Cytotoxicity screening of Melastoma malabathricum extracts on human breast cancer cell lines in vitro
2014-03-23NurfarizaAhmadRoslenNurAizuraMatAlewiHadjiAhamadaMohammadSyaifulBahariAbdullRasad
Nurfariza Ahmad Roslen, Nur Aizura Mat Alewi, Hadji Ahamada, Mohammad Syaiful Bahari Abdull Rasad
Department of Biomedical Science, Kulliyyah of Allied Health Sciences, IIUM, 25200 Kuantan, Pahang, Malaysia
Cytotoxicity screening of Melastoma malabathricum extracts on human breast cancer cell lines in vitro
Nurfariza Ahmad Roslen, Nur Aizura Mat Alewi, Hadji Ahamada, Mohammad Syaiful Bahari Abdull Rasad*
Department of Biomedical Science, Kulliyyah of Allied Health Sciences, IIUM, 25200 Kuantan, Pahang, Malaysia
PEER REVIEW
Peer reviewer
Dr. Mohd. Arifin Bin Kaderi, PhD, IARC Postdoctoral Fellow, International Agency for Research on Cancer (WHO-IARC), 150 cours Albert Thomas, 69372 Lyon, France.
Tel: +33 (0)4 7273 8354
Fax: +33 (0)4 7273 8388
Comments
This is an interesting research work in which the authors had demonstrated the in vitro cytotoxic activity of the methanol extract of the leaves of M. malabatricum on the MCF-7 cancer cell-line, as well as moderate cytotoxic activity of the methanol and chloroform extract of the flowers. This appears to provide interesting data that these extracts of the plant may become an interesting anticancer agent against breast cancer. The promising potential of this plant extract should be further studied.
(Details on Page 548
Objective:To screen the cytotoxic activity of Melastoma malabathricum (M. malabathricum) against human breast cancer cell line (MCF-7) in vitro.
Melastoma malabathricum, Cytotoxicity, Human breast cancer, MCF-7
1. Introduction
Malaysia is well known as a tropical rainforest country that has abundant sources of medicinal plants.Melastoma malabathricum(M. malabathricum) belongs to the family Melastomataceae and has many common names includingsendudukin the native language of Malaysia.M. malabathricumis a small shrub commonly found in cleared land, wasteland and roadside throughout the Southeast Asian countries and other parts of the globe. Melastomataceae is a family of plants with a total of more than 4 000 species in the world.
In the Southeast Asian region alone, the genusMelastomacomprises of 22 species, 2 subspecies and 3 varieties. It is a meter tall shrub with lots of branches and can grow up to a height of about 3 to 6 m[1]. As described by Siratet al., there are 3 varieties ofM. malabathricumclassified by the colors of their petalsi.e.pink-magenta petals, white petals, and purple-magenta petals[2]. The attractive flowers produced byM. malabathricumhave 5 petals with the pink-magenta as the commonest variety found in Malaysia and Indonesia. The fruits ofM. malabathricumare soft, dark purple with numerous orange seeds inside. The seeds are tasteless and can be eaten and stain the tongue to black.
Traditionally, it is believed that many parts ofM. malabathricumhave been used in herbal remedies for the treatment of various human ailments. The Malay population has used the leaves and roots ofM. malabathricumfor thetreatment of wounds, post-natal care, and prevention of scars from small pox infection, stomach ulcers, dysentery and diarrhea[3]. Previous studies have shown that the family Melastomataceae demonstrates various bioactivities including antiviral and cytotoxic activities[4], anti-oxidant and anticancer activities[2]. In addition to its anti-hypertensive effect[3], it has anti-nociceptive, anti-inflammatory and antipyretic properties[5], as well as antibacterial and antifungal activity[6,7]. The plant has many attributes, multiple uses and considerable potentiality.
