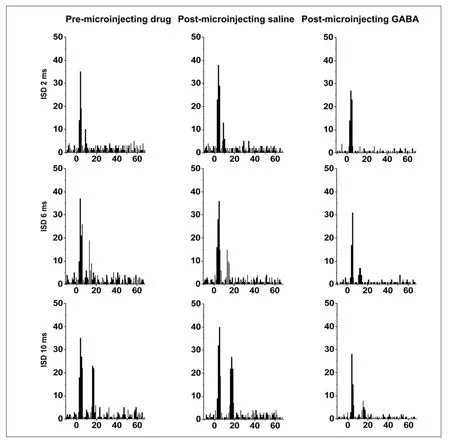
Figure 3 Post-stimulus time histograms of the responses of a single inferior colliculus neuron to the paired sounds when the leading and lagging sounds were both ipsilateral.
Sound signals sent to the inferior colliculus arise from monaural or binaural upstream nuclei. Binaural encoding plays a leading role in environments with echoes[43-44]. The proportion of excitatory and inhibitory signals transmitted to the inferior colliculus affects binaural sound processing and further determines sound perception[28,31-32]. Projections to the inferior colliculus that originate from the lateral superior olivary nucleus and the dorsal nucleus of the lateral lemniscus are purely inhibitory or both excitatory and inhibitory[45]. Of these, the terminals originating from the dorsal nucleus of the lateral lemniscus and the superior paraolivary nucleus are mostly gamma-aminobutyric acid ergic. Several previous histomorphological studies have demonstrated gamma-aminobutyric acid-ergic cells in the inferior colliculus and gamma-aminobutyric acid-ergic projections to inferior colliculus abound in different species[36,41]. Increases or decreases in gamma-aminobutyric acid levels thus alter the response properties of neurons in the inferior colliculus. For example, gamma-aminobutyric acid inhibition shapes the tuning curves, frequency-modulation directions, and response selectivity to complex signals[26,33,37-38,46].
Auditory precedence effect in the inferior colliculus and the possible neuronal mechanism
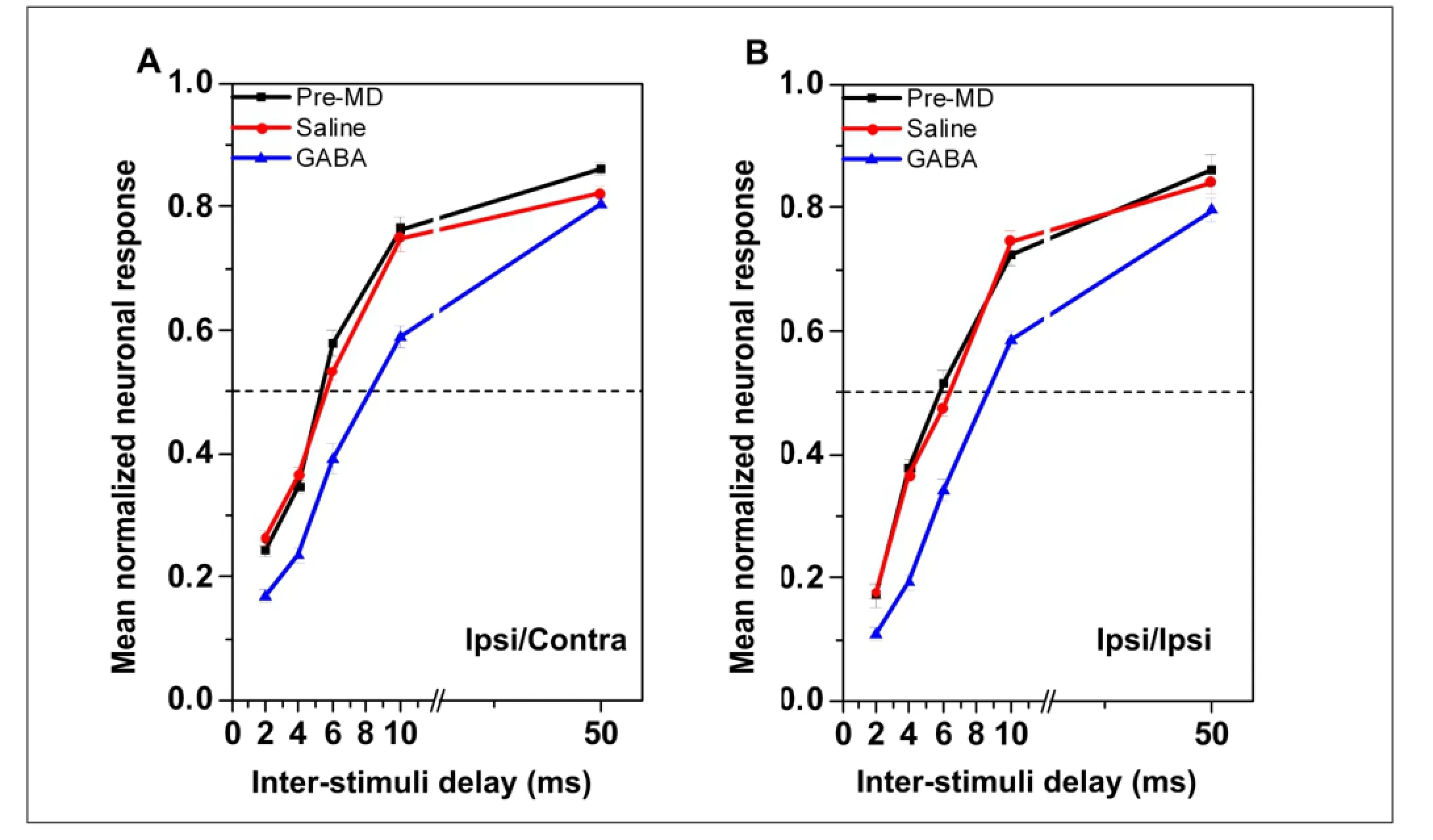
Figure 4 Normalized responses to the lagging sound under the three recording conditions when the leading sound was ipsilateral to the recording site.
The precedence effect is a physiological phenomenon in which the auditory system selectively suppresses the directional information of lagging sounds to make localizing the initial source accurate[22-23,47-48]. The existence of precedence effect is therefore a prerequisite for the faithful sound localization in natural reverberant environments[12,16,49-50].
