Electro-acupuncture at Conception and Governor vessels and transplantation of umbilical cord bloodderived mesenchymal stem cells for treating cerebral ischemia/reperfusion injury
2014-03-23HaiboYuPengdianChenZhuoxinYangWenshuLuoMinPiYonggangWuLingWang
Haibo Yu, Pengdian Chen, Zhuoxin Yang, Wenshu Luo, Min Pi, Yonggang Wu, Ling Wang
1 Af fi liated Shenzhen Traditional Chinese Medicine Hospital, Guangzhou University of Chinese Medicine, Shenzhen, Guangdong Province, China
2 Af fi liated Shenzhen Maternity & Child Healthcare Hospital, Southern Medical University, Shenzhen, Guangdong Province, China
Electro-acupuncture at Conception and Governor vessels and transplantation of umbilical cord bloodderived mesenchymal stem cells for treating cerebral ischemia/reperfusion injury
Haibo Yu1, Pengdian Chen1, Zhuoxin Yang2, Wenshu Luo1, Min Pi1, Yonggang Wu1, Ling Wang1
1 Af fi liated Shenzhen Traditional Chinese Medicine Hospital, Guangzhou University of Chinese Medicine, Shenzhen, Guangdong Province, China
2 Af fi liated Shenzhen Maternity & Child Healthcare Hospital, Southern Medical University, Shenzhen, Guangdong Province, China
Pengdian Chen and Haibo Yu contributed equally to this study.
Mesenchymal stem cell transplantation is a novel means of treating cerebral ischemia/reperfusion, and can promote angiogenesis and neurological functional recovery. Acupuncture at Conception and Governor vessels also has positive effects as a treatment for cerebral ischemia/ reperfusion. Therefore, we hypothesized that electro-acupuncture at Conception and Governor vessels plus mesenchymal stem cell transplantation may have better therapeutic effects on the promotion of angiogenesis and recovery of neurological function than either treatment alone. In the present study, human umbilical cord blood-derived mesenchymal stem cells were isolated, cultured, identified and intracranially transplanted into the striatum and subcortex of rats at 24 hours following cerebral ischemia/reperfusion. Subsequently, rats were electro-acupunctured at Conception and Governor vessels at 24 hours after transplantation. Modified neurological severity scores and immunohistochemistry fi ndings revealed that the combined interventions of electro-acupuncture and mesenchymal stem cell transplantation clearly improved neurological impairment and up-regulated vascular endothelial growth factor expression around the ischemic focus. The combined intervention provided a better outcome than mesenchymal stem cell transplantation alone. These fi ndings demonstrate that electro-acupuncture at Conception and Governor vessels and mesenchymal stem cell transplantation have synergetic effects on promoting neurological function recovery and angiogenesis in rats after cerebral ischemia/reperfusion.
nerve regeneration; acupuncture; human umbilical cord blood-derived mesenchymal stem cells; electro-acupuncture; cerebral ischemia/reperfusion; vascular endothelial growth factor; angiogenesis; Conception vessel; Governor vessel; modified neurological severity score; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 81072877; Key Laboratory Project of Condition and Platform Construction Plan of Shenzhen Scientific Research Fund, No. CXB201111250113A; Shenzhen Scientific and Technology Development Program, No. 201203149
Yu HB, Chen PD, Yang ZX, Luo WS, Pi M, Wu YG, Wang L. Electro-acupuncture at Conception and Governor vessels and transplantation of umbilical cord blood-derived mesenchymal stem cells for treating cerebral ischemia/reperfusion injury. Neural Regen Res. 2014;9(1):84-91.
Introduction
Despite advances in medical treatment, cerebral ischemia/ reperfusion is still a major cause of adult morbidity, mortality and disability, and the only approved therapy is thrombolysis within 3.0–4.5 hours after symptom onset[1-2]. Besides thrombolysis and neuroprotection, vascular protection is also of signi fi cance for the treatment of cerebral ischemia injury[3]. Vascular endothelial growth factor, a potent angiogenetic and neurotrophic factor, is known to be involved in the regulation of the vascular permeability of the blood-brain barrier[4-5].