2. Materials and methods
2.1. Preparation of extracts
Leaves, stems and flowers ofM. malabathricumwere cleaned and dried in a drying cabinet (Protech, Malaysia). The air dried powdered leaves (314 g), stems (426.7 g) and flowers (173.2 g) ofM. malabathricumwere extracted respectively withn-hexane, chloroform, and methanol in a Soxhlet apparatus. The extracts were concentrated using rotary evaporator (Eyela, UK) and evaporated to dryness in a fume cupboard. The leaves yieldedn-hexane (7.108 g, 69.8%), chloroform (4.019 g, 57.9%) and methanol (38.967 g, 47.6%) extracts as brown sticky liquid. The stems gaven-hexane (2.429 g, 23.8%), chloroform (1.775 g, 25.6%) and methanol (24.965 g, 30.5%) extracts as light green liquid. The flower producedn-hexane (0.641 g, 6.4%), chloroform (1.141 g, 16.5%) and methanol (17.913 g, 21.9%) as green sticky liquid. The extracts were dissolved in dimethyl sulfoxide to a final concentration that ranging from 6.25 to 100 µg/mL.
2.2. Cell lines and culture medium
Human breast cancer cell line (MCF-7) was obtained from American Type Cell Culture (ATCC, USA). They were cultured in Dulbecco’s Modified Eagle’s Medium. All media were supplemented with 10% (v/v) fetal bovine serum, 100 µg/mL of 1% (v/v) penicillin-streptomycin (PenStrep), and 4 mmol/ L l-glutamine. The cell cultures were maintained in an incubator containing 5% (v/v) CO2at 37 °C.
2.3. In vitro cytotoxicity assay
Confluent stock cultures of cells were harvested with 0.05% (v/v) trypsin-EDTA and plated onto 96-well plates at the cell density of 5×104cells/well. Cell viability before plating was routinely determined by trypan blue exclusion test. The cells were allowed to attach onto the plate by incubation for approximately 24-48 h. When the cells reached confluency between 80%-90%, the medium was replaced with fresh medium containing 0.5% (v/v) fetal bovine serum. The cells were incubated for another 4 h to achieve dormant state. The cells were then treated with different concentrations of methanol, chloroform andn-hexane extracts fromM. malabathricum. One column of wells was treated with only 0.1% of dimethyl sulfoxide that was used to dissolve the crude extracts in the stock solutions; this served as a control. After treatment, the plates were incubated for a further 72 h.
2.4. Cell viability determination
Viability of cells were determined by using methylene blue staining method[8]. Methylene blue only stains viable cells. Briefly, glutaraldehyde was added to each well to a final concentration of 2.5% (v/v) and the viable cells were fixed for 15 min. After washing with 0.15 mol/L sodium chloride (NaCl) and removing the dead cells, the fixed cells were stained with 0.1 mL of 0.05% (w/v) methylene blue solution for 15 min. After washing off the excess dye with 0.15 mol/L sodium chloride solution, dye solution was carried out with 0.2 mL of 0.33 mol/ L hydrochloric acid (HCl). The absorbance was then read at 650 nm using Vmax Kinetic Microplate Reader (TECAN, Germany).
2.5. Calculations and statistics
Percentage inhibition of cancer cell line was calculated using the following equation:
%Inhibition=[(Abs. Control-Abs. Sample)/(Abs. Control)]×100 where Abs.=Absorbance.
Experiments were performed in three replicate. Results were expressed as percentage growth inhabitation of control. The IC50values for each of the active extracts were determined by plotting the percentage inhibition values against the respective concentrations of the extracts. A linear equation for the resulting plot was obtained. Theyin the equation was substituted with 50 to obtain thexvalue which represents the IC50. The IC50is the concentration of the extracts, at which growth was inhibited in 50% of the cells population. Statistical significant difference was determined using One-way ANOVA and differences were considered to be significant ifP-value is less than 0.05.
3. Results
The growth inhibitions of various extracts from leaves, stems and flowers ofM. malabathricumwere evaluated using methylene blue assay. The determinations of cytotoxicity were done using dose-response curve obtained by non linear regression analysis. The results showed that methanol leaf extract caused 81.23% of growth inhibition against MCF-7 cell line (Table 1) followed by methanol flower extract (77.97%) and chloroform flower extract (63.06%) at concentration of 100 µg/mL respectively (Table 2). All stem extracts produced very low inhibition against MCF-7 cell line with less than 47% growth inhibition for every concentration used (Table 3).