Behavioral studies show the precedence effect in human and other mammals such as rabbits, rats, and cats[9,24,51-52]. Kelly[53]found that rats could be trained localize single clicks and paired clicks. Their best responses to the paired clicks occurred when the clicks were temporally separated by 4 ms. Blauert thought that during the precedence window, the presence of the lagging sounds could still be detected even though they were not heard as separate sounds with distinct locations[54]. Supporting this claim, studies have shown that listeners are as sensitive to intensity decreases in the lagging sound as to intensity increases in the leading sound, indicating that intensity information is not sup-pressed from the lagging sounds[47-48]. Thus, the precedence effect results from suppression of lagging-sound directional information, but not other factors.
The inferior colliculus has been widely examined with regard to the precedence effect because of its major converging inputs from upstream brain stem nuclei[20,55]. Moreover, many inferior colliculus neurons are sensitive to sound location, evidenced by directional selectivity[56-57]. Inhibitory inputs to the inferior colliculus from upstream brain stem nuclei may contribute to the precedence effect observed in inferior colliculus. The inhibitory projections to the inferior colliculus mainly arise from the lateral superior olive and the dorsal nucleus of the lateral lemniscus. Of these, the terminals from the dorsal nucleus of the lateral lemniscus are mostly gamma-aminobutyric acid-ergic[28,35].
The echo threshold is defined as the minimum interstimulus delay that results in perception and localization of both leading and lagging sources[9,22,58]. The half-maximal inter-stimulus delay is the delay time at which neurons respond to the lagging stimulus 50% of the time, and is often used to estimate neural echo thresholds (that are not concerned with perception). At the level of the inferior colliculus, localization dominance is correlated with the degree to which the response to the lagging source decreases and the response to the leading source dominates[22-24,34-35]. Therefore, the half-maximal inter-stimulus delays exhibited by neurons in the inferior colliculus are used to approximate echo thresholds, and are thought to be the neural correlates of the precedence effect. Here, we observed changes in half-maximal interstimulus delays after local gamma-aminobutyric acid application.