In recent years, progress in stem cell biology has opened up avenues to therapeutic strategies for the treatment of neurological diseases. Mesenchymal stem cells are an attractive candidate for cell therapy since they are readily obtained and can be expanded in vitro. Some transplanted mesenchymal stem cells differentiate into neuron-like cells and endothelial cells in recipient brains[6]. In addition, these cells can be induced to differentiate into vascular endothelial cells showing up-regulated vascular endothelial growth factor expression, with autocrine and paracrine modes of action, in the brains of cerebral ischemia/reperfusion rats; consequently, they promote angiogenesis and ameliorate neurological functional de fi cits[7-10].

Figure 1 Flow cytometric analysis of human umbilical cord blood-derived mesenchymal stem cells at passage 3.
Acupuncture has been widely applied as a treatment or as an adjuvant modality for apoplexy patients in China for centuries. A previous study by our laboratory[11]showed that electro-acupuncture at Conception and Governor vessels continuously promotes the proliferation and differentiation of endogenous neural stem cells into neurons in cerebral ischemia/reperfusion rats, which is beneficial for neural regeneration[12]. Recent studies have also found that electro-acupuncture induces up-regulation of vascular endothelial growth factor expression and reduces neurological de fi cit scores in cerebral ischemia/reperfusion rats[13-14]. Therefore, we hypothesized that electro-acupuncture at Conception and Governor vessels may have additive or synergetic effects with mesenchymal stem cell transplantation, on the up-regulation of vascular endothelial growth factor expression and improved neurological functional recovery, which could provide a novel treatment for cerebral ischemia/reperfusion.
To investigate the combinatorial effects of electro-acupuncture and mesenchymal stem cells in the treatment of cerebral ischemia/reperfusion, human umbilical cord blood-derived mesenchymal stem cells (HUCB-MSCs) were isolated, cultured and transplanted into the brains of cerebral ischemia/reperfusion rats. Electro-acupuncture was also applied. We examined their combinatorial effects on neurological severity scores and vascular endothelial growth factor expression in the rats.
Results
Quantitative analysis of experimental animals
A total of 96 rats were equally and randomly divided into the following four groups: a sham group (no ischemia/reperfusion), model group (ischemia/reperfusion plus PBS transplantation), HUCB-MSC group (ischemia/reperfusion plus mesenchymal stem cells transplantation), and electro-acupuncture group (ischemia/reperfusion, mesenchymal stem cells transplantation and electro-acupuncture). In terms of the time period after cerebral ischemia/reperfusion, each group was further divided into three subgroups of 7, 14 and 28 days. Thus, each subgroup contained eight rats. All 96 rats were involved in the fi nal analysis.
Identi fi cation of HUCB-MSCs
According to the identi fi cation criteria of mesenchymal stem cells proposed by the International Society for Cellular Therapy[15], we selected specific cell surface markers to identify these cells. Flow cytometric analysis revealed that HUCBMSCs at passage three highly expressed mesenchymal stem cell antigens, including CD90 (100%), CD73 (96.4%) and CD105 (99.5%), and adhesion molecules CD44 (100%) and CD29 (98.2%), but expressed low levels of CD45 (0.9%), CD34 (0.3%), CD19 (0.9%), CD14 (0.6%) and human leukocyte antigen-DR (0.8%). These fi ndings indicate that the major population of the adherent cells cultured was HUCBMSCs (Figure 1).
Electro-acupuncture and HUCB-MSC transplantation improved neurological de fi cits of rats following cerebral ischemia/reperfusion
The modi fi ed neurological severity score in the sham group was 0 because no neurological deficits were found. All rat models of cerebral ischemia/reperfusion injury in the other three groups showed severe neurological deficits, which gradually improved as time proceeded. The HUCB-MSC group had a lower score than the model group on days 7, 14 and 28 (P < 0.01), but a higher score than the electro-acupuncture group (P < 0.01). These fi ndings suggest that electro-acupuncture combined with HUCB-MSC treatment is superior to HUCB-MSC transplantation alone in improving neurological functions (Figure 2).