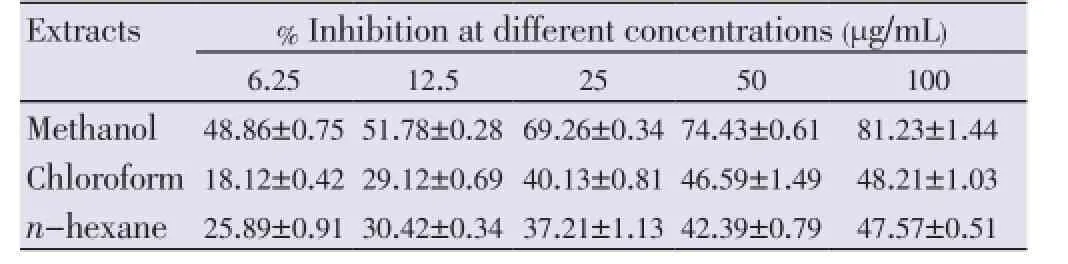
Table 1 Percentage inhibition of leaf extracts on MCF-7 cell lines after 72 h of treatment.
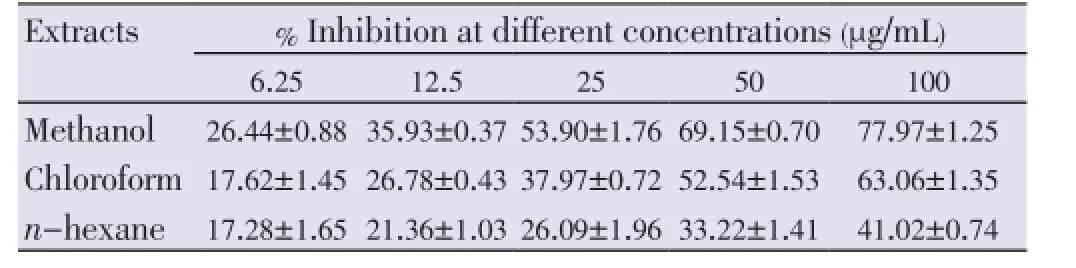
Table 2 Percentage inhibition of flower extracts on MCF-7 cell lines after 72 h of treatment.
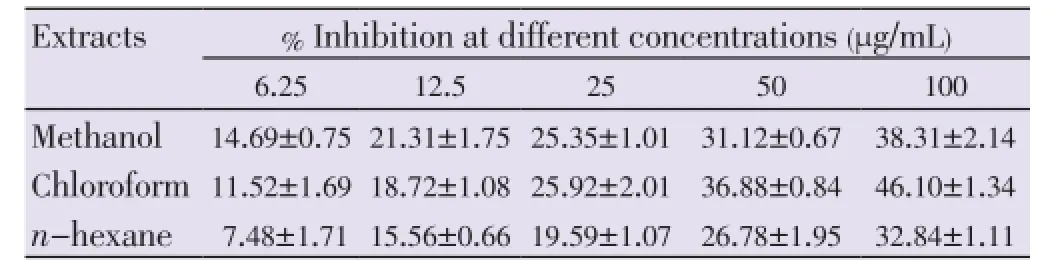
Table 3 Percentage inhibition of stem extracts on MCF-7 cell lines after 72 h of treatment.
A dose-dependent response was observed between the concentration of all extracts and their growth-inhibition activities. Almost all extracts exhibited increased activity after prolonged treatment. The results obtained showed that methanol leaf extract appeared to be cytotoxic with IC50value of 7.14 µg/mL (Figure 1) followed by methanol flower extract with IC50value of 33.63 µg/mL (Figure 2) respectively after 72 h of treatment. All results obtained showed a significant difference between solvents and plant parts withP-values less than 0.05 except stems with undetermined IC50(Figure 3).