Gamma-aminobutyric acid inhibition shapes the recovery curve of the lagging sound
In the present study, normalized responses to lagging stimuli decreased signi fi cantly, and half-maximal interstimulus delays increased signi fi cantly after gamma-aminobutyric acid microinjection into inferior colliculus, demonstrating thatlocal gamma-aminobutyric acid inhibition can prolong the recovery time for responses to lagging stimuli, thus leading to the precedence effect.
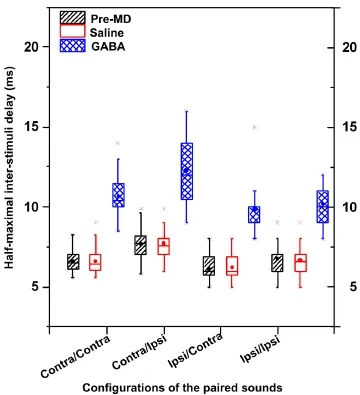
Figure 5 Box graphs of the half-maximal inter-stimulus delays for the inferior colliculus neurons before and after drug application under four stimulus con fi gurations.
Scholars[24,34]studied the prece-dence effect in the inferior colliculus of awake rabbits and anesthetized cats respectively. They postulated that pentobarbital anesthesia was responsible for the prolonged recovery times associated with lagging stimuli found in the inferior colliculus. Furthermore, Song et al.[40]observed the alteration of neuronal responses to the lagging sound after intraperitoneal administration of pentobarbital sodium. They suggested that pentobarbital anesthesia prolongs the recovery time of responses to lagging stimulus. The effects of pentobarbital anesthesia on the precedence effect stem from decreased dissociation of gamma-aminobutyric acid from its receptor (i.e., it is a gamma-aminobutyric acid agonist). Our fi ndings are consistent with the results of these previous studies.
Gamma-aminobutyric acid-ergic inputs to the inferior colliculus play a major role in mediating lagging suppression recorded from the inferior colliculus. Lesioning the dorsal nucleus of the lateral lemniscus or afferent inputs decreased the responses to lagging stimuli and prolonged the recovery of responses to lagging sounds in the contralateral inferior colliculus and had a small effect in the ipsilateral inferior colliculus[34]. Thus, projections from the contralateral dorsal nucleus of the lateral lemniscus likely play a major role in the precedence effect-like responses of the inferior colliculus. The neurons of both the dorsal nucleus of the lateral lemniscus and the inferior colliculus are sensitive to binaural cues used in sound location. Most neurons in the inferior colliculus show excitatory responses to contralateral stimuli[26,31-32]. These fi ndings are consistent with those indicating that the degree to which responses to lagging sounds are reduced depends on the binaural properties of the leading source[34,47].
In conclusion, when simulating the precedence effect by presenting leading and lagging sounds, local application of gamma-aminobutyric acid into the recording site in the rat inferior colliculus decreased neural responses to the lagging stimuli, raised the half-maximal inter-stimulus delay of the inferior colliculus neurons, and prolonged the recovery period for responses to the lagging stimuli. Thus, we consider that gamma-aminobutyric acid inhibition is an important mechanism underlying the precedence effect activity in the inferior colliculus. This fi nding could help to understand the neural correlates of the precedence effect and the mechanisms of sound localization in a complex auditory environment with the reverberation.
Materials and Methods
Design
An animal experiment using neurophysiology to study psychoacoustics.
Time and setting
This study was performed at the Neurophysiology Laboratory, Department of Neurophysiology, Capital Medical University, China from March 2011 to April 2012.
Materials
Animals
Twenty-two speci fi c-pathogen free adult male Sprague-Dawley rats aged 8 weeks and weighing 200–250 g were provided by the Laboratory Animal Services Center of Capital Medical University in China, license No. SCXK2011-0011. The animals were housed at room temperature with a natural light cycle and quiet conditions and given free access to water and a regular rat diet. All procedures were approved by the Animal Research Committee of Capital Medical University for the care and use of animals, and complied with the National Institutes of Health guidelines for animal use.
Instruments
Experiments took place in a sound-attenuating chamber (1.2 m × 1.0 m × 0.9 m), which was dimly illuminated, electrically shielded, and put on a vibration isolation table. The four walls of the chamber were lined with sound-absorbing foam, as was the equipment. Rats were placed in the center of the chamber.