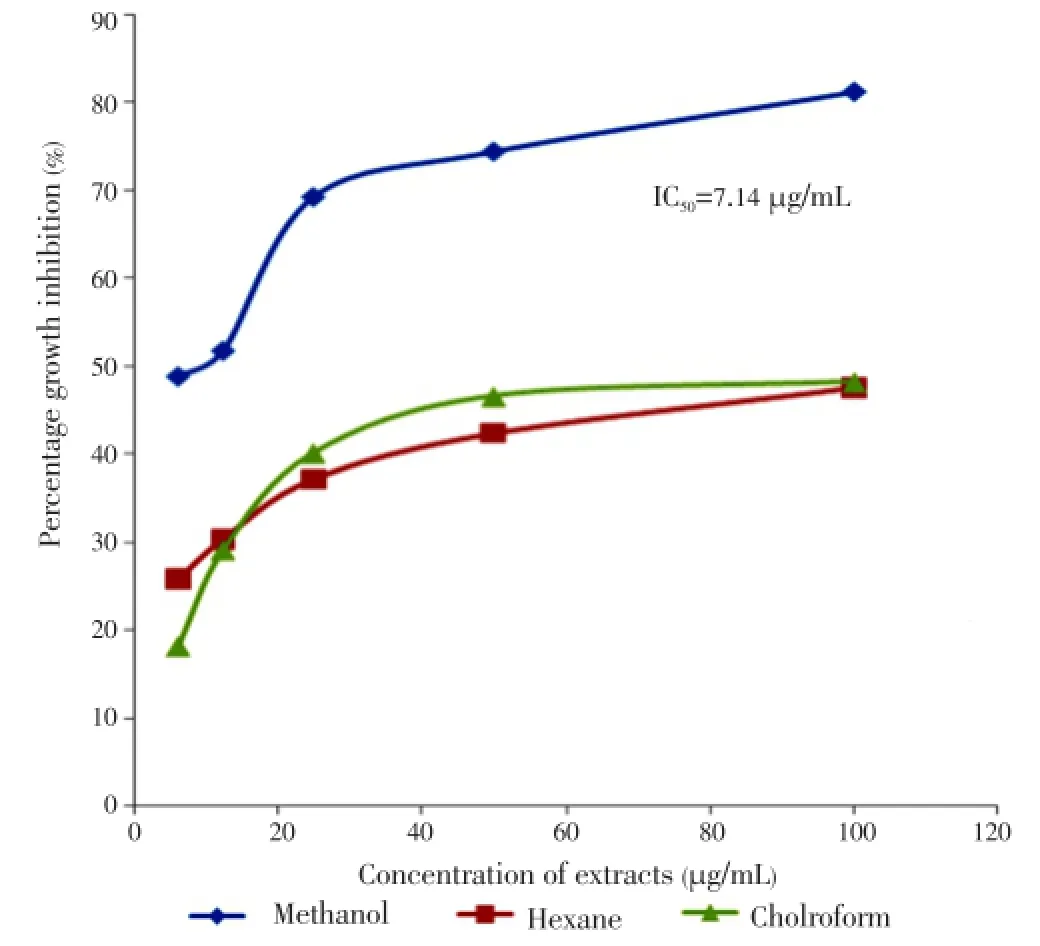
Figure 1. Growth inhibition of leaf extracts on MCF-7 cell line after 72 h of treatment.
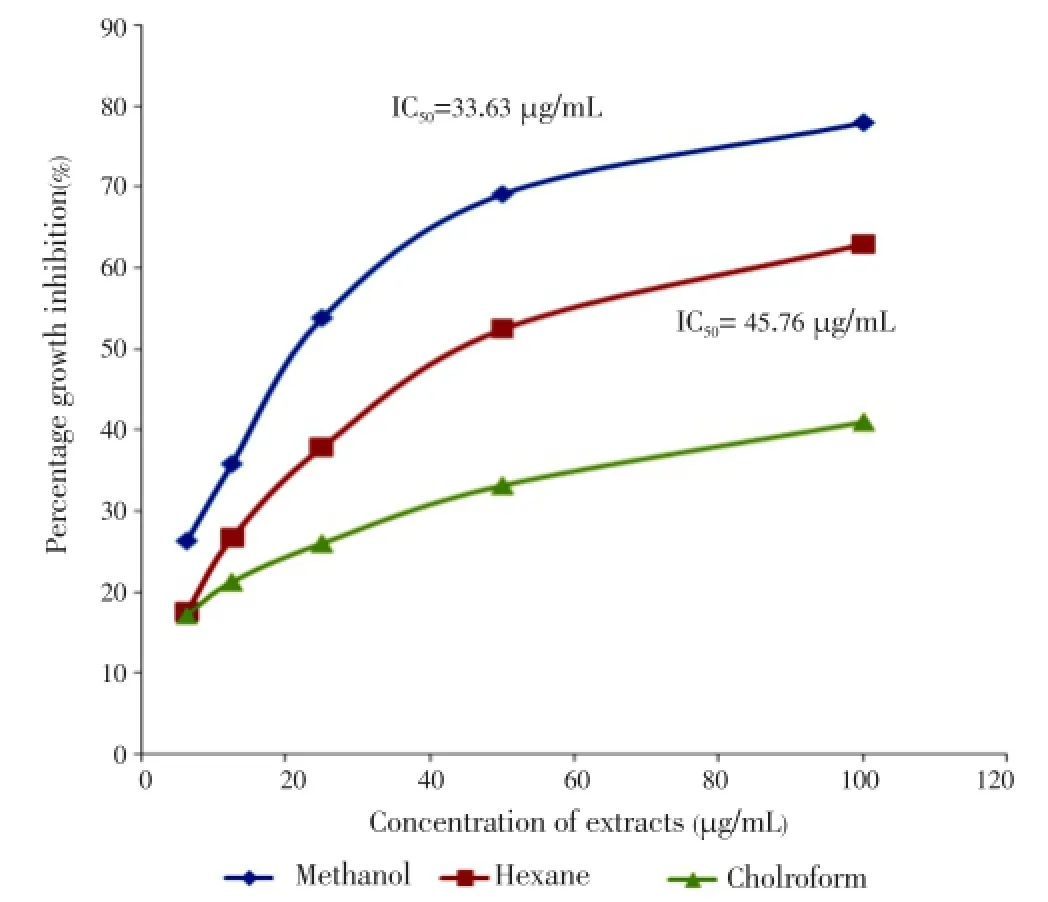