Methods
Inferior colliculus location
Rats were weighted, anesthetized with sodium pentobarbital (50 mg/kg, i.p.), and fi xed in a small-animal stereotaxic holder (RWD Life Science Co., Ltd., Shenzhen, Guangdong Province, China). The incisor bar was adjusted until the heights of lambda and bregma were equal (flat skull). Zygomatic archbars (Patent application No. 201020600962.8) were employed to replace the ear bars for fastening the head[40]. The following coordinates were used for locating the inferior colliculus: antero-posterior = −8.0 to −9.0 mm (according to the bregma), medio-lateral = 1.0 to 2.8 mm (according to the midsagittal suture) and dorso-ventral = 3.5 mm (according to the surface of the brain)[59]. A small hole was drilled in the skull and the dura was removed to expose the surface of the cortex[60]. Body temperature (37–39°C) was maintained using a thermostatically controlled heating blanket. Liquid paraf fi n was dropped onto the skull opening to prevent dehydration of the brain before recording.
Sound stimuli
The sound stimuli included a single white noise stimulus of 40 ms (as a search stimulus) and two successive and identical 4 ms white noise bursts with equal levels and coherent in phase (simulating echoes in a reverberant environment). These stimuli were digitally generated at a sampling rate of 192 kHz by Adobe Audition 3.0 (Adobe Systems Inc., San Jose, CA, USA). Two electrostatic speakers (EC-1 electrostatic speakers; Tucker-Davis Tech., Alachua, FL, USA) were located along a 25 cm radius measured from the center of the rats’ heads and were held constant at ± 45° on the horizontal and median sagittal planes in the chamber. The single 40 ms stimulus was presented from the two loudspeakers simultane-ously and each of the paired stimuli from one of the two loudspeakers. The successive stimuli were presented with different inter-stimulus delays. The leading stimulus was the fi rst 4 ms burst, and the lagging stimulus was the second one[24]. The outputs of the speakers were quan-tified with a sound calibrator (Type 4231, Brüel & Kjær Sound & Vibration Measurement A/S, Copenhagen, Denmark) and expressed in decibels of sound pressure level referred to 20 µPa root mean square.
Local gamma-aminobutyric acid application to the recording site in the inferior colliculus
Iontophoretic application of gamma-aminobutyric acid to the inferior colliculus neurons was given with a five-barrel glass capillary (World Precision Instrument, Sarasota, FL, USA) pulled to a tip diameter of 6–11 µm by a vertical microelectrode puller (Narishige Scienti fi c Instrument Lab, Tokyo, Japan)[61]. The central barrel of the multibarrel electrode (impedance 4–10 MΩ) was used to record neural responses. The other side-barrels (impedance 20–60 MΩ) were used for microinjecting drug, controlling, balancing, and grounding[62].
Gamma-aminobutyric acid (Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 0.2 mol/L was dissolved in 0.16 mol/L sodium chloride with the pH value adjusted to 3.5–4.0. The gamma-aminobutyric acid solution was prepared freshly just prior to each experiment[37]. One side barrel of the multi-barrel electrode was filled with gamma-aminobutyric acid solution before use and another with 2 mol/L sodium chloride (pH 7.0) as a control. The remaining two side-barrels were each fi lled with 1 mol/L sodium chloride (pH 7.0); one was used as ground and the other as a balance.
The barrels filled with drugs were connected via a silver-silver chloride electrode to a microiontophoresis constant current generator (Neurophore BH-2 Ontophoretic System, Harvard Medical Systems Co., Holliston, MA, USA). Gamma-aminobutyric acid was ejected at positive currents varying from 50 to 100 nA for 3 to 6 minutes, and a retaining current was applied with positive currents ranging from 5 to 10 nA. The ejecting and retaining currents for the control sodium chloride solution were the same as for gamma-aminobutyric acid. The balance electrode was connected to the balance module of the constant current generator. During electrophoresis, equal and opposite polarity currents were given to the balance barrel to equalize the influence of the currents. The ground barrel was connected to the ground wire of the constant current generator.