Figure 2. Growth inhibition of flower extracts on MCF-7 cell line after 72 h of treatment.
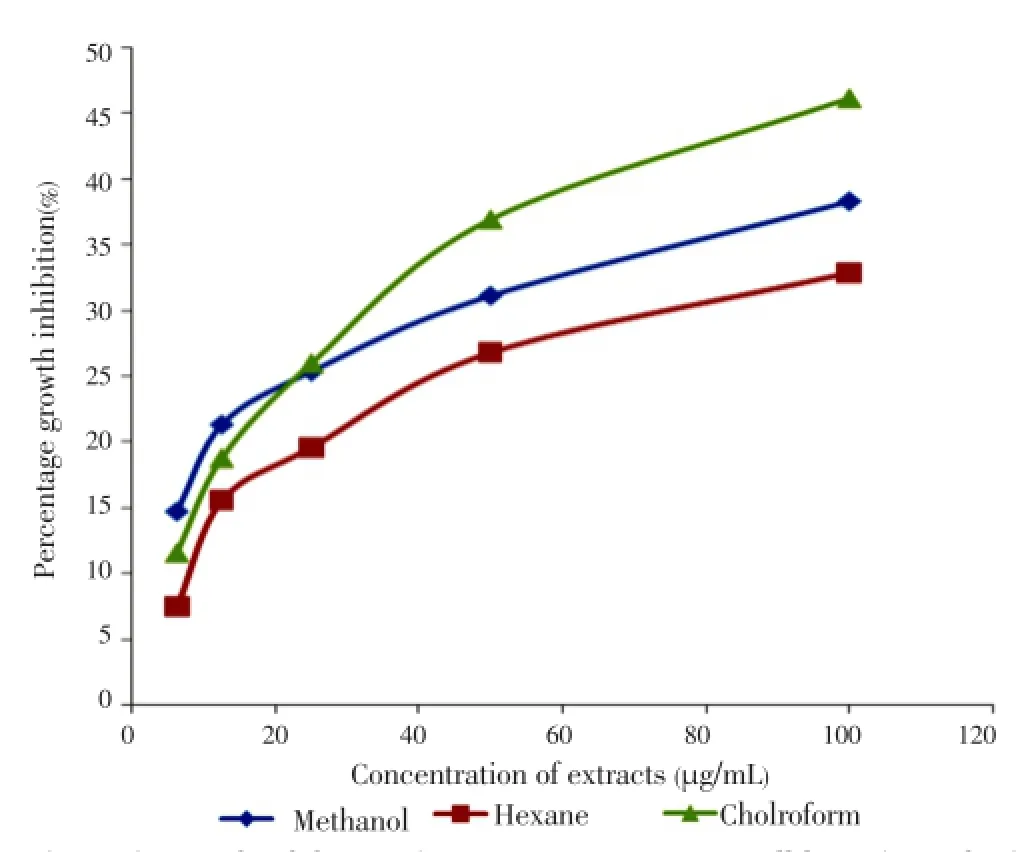
Figure 3. Growth inhibition of stem extracts on MCF-7 cell line after 72 h of treatment.