Extracellular recording in the rat inferior colliculus
Extracellular recording was used to record the firing of neurons in the rat inferior colliculus. The central barrel of the fi ve-barrel electrode (impedance 4–10 MΩ) was fi lled with 1% pontamine sky blue dissolved in 3 mol/L sodium chloride (pH 7.0). Using a microelectrode holder, the electrode was vertically penetrated into the inferior colliculus from the surface of the brain. Electrodes were advanced using a precision digital micromanipulator (PF5–1, Narishige Scienti fi c Instrument Lab, Tokyo, Japan). Spikes were amplified (2,400 A, Dagan Co., Minneapolis, MN, USA), fi ltered (0.3–10 kHz), and then converted to digital signals through an analog-to-digital converter (PowerLab 4/30, AD Instruments Pty Ltd., Sydney, Australia).
After inserting the electrode into the inferior colliculus and starting recording, a 2% agar solution was applied to cover the surface of the brain above inferior colliculus. At the end of each experiment, the recording site was stained via iontophoretic ejection of pontamine sky blue from the tip of recording electrode (taking negative pole, 0.1 nA, 10 minutes; and then taking positive pole, 0.1 nA, 6 minutes). The animal was given an overdose of sodium pentobarbital and then perfused with 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4). The brain was removed and further fi xed in 4% paraformaldehyde for about 24 hours at 4°C, followed by cryoprotection (30% sucrose in 0.1 mol/L phosphate buffer for 1–3 days at 4°C). Coronal sections were cut with a freezing microtome, mounted on slides, and stained with Nissl to identify the recording site.
Data acquisition and analysis
The digital signals from the analog-to-digital converter were stored in a computer using LabChart 7.3 software (AD Instruments Pty Ltd.) at a 20 kHz sampling rate. The sound stimuli were also recorded by the PowerLab converter as triggers for neuron spiking. Data analysis was performed online and off-line.
The 40 ms white noise was presented from the two loudspeakers simultaneously as the electrode was advanced through the inferior colliculus to search for auditory neurons. After isolating a neuron, its minimum activation threshold was determined. Then, the neural responses to a single 4 ms noise burst in isolation (contralateral or ipsilateral) were collected for normalization and comparisonpurposes. Injections were then given (gamma-aminobutyric acid or saline), and recordings were made as the successive bursts were delivered at varying inter-stimulus delays (2, 4, 6, 10, and 50 ms) and four locational con fi gurations (contralateral or ipsilateral leading and contralateral or ipsilateral lagging). The noise bursts were presented at a constant intensity 30 dB above threshold either to the single or to the paired sounds. Successive bursts were repeated 50 times with 500 ms intervals separating each pair or bursts.
The neural responses to lagging stimulus were normalized by the responses to a single stimulus. A normalized response of 1.0 indicates that the leading stimulus did not affect responses to lagging stimulus, whereas values < 1.0 indicate that responses to the lagging stimulus were suppressed[9,22]. For the 2 ms interstimulus delays, the stimuli overlapped and the responses to lagging stimulus were estimated by subtracting a neuron’s response to the single stimulus from that to the paired stimuli. The inter-stimulus delay at which responses to the lagging stimulus occurred 50% of the time (half-maximal inter-stimulus delay) was calculated[34].
Data were analyzed and graphed using Origin Pro 8 (Origin Lab Co., Northampton, MA, USA). One-way repeated-measures analysis of variance (ANOVA) was used to assess the effect of local gamma-aminobutyric acid application on normalized responses to lagging stimuli at different inter-stimulus delays. One-way ANOVA was also used to analyze the half-maximal interstimulus delays. Least signi fi cant difference test was applied for multiple comparisons. A value of P < 0.05 was considered statistically signi fi cant.
Acknowledgments:We are grateful to Dr. Sun ZL from Beijing Anding Hospital, Capital Medical University in China for the extracellular recordings, and Chen X from AD Instruments Limited Company in Australia for data analysis with Labchart software. Special thanks to Dr. Song PL from First Affiliated Hospital, Harbin Medical University in China for expert technical assistance and the insights into the mechanism of the precedence effect.
Author contributions:Due care has been taken to ensure the integrity of the work. The first author Wang YJ performed the experiment, analyzed the data, and wrote the manuscript. The corresponding author Wang NY was the director of the study, obtained the funding, and contributed to the experimental design. Jia J and Xie Y provided the instructions for the design and methods for the electrophysiological experiment. Wang D, Liu JF, Wen XH, and Li XT participated in data collection and data analysis. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Peer review:Extracellular recording was used to examine response properties in auditory neurons of the inferior colliculus. The results help to explain the difficulty in sound localization and speech perception that patients with hearing aids exhibit in complex sound environments.
[1] Vincent C, Bébéar JP, Radafy E, et al. Bilateral cochlear implantation in children: localization and hearing in noise bene fi ts. Int J Pediatr Otorhinolaryngol. 2012;76(6):858-864.
[2] Kerber S, Seeber BU. Sound localization in noise by normal-hearing listeners and cochlear implant users. Ear Hear. 2012;33(4):445-457.
[3] Yasin I, Henning GB. The effects of noise-bandwidth, noise-fringe duration, and temporal signal location on the binaural masking-level difference. J Acoust Soc Am. 2012;132(1):327-338.
[4] Mueller MF, Kegel A, Schimmel SM, et al. Localization of virtual sound sources with bilateral hearing aids in realistic acoustical scenes. J Acoust Soc Am. 2012;131(6):4732-4742.
[5] Gourévitch B, Brette R. The impact of early re fl ections on binaural cues. J Acoust Soc Am. 2012;132(1):9-27.
[6] Schwartz A, McDermott JH, Shinn-Cunningham B. Spatial cues alone produce inaccurate sound segregation: the effect of interaural time differences. J Acoust Soc Am. 2012;132(1):357-368.
[7] Verhaert N, Lazard DS, Gnansia D, et al. Speech performance and sound localization abilities in Neurelec Digisonic® SP binaural cochlear implant users. Audiol Neurootol. 2012;17(4):256-266.
[8] Agterberg MJ, Snik AF, Hol MK, et al. Contribution of monaural and binaural cues to sound localization in listeners with acquired unilateral conductive hearing loss: improved directional hearing with a bone-conduction device. Hear Res. 2012;286(1-2):9-18.
[9] Litovsky RY, Colburn HS, Yost WA, et al. The precedence effect. J Acoust Soc Am. 1999;106(4 Pt 1):1633-1654.
[10] Tollin DJ, Yin TC. Psychophysical investigation of an auditory spatial illusion in cats: the precedence effect. J Neurophysiol. 2003;90(4):2149-2162.
[11] Goupell MJ, Yu G, Litovsky RY. The effect of an additional reflection in a precedence effect experiment. J Acoust Soc Am. 2012;131(4):2958-2967.
[12] Sayegh R, Aubie B, Fazel-Pour S, et al. Recovery cycle times of inferior colliculus neurons in the awake bat measured with spike counts and latencies. Front Neural Circuits. 2012;6:56.
[13] London S, Bishop CW, Miller LM. Spatial attention modulates the precedence effect. J Exp Psychol Hum Percept Perform. 2012;38(6):1371-1379.
[14] Seeber BU, Hafter ER. Failure of the precedence effect with a noise-band vocoder. J Acoust Soc Am. 2011;129(3):1509-1521.
[15] Akeroyd MA, Guy FH. The effect of hearing impairment on localization dominance for single-word stimuli. J Acoust Soc Am. 2011;130(1):312-323.
[16] Litovsky RY, Godar SP. Difference in precedence effect between children and adults signifies development of sound localization abilities in complex listening tasks. J Acoust Soc Am. 2010;128(4):1979-1991.
[17] Sanders LD, Joh AS, Keen RE, et al. One sound or two? Object-related negativity indexes echo perception. Percept Psychophys. 2008;70(8):1558-1570.
[18] Faller C, Merimaa J. Source localization in complex listening situations: selection of binaural cues based on interaural coherence. J Acoust Soc Am. 2004;116(5):3075-3089.
[19] Lee N, Elias DO, Mason AC. A precedence effect resolves phantom sound source illusions in the parasitoid fl y Ormia ochracea. Proc Natl Acad Sci U S A. 2009;106(15):6357-6362.
[20] Dent ML, Tollin DJ, Yin TC. In fl uence of sound source location on the behavior and physiology of the precedence effect in cats. J Neurophysiol. 2009;102(2):724-734.
[21] Chiang YC, Freyman RL. The in fl uence of broadband noise on the precedence effect. J Acoust Soc Am. 1998;104(5):3039-3047.
[22] Litovsky RY, Yin TC. Physiological studies of the precedence effect in the inferior colliculus of the cat. I. Correlates of psychophysics. J Neurophysiol. 1998;80(3):1285-1301.
[23] Yin TC. Physiological correlates of the precedence effect and summing localization in the inferior colliculus of the cat. J Neurosci. 1994;14(9):5170-5186.
[24] Fitzpatrick DC, Kuwada S, Batra R, et al. Neural responses to simple simulated echoes in the auditory brain stem of the unanesthetized rabbit. J Neurophysiol. 1995;74(6):2469-2486.
[25] Spierer L, Bourquin NM, Tardif E, et al. Right hemispheric dominance for echo suppression. Neuropsychologia. 2009;47(2):465-472.
[26] Li L, Yue Q. Auditory gating processes and binaural inhibition in the inferior colliculus. Hear Res. 2002;168(1-2):98-109.
[27] Blauert J. Modeling binaural processing: what next? J Acoust Soc Am. 2012;132(3):1911.
[28] Kelly JB, Li L. Two sources of inhibition affecting binaural evoked responses in the rat’s inferior colliculus: the dorsal nucleus of the lateral lemniscus and the superior olivary complex. Hear Res. 1997;104(1-2):112-126.
[29] Pérez-González D, Hernández O, Covey E, et al. GABA(A)-mediated inhibition modulates stimulus-specific adaptation in the inferior colliculus. PLoS One. 2012;7(3):e34297.
[30] Caspary DM, Havey DC, Faingold CL. Effects of microiontophoretically applied glycine and GABA on neuronal response patterns in the cochlear nuclei. Brain Res. 1979;172(1):179-185.
[31] Yang L, Pollak GD. Features of ipsilaterally evoked inhibition in the dorsal nucleus of the lateral lemniscus. Hear Res. 1998;122(1-2):125-141.
[32] Bauer EE, Klug A, Pollak GD. Features of contralaterally evoked inhibition in the inferior colliculus. Hear Res. 2000;141(1-2):80-96.
[33] Pollak GD, Xie R, Gittelman JX, et al. The dominance of inhibition in the inferior colliculus. Hear Res. 2011;274(1-2):27-39.
[34] Tollin DJ, Populin LC, Yin TC. Neural correlates of the precedence effect in the inferior colliculus of behaving cats. J Neurophysiol. 2004;92(6):3286-3297.
[35] Litovsky RY, Yin TC. Physiological studies of the precedence effect in the inferior colliculus of the cat. II. Neural mechanisms. J Neurophysiol. 1998;80(3):1302-1316.
[36] Merchán M, Aguilar LA, Lopez-Poveda EA, et al. The inferior colliculus of the rat: quantitative immunocyto-chemical study of GABA and glycine. Neuroscience. 2005;136(3):907-925.
[37] Yin S, Chen Z, Yu D, et al. Local inhibition shapes duration tuning in the inferior colliculus of guinea pigs. Hear Res. 2008;237(1-2):32-48.
[38] Jen PH, Feng RB. Bicuculline application affects discharge pattern and pulse-duration tuning characteristics of bat inferior collicular neurons. J Comp Physiol A. 1999;184(2):185-194.
[39] Wu F, Jen PH. Involvement of GABA-mediated inhibition in shaping the frequency selectivity of neurons in the inferior colliculus of the big brown bat, Eptesicus fuscus. Chin J Physiol. 2009;52(1):38-46.
[40] Song P, Wang N, Wang H, et al. Pentobarbital anesthesia alters neural responses in the precedence effect. Neurosci Lett. 2011; 498(1):72-77.
[41] Caspary DM, Ling L, Turner JG, et al. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211(Pt 11):1781-1791.
[42] Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20(1):485-494.
[43] Blauert J. Binaural localization. Scand Audiol Suppl. 1982;15:7-26.
[44] Slee SJ, Young ED. Information conveyed by inferior colliculus neurons about stimuli with aligned and misaligned sound localization cues. J Neurophysiol. 2011;106(2):974-985.
[45] Sun H, Wu SH. The physiological role of pre- and post-synaptic GABA(B) receptors in membrane excitability and synaptic transmission of neurons in the rat’s dorsal cortex of the inferior colliculus. Neuroscience. 2009;160(1):198-211.
[46] Pecka M, Zahn TP, Saunier-Rebori B, et al. Inhibiting the inhibition: a neuronal network for sound localization in reverberant environments. J Neurosci. 2007;27(7):1782-1790.
[47] Freyman RL, McCall DD, Clifton RK. Intensity discrimination for precedence effect stimuli. J Acoust Soc Am. 1998;103(4):2031-2041.
[48] McCall DD, Freyman RL, Clifton RK. Sudden changes in spectrum of an echo cause a breakdown of the precedence effect. Percept Psychophys. 1998;60(4):593-601.
[49] Bishop CW, London S, Miller LM. Visual in fl uences on echo suppression. Curr Biol. 2011;21(3):221-225.
[50] Wolf M, Schuchmann M, Wiegrebe L. Localization dominance and the effect of frequency in the Mongolian Gerbil, Meriones unguiculatus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196(7):463-470.
[51] Sanders LD, Zobel BH, Freyman RL, et al. Manipulations of listeners’ echo perception are re fl ected in event-related potentials. J Acoust Soc Am. 2011;129(1):301-309.
[52] Litovsky RY, Delgutte B. Neural correlates of the precedence effect in the inferior colliculus: effect of localization cues. J Neurophysiol. 2002;87(2):976-994.
[53] Kelly JB. Localization of paired sound sources in the rat: small time differences. J Acoust Soc Am. 1974;55(6):1277-1284.
[54] Blauert J. Localization and the law of the fi rst wavefront in the median plane. J Acoust Soc Am. 1971;50(2):466-470.
[55] Agaeva MIu. Echo thresholds of the precedence effect in the vertical plane. Zh Vyssh Nerv Deiat Im I P Pavlova. 2008;58(4):443-448.
[56] Ashida G, Carr CE. Sound localization: Jeffress and beyond. Curr Opin Neurobiol. 2011;21(5):745-751.
[57] Burger RM, Fukui I, Ohmori H, et al. Inhibition in the balance: binaurally coupled inhibitory feedback in sound localization circuitry. J Neurophysiol. 2011;106(1):4-14.
[58] Xia J, Shinn-Cunningham B. Isolating mechanisms that in fl uence measures of the precedence effect: theoretical predictions and behavioral tests. J Acoust Soc Am. 2011;130(2):866-882.
[59] Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic Press, 1997.
[60] Pinault D. A new stabilizing craniotomy-duratomy technique for single-cell anatomo-electrophysiological exploration of living intact brain networks. J Neurosci Methods. 2005;141(2):231-242.
[61] Havey DC, Caspary DM. A simple technique for constructing‘piggy-back’ multibarrel microelectrodes. Electroencephalogr Clin Neurophysiol. 1980;48(2):249-251.
[62] Satake S, Yamada K, Melo LL, et al. Effects of microinjections of apomorphine and haloperidol into the inferior colliculus on prepulse inhibition of the acoustic startle re fl ex in rat. Neurosci Lett. 2012;509(1):60-63.
Copyedited by Phillips A, Li KY, Wang X, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.128250
Ningyu Wang, M.D., Department of Otorhinolaryngology Head and Neck Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020; College of Otolaryngology, Capital Medical University, Beijing 100069, China; Key Laboratory of Otolaryngology Head and Neck Surgery, Capital Medical University, Ministry of Education, Beijing 100069, China, wny0108@hotmail.com.
http://www.nrronline.org/
Accepted: 2013-02-26
杂志排行
中国神经再生研究(英文版)的其它文章
- Posterior quadrantic disconnection maintains the activity of isolated temporal-parietal-occipital nerve tissue: neuroprotective measures in the surgical treatment of epilepsy
- Examination of Huntington’s disease in a Chinese family
- Circadian fl uctuations in three types of sensory modules in healthy subjects
- 7.0T nuclear magnetic resonance evaluation of the amyloid beta (1–40) animal model of Alzheimer’s disease: comparison of cytology veri fi cation
- Compound Formula Rehmannia alleviates levodopainduced dyskinesia in Parkinson’s disease
- The Pael-R gene does not mediate the changes in rotenone-induced Parkinson’s disease model cells