4. Discussion
This study was aimed at screening for the potential cytotoxicity activity against human breast cancer cell line (MCF-7)in vitro. Leaves, stems and flowers were extracted using three solvents, namely,n-hexane, chloroform and methanol. The cytotoxicity was determined using methylene blue assay[8]. The results obtained were analyzed by determining the mean absorbance and standard deviation for each concentration. The percentage growth inhibition and half maximal inhibitory concentration (IC50) were determined using SPSS version 17 and Excel (point to point analysis). Significant results were compared using Oneway ANOVA and pairedt-test. The criterion of cytotoxic and non-cytotoxic was adapted from the guideline set by the National Cancer Institute of America. The screening protocol has indicated that plant extracts with IC50less than 30 µg/mL were considered to be cytotoxic and non cytotoxic if otherwise[9].
Similar study was reported by Siratet al. that methanol extract of flower fromM. malabathricumyielded kaempferol-3-O-(2”,6”-di-O-p-trans-coumaroyl) and ethyl acetate extract of the same flower gave naringenin as an isolated compounds[2]. Both compounds were found to be active in inhibiting cell proliferation of MCF-7 with IC50values of 0.28 µmol/L and 1.3 µmol/L respectively. It is important to note that the reported IC50value of this kaempferol-3-O-(2”,6”-di-O-p-trans-coumaroyl) was lower than the positive control used, tamoxifen (0.76 µmol/ L), a prominent breast cancer drug in the market. It was also mentioned that this cytotoxic activity affects the cells proliferation and morphology and act in a dose-dependent manner. Furthermore, Mohd Joffryet al. observed that methanol extract of leaf fromM. malabathricumshowed cytotoxic activity against human cancers cell line (MCF-7) with an IC50value less than 25 µg/mL[3]. This present study contributes to the mounting evidence that the leaf and flower extracts ofM. malabathricumhave potential anticancer activity.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors would like to thank International Islamic University Malaysia (IIUM) for research grants support (EDW B11-024-0502 and EDW B11-216- 0694) and Ministry of Higher Education (MOHE) for funding through grant FRGS No.: 11-039-0188.
Comments
Background
Breast cancer is one of the most important malignancy among women in the developed and developing countries. It accounts for almost a quarter of all cancers among female. The potential of natural biological products from the tropical rainforest has become subjects of intense research in recent years. As a source of tremendous diversities of fauna, systematic scientific studies on these natural resources may provide discovery towards potential novel pharmaceutical agents against breast cancer.
Research frontiers
The present research work explored the cytotoxic activity of several extracts from various parts ofM. malabathricumon human breast cancer cell line, the MCF-7. The study seems to provide some initial interesting data on the potential of certain extracts of the plant to inhibit the growth of the cell linein vitro.
Related reports
Several extracts ofM. malabathricumhad been previously reported to contain various bioactive phytochemical and had been demonstrated to possesses anti-inflammatory, antioxidative and wound-healing properties. This may support the findings of the present research, as these pathways are also involved in the development of neoplasia and malignancy.
Innovations and breakthroughs
M. malabatricum, known commonly assendudukin the native language of Malaysia, is a plant used to stop bleeding and for wound healing among rural inhabitants. In the present study, the authors provided initial data on the cytotoxic activity of certain extracts of the plant.
Applications
M. malabatricumis a common shrub, which is ubiquitous throughout tropical and sub-tropical regions. The findings from the present study, if confirmed in further detailed analyses and proven safe to be developed as chemotherapeutic agent against breast cancer, may provide interesting application of this abundant natural resource from the tropical forest.
Peer review
This is an interesting research work in which the authors had demonstrated thein vitrocytotoxic activity of the methanol extract of the leaves ofM. malabatricumon the MCF-7 cancer cell-line, as well as moderate cytotoxic activity of the methanol and chloroform extract of the flowers. This appears to provide interesting data that these extracts of the plant may become an interesting anticancer agent against breast cancer. The promising potential of this plant extract should be further studied.
[1] bin Zakaria M, Mohd MA. Traditional Malay medicinal plants. Kuala Lumpur: Institut Terjemahan Negara Malaysia Berhad; 2010, p. 120-128.
[2] Sirat HM, Susanti D, Ahmad F, Takayama H, Kitajima M. Amides, triterpene and flavonoids from the leaves of Melastoma malabathricum L. J Nat Med 2010; 64(4): 492-495.
[3] Joffry SM, Yob NJ, Rofiee MS, Affendi MM, Suhaili Z, Othman F, et al. Melastoma malabathricum (L.) Smith ethnomedicinal uses, chemical constituents and pharmacological properties: a review. Evid Based Complement Alternat Med 2012; doi: 10.1155/2012/258434.
[4] Türkez H, Aydin E, Aslan A. Effects of lichenic extracts (Hypogymnia physodes, Ramalina polymorpha and Usnea florida) on human blood cells: cytogenetic and biochemical study. Iran J Pharm Res 2012; 21(11): 889-896.
[5] Zakaria ZA, Rofiee MS, Mohamed AM, Teh LK, Salleh MZ. In vitro antiproliferative and antioxidant activities and total phenolic contents of the extracts of Melastoma malabathricum leaves. J Acupunct Meridian Stud 2011; 4(4): 248-256.
[6] Goyal M, Sasmal D, Nagori BP. Review on medicinal plants used by local community on Jodhpur District of Thar Desert. Int J Pharmacol 2011; 7: 333-339.
[7] Siti Nurhadis CO, Janna Ong A, Khairul Anuar K, Sieo CC, Mujahir H. Effects of flower and fruit extracts of Melastoma malabathricum Linn. on growth of pathogenic bacteria: Listeria monocytogenes, Staphylococccus aureus, Escherichia coli, and Salmonella typhimurium. Evid Based Complement Alternat Med 2013; doi: 10.1155/2013/459089.
[8] Leong OK, Muhammad TS, Sulaiman SF. Cytotoxic activities of Physalis minima L. chloroform extract on human lung adenocarcinoma NHI-H23 cell lines by induction of apoptosis. Evid Based Complement Alternat Med 2011; doi: 10.1093/ecam/ nep057.
[9] Ramasamy S, Wahab NA, Abidin NZ, Manickam S, Zakaria Z. Growth inhibition of human gynecologic and colon cancer cells by Phyllanthus watsonii through apoptosis induction. PLoS ONE 2012; 7(4): e34793.
10.12980/APJTB.4.2014C658
*Corresponding author: Mohammad Syaiful Bahari Abdull Rasad, Department of Biomedical Science, Kulliyyah of Allied Health Sciences, International Islamic University Malaysia (IIUM), 25200 Kuantan, Pahang, Malaysia.
Tel: +609-5705253
Fax: +609-5706776
E-mail: syaiful@iium.edu.my
Foundation Project: Supported by International Islamic University Malaysia (EDW B11-024-0502 and EDW B11-216- 0694).
Article history:
Received 19 Jan 2014
Received in revised form 27 Jan, 2nd revised form 2 Feb, 3rd revised form 7 Feb 2014
Accepted 13 Mar 2014
Available online 5 Apr 2014
Methods:A three steps extraction protocol using n-hexane, chloroform and methanol as the solvents systems was carried out on leaves, stems and flowers of M. malabathricum. Dimethyl sulfoxide was used in extracts dilution and serial dilutions were conducted to obtain five different extract concentrations (100 µg/mL, 50 µg/mL, 25 µg/mL, 12.5 µg/mL and 6.25 µg/mL). The evaluation of cell growth was determined using methylene blue assay.
Results:Methanol extract from the leaves showed significant anticancer activity against MCF-7 cell lines with the IC50value of 7.14 µg/ml while methanol and chloroform extract from the flowers exhibited a moderate activity towards MCF-7 cell line with the IC50value of 33.63 µg/mL and 45.76 µg/mL respectively after 72 h of treatment.
Conclusions:The extracts from leaves and flowers of M. malabathricum showed promising anticancer activity toward human breast cancer cell lines with the lowest IC50at 7.14 µg/mL while the extracts from stems showed less growth inhibition activity.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antimicrobial activity against periodontopathogenic bacteria, antioxidant and cytotoxic effects of various extracts from endemic Thermopsis turcica
- Proteomics analysis of antimalarial targets of Garcinia mangostana Linn.
- The presence of eucalyptol in Artemisia australis validates its use in traditional Hawaiian medicine
- Jeju seaweeds suppress lipopolysaccharide-stimulated proinflammatory response in RAW 264.7 murine macrophages
- Antioxidant potential of Rumex vesicarius L.: in vitro approach
- In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines