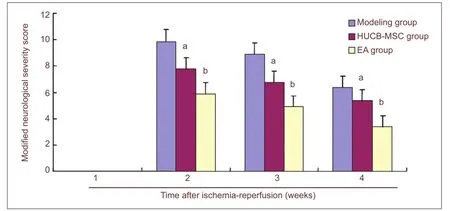
Figure 2 Effect of electro-acupuncture (EA) plus human umbilical cord blood-derived mesenchymal stem cell (HUCB-MSC) treatments on neurological function in cerebral ischemia rats.
Electro-acupuncture and HUCB-MSC transplantation up-regulated vascular endothelial growth factor expression around the ischemic focus in rats following cerebral ischemia/reperfusion
Under a light microscope, vascular endothelial growth factor-positive cells were observed with yellow or brown cytoplasm. There were no signi fi cant differences in the numbers of positive cells in the sham group among the various time points (Figure 3). However, the numbers of vascular endothelial growth factor-positive cells in the other three groups gradually decreased from a peak at 7 days after injury, being significantly decreased at 14 days and further decreased at 28 days after cerebral ischemia/reperfusion (P < 0.01; Figure 3).
The numbers of vascular endothelial growth factor-positive cells in the HUCB-MSC and electro-acupuncture groups were signi fi cantly higher than that in the model group at 7, 14 and 28 days (P < 0.01). There was no signi fi cant difference in cell numbers between the HUCB-MSC group and the electro-acupuncture group at 7 days (P > 0.05). However, with prolonging of the reperfusion period, there were significantly more vascular endothelial growth factor-positive cells in the electro-acupuncture group compared with HUCB-MSCs group at 14 and 28 days (P < 0.05; Figure 3).
Discussion
Mesenchymal stem cells offer promise for cell therapy procedures owing to their multipotent abilities, which means they could be induced to secrete diverse cytokines and growth factors and promote neurological functional recovery following cerebral ischemia/reperfusion injuries[6]. Compared with human bone marrow-derived MSCs, HUCB-MSCs have many advantages such as an abundant source that can be accessed without pain, easy harvesting, low immunogenicity and a low possibility of being polluted by viruses and bacteria[7]. Accordingly, in the present study, we selected the HUCB-MSCs. Our data indicated that the cultured adherent HUCB-MSCs exhibited a spindle shape and expressed mesenchymal stem cell-speci fi c markers.
In our previous studies, electro-acupuncture at Conception and Governor vessels continuously promoted the proliferation and differentiation of neural stem cells into neurons in cerebral ischemia/reperfusion rats[11]. Furthermore, electro-acupuncture at the Conception vessel up-regulated the expression levels of neurotrophic factors such as basic fibroblast growth factor and epidermal growth factor[16-17]. Accumulating evidence suggests that electro-acupuncture at the Governor vessel enhances vascular endothelial growth factor expression and inhibits cellular apoptosis following acute cerebral ischemia/reperfusion injuries[14,18-19]. Based on classic acupuncture texts and other generally acknowledged traditional Chinese medicine sources as well as the results of clinical practice[20-22], we selected Qihai (CV6), Guanyuan (CV4) and Chengjiang (CV24) acupoints from the Conception vessel and Baihui (GV20), Dazhui (GV14) and Shuigou (GV26) acupoints from the Governor vessel in the present study.

Figure 3 Effect of electro-acupuncture (EA) plus human umbilical cord blood-derived mesenchymal stem cells (HUCB-MSCs) on the number of vascular endothelial growth factor (VEGF)-positive cells around the ischemic focus in rats.
Vascular endothelial growth factor is a chemokine and specific mitogen of endothelial cells, exerting its biological functions via three related receptor tyrosine kinases (Flt-1, Flk-2 and Flt-4), promoting endotheliosis in vitro and inducing angiogenesis in vivo[23]. In addition, it has been shownto stimulate axonal outgrowth and enhance cell viability[24], promote the proliferation and differentiation of endothelial cells, and inhibit cellular apoptosis via inducing Bcl-2 and Survivin expression and down-regulating caspase-3 expression[25-26]. Moreover vascular endothelial growth factor acts directly on neural stem cells and repairs the brain through inducing other growth factors such as brain-derived neurotrophic factor[27-28]. Hence, vascular endothelial growth factor plays a role in angiogenesis and has a neurotrophic effect in the treatment of cerebral ischemia/reperfusion. The results from the present study reveal that vascular endothelial growth factor expression is up-regulated following cerebral ischemia/reperfusion, and that electro-acupuncture plus HUCB-MSCs transplantation can maintain this increase in vascular endothelial growth factor expression. This evidence is consistent with the results of previous studies showing bene fi cial effects of human bone marrow-derived mesenchymal stem cells in experimental cerebral ischemic models[29]. With prolonging of the reperfusion period, the effects were superior to that of HUCB-MSC transplantation alone.
A number of studies have shown functional improvement in focal cerebral ischemia/reperfusion rats following intracerebral delivery of mesenchymal stem cells[30]. However, the mechanisms by which mesenchymal stem cell transplantation improves stroke deficits remain uncertain. Diverse mechanisms may be responsible for properties such as increasing neovascularization, integrating into the host circuitry, reducing apoptosis of host cells, inducing host brain plasticity, and attenuating inflammation[31-32]. Accumulating evidence indicates that mesenchymal stem cells protect against cerebral injuries and promote angiogenesis via differentiating into vascular endothelial cells, extracellular matrix or laminin[33]. Interestingly, the modified neurological severity score results in this study showed that electro-acupuncture plus HUCB-MSC transplantation better improved neurological functions than did HUCB-MSC transplantation alone. This may be a consequence of the up-regulation of vascular endothelial growth factor expression. Considering the angiogenic effects of electro-acupuncture and mesenchymal stem cells, we tentatively infer that electro-acupuncture has additional or synergetic effects on mesenchymal stem cell transplantation, to further improve neurological functions following cerebral ischemia/reperfusion. According to traditional Chinese medicine theories, the Conception vessel governs embryonic pregnancy and controls the growth and development of the fetus, and may enhance the expression levels of endogenous neurotrophic factors such as vascular endothelial growth factor and promote functional recovery. The Governor vessel manages and activates yang qi, and inhibits cellular apoptosis following cerebral ischemia/reperfusion injuries[34].
In conclusion, electro-acupuncture enhances the therapeutic potency of mesenchymal stem cells including neurological improvement, possibly through induction of angiogenesis. A combination of electro-acupuncture plus HUCBMSC transplantation represents a new potential therapeutic strategy for the treatment of cerebral ischemia/reperfusion.
However, the present study lacked a group receiving electro-acupuncture only, so we could not rule out the possibility that electro-acupuncture alone would have a similar action to the combined treatments; this possibility needs further investigation. In addition, the mechanisms of action of the combined treatments for cerebral ischemia/reperfusion are not entirely understood. Further studies addressing the combinatorial effects on the transformation of mesenchymal stem cells into neurons, neurotrophic factor expression and cellular apoptosis are warranted.
Materials and Methods
Design
A randomized, controlled, animal experiment.
Time and setting
The experiments were performed in the Research Center for Neural Engineering, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, China from June 2011 to October 2012.
Materials
Animals
Ninety-six adult male Sprague-Dawley rats, weighing 220–250 g were purchased from Guangdong Provincial Experimental Animal Center, Guangdong Province, China (license No. SCXK (Yue) 2008-0002). The rats were housed (four or fi ve per cage) at 23 ± 1°C, in an alternating 12-hour light/dark cycle, and allowed free access to food and water. All experimental protocols and procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[35].
Cells
Human umbilical cord blood samples were collected from the umbilical veins of deliveries, from the Maternal Department, Affiliated Shenzhen Traditional Chinese Medicine Hospital, Guangzhou University of Chinese Medicine, China, with informed written maternal consent. Parturient infected with hepatitis virus, HIV or Treponema pallidum, and samples from fetuses with deformities, were excluded from the study.
Methods
Isolation and culture of HUCB-MSCs

Figure 4 Morphology of human umbilical cord blood-derived mesenchymal stem cells at primary passage at days 11 (A) and 28 (B).
With 75% alcohol disinfection, the needle of an umbilical cord blood collection bag containing 0.74 g of sodium citrate (Sichuan Nigale Biomedical Co., Ltd., Sichuan Province, China) was inserted into the umbilical vein near placentas and human umbilical cord blood was collected by gravity. When the blood was collected, we put the bag lower than the umbilical cord. According to rules of gravity, the blood fl ows from the umbilical cord (higher position) to the bag (lower position). Isolation and expansion of HUCBMSCs were conducted as previously reported[36]. In brief, mononuclear cells were isolated in lymphocyte separation by Ficoll-Hypaque gradient centrifugation (speci fi c gravity 1.077 g/cm3; Pharmacia, Piscataway, NJ, USA). The separated mononuclear cells were washed and suspended in Dulbecco’s modi fi ed Eagle’s medium/nutrient mixture F-12 (Gibco, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin-streptomycin (Hyclone, Logan, UT, USA), 1% glutamine (Gibco), 10 ng/mL human epidermal growth factor (ProSpec, Ness-Ziona, Israel) and 10 ng/mL recombinant human fi broblast growth factor-basic (PeproTech, USA). The culture medium served as the maintenance and expansion system and cells were seeded at 1 × 106cells/cm2onto 75-cm2plastic culture flasks (Corning Incorporated, NY, USA). Cultures were maintained at 37°C in a humidi fi ed atmosphere containing 5% CO2. After 1 week, non-adherent cells were removed. The medium was replaced with the same fresh medium twice a week, and the onset of fibroblast-like adherent cells was observed. At 3–4 weeks, when the monolayer of mesenchymal stem cell colonies reached 80% confluence, cells were trypsinized with 0.25% trypsin-EDTA (Gibco), washed, resuspended in culture medium and subcultured at 3,000 cells/cm2(Figure 4). Mesenchymal stem cells of each umbilical cord blood harvested were expanded ex vivo by successive subcultivation under the same condition. HUCB-MSCs at passage three, at 1 × 105/μL, were used for transplantation.
Before transplantation, flow cytometric analyses of HUCB-MSCs at passage 3 were performed to identify the cells. In brief, cell suspensions were washed twice with PBS. For direct assays, aliquots of cells at 2 × 105/mL were immuno-labeled at 4°C for 30 minutes with the following mouse anti-human monoclonal antibodies (7 μL each, 0.2 mg/mL): phycoerythrin-conjugated CD73, CD29, CD34, fluorescein isothiocyanate-conjugated CD90, CD14, CD44, CD45, allophycocyanin-conjugated CD19, CD105 and Peridinin-Chlorophyll-Protein Complex-conjugated human leukocyte antigen-DR. All antibodies were purchased from Becton Dickinson (BD) Biosciences (San Diego, CA, USA) and the labeled cells were analyzed using a FACSCalibur fl ow cytometer (Arial, BD Biosciences).
Establishing cerebral ischemia/reperfusion models
Transient cerebral ischemia/reperfusion was induced as described previously[37]. Brie fl y, after anesthesia with intraperitoneal injection of 300 mg/kg chloral hydrate, the rats were placed in a supine position. Using a surgical microscope (STEMI DV4, USA), the skin was incised along the midline of the neck and the right common carotid artery, external carotid artery and internal carotid artery were exposed through a ventral midline incision. The occipital artery and thyroid artery were electrocauterized; and the right external carotid artery distal to the heart was ligated. The common carotid artery and internal carotid artery distal to the heart were then clamped. The right external carotid artery was cut off at the ligation site, and the internal carotid artery and external carotid artery were intersected and aligned. A gap was made at the bifurcation of the external carotid artery adjacent to the common carotid artery, and a 4-0 mono fi lament suture with its tip rounded by heating near a fl ame was inserted from the incision and slowly advanced to the middle cerebral artery (depth 18 ± 2 mm). Rectal temperature was maintained at 37°C throughout the surgical procedure until the anesthesia ended. After 2 hours, the animals were re-anesthetized, the fi lament was withdrawn out of internal carotid artery for reperfusion, and the wound was sutured. Model establishment was considered successful with the appearance of contralateral hemiplegia (limb disturbance such as flexion and adduction) and contralateral circling while walking. For the sham surgery group, the operating procedure was the same as described above without fi lament insertion into the internal carotid artery.
HUCB-MSC transplantation
HUCB-MSCs at passage 3 were harvested, suspended in PBS and placed in an ice bath before cell transplantation. Transplantation of cells was conducted as previously reported with some modi fi cation[38]. Namely, after 24 hours of reperfusion, rats were anesthetized and tethered to a stereotactic apparatus (World Precision Instruments Inc., Sarasota, FL, USA). A piece of paper was used to cover the two eyes, and the cranium was exposed through a midline skin incision. A burr hole was made using a small high-speed dental drill, and a suspension of 10 μL of cells containing a total of 106HUCB-MSCs was injected stereotactically into the right striatum (anteroposterior = 0 mm, mediolateral = 2.0 mm, dorsoventral = 4.5 mm) and right subcortex (anteroposterior = 0 mm, mediolateral = 2.0 mm, dorsoventral = 2.0 mm) using a 25-μL microsyringe (Hamilton, Switzerland), with 7 μL being injected into the striatum and 3 μL into the subcortex. Cells were injected at a speed of 1 μL/minute with an automatic microinjection pump and the needle was left in situ for 5 minutes post-injection before being slowly removed. An equal volume of PBS was administered in the model group using the same procedure.
Electro-acupuncture treatment
At 24 hours after transplantation, rats in the electro-acupuncture group were electro-acupunctured at Shuigou (GV26; 1 mm below nasal tip, depth of insertion: 1 mm, upward), Baihui (GV20; center of parietal bone, depth of insertion: 4 mm, backward), Dazhui (GV14; below the spinous process of the 7thcervical and 1stthoracic vertebrae, depth of insertion: 5 mm, vertically), Chengjiang (CV24, 1 mm below pubic margin of low lip, depth of insertion: 1 mm, downward), Guanyuan (CV4, 25 mm below the umbilicus, depth of insertion: 5 mm, vertically) and Qihai (CV6, middle point between the umbilicus and Guanyuan, depth of insertion: 5 mm, vertically) according to the document[39]. Huanqiu Brand sterile acu-puncture needles (0.3 mm in diameter and 25 mm in length, Suzhou Acupuncture Supplies, Suzhou, Jiangsu Province, China) were used by Doctor Chen, who has 5 years of acupuncture experience. In addition, electrical stimulation was given from a medical electro-acupuncture apparatus (type SDZ-II, Suzhou Medical Appliance Factory, Suzhou, Jiangsu Province, China), with a frequency of 30 Hz disperse waves and 100 Hz dense waves and 5 V intensity for 20 minutes below the level that induced visible muscle contraction.
Neurological function assessment
Neurological function was assessed on days 7, 14 and 28 using a modi fi ed neurological severity score by two individuals blinded to the experimental groups, as described previously[40-41]. In brief, this score is derived by assessing animals for hemiparesis (response to raising the rat by the tail or placing the rat on a fl at surface), sensory de fi cits (i.e., visual, tactile, placing, proprioception), beam balance tests (response to placement and posture on a narrow beam and time before dropping), absent re fl exes (pinna, corneal, startle), and abnormal movement (seizure, myoclonus, myodystony). One point is awarded for the inability to perform a task or for the lack of a tested re fl ex. Neurological functions were graded on a scale of 0 to 18 (normal score = 0; maximal de fi cit score = 18), and higher scores represent a more serious nerve injury.
Immunohistochemistry for vascular endothelial growth factor
After anesthesia, rat brains were fi xed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde (pH 7.4; Sigma-Aldrich, St. Louis, MO, USA). Brains were removed carefully and fixed in paraformaldehyde solution. After paraffin embedding, a series of adjacent 3-μm-thick coronal cryostat sections were cut from the brain tissue (anteroposterior = –1.0 to 1.0 from the needle hole). Following a series of procedures, including xylene dewaxing, gradient ethanol hydration and tap water washing, the slices were boiled in antigen retrieval solution (EDTA buffer, pH 6.0). The glass slides were cooled to room temperature and rinsed three times with PBS. The sections were incubated in mouse anti-vascular endothelial growth factor monoclonal antibody (1:3,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. The following day, the slices were washed three times with PBS for 5 minutes each time. Each slice was incubated with 50 μL MaxVision™reagent (containing goat anti-mouse IgG complex; Fuzhou Maxim Biotech, Fuzhou, Fujian Province, China) at room temperature for 20 minutes, followed by three PBS rinses. Each slice was supplemented with 100 μL of enhanced fresh 3,3′-diaminobenzidine solution (Fuzhou Maxim Biotech, China). The sections were rinsed with tap water, counterstained with hematoxylin, rinsed with tap water, and then processed by gradient ethanol hydration, xylene transparency, and mild gum mounting. Then, the region around the cerebral ischemia focus was observed under a light microscope (Leica DC, Germany) with 200 × magni fi cation. The mean number of positive cells per six non-overlapping fi elds from each rat was counted.
Statistical analysis
Measurement data were analyzed using SPSS 15.0 software (SPSS, Chicago, IL, USA), and are expressed as mean ± SD. Signi fi cance was analyzed using one-way analysis of variance and the least signi fi cant difference test, and a level of P < 0.05 was considered statistically signi fi cant.
Acknowledgments:We would like to show our deepest gratitude to Professor Wang LP (Research Center for Neural Engineering, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences), Professor Xiong YQ, Professor Liu XL, Ms. Mu GP (Department of Central Laboratory, Shenzhen Traditional Chinese Medicine Hospital), Professor Shao MM (Department of Pathology, Shenzhen Traditional Chinese Medicine Hospital) and Professor Feng J (Department of Maternity, Shenzhen Traditional Chinese Medicine Hospital) for excellent advanced technical assistance.
Author contributions:Yu HB conceived and designed the study, conducted experiments, collected and interpreted the data, and wrote the manuscript. Chen PD conceived and designed the study and wrote the manuscript. Yang ZX conducted the experiments, and collected and interpreted the data. Luo WS conceived the study, interpreted the data, and wrote the manuscript. Pi M assisted with study implementation. Wu YG and Wang L conducted the experiments, and collected and interpreted the data. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Peer review:The present study indicated that electro-acupuncture at Conception and Governor vessels combined with HUCBMSC transplantation could up-regulate VEGF expression and improve neurological deficit function for cerebral ischemia/reperfusion rats, which provide novel ideas and experimental evidence for transformed medicine research in the combined treatments for cerebral ischemia.
[1] Lavados PM, Hennis AJ, Fernandes JG, et al. Stroke epidemiology, prevention, and management strategies at a regional level: Latin America and the Caribbean. Lancet Neurol. 2007;6(4):362-372.
[2] Baldwin K, Orr S, Briand M, et al. Acute ischemic stroke update. Pharmacotherapy. 2010;30(5):493-514.
[3] Del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354(6):553-555.
[4] Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156(3):965-976.
[5] Carmeliet P, Coleen D. Molecular analysis of blood vessel formation and disease. Am J Physiol. 1997;273(5 Pt 2):H2091-H2104.
[6] Chen J, Li Y, Wang L, et al. Therapeutic bene fi t of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005-1011.
[7] Chung DJ, Choi CB, Lee SH, et al. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J Neurosci Res. 2009;87(16):3554-3567.
[8] Liao W, Xie J, Zhong J, et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation. 2009;87(3):350-359.
[9] Lin YC, Ko TL, Shih YH, et al. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42(7):2045-2053.
[10] Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1(2):92-100.
[11] Luo WS, Yu HB, Yang ZX, et al. In fl uence of Ren and Du meridian electro-acupuncture on neural stem cell proliferation and extracellular signal-regulated kinase pathway in a rat model of focal cerebral ischemia injury. Neural Regen Res. 2010;5(6):433-438.
[12] Yang ZX, Chen PD, Yu HB, et al. Research advances in treatment of cerebral ischemic injury by acupuncture of conception and governor vessels to promote nerve regeneration. J Chin Integr Med. 2012;10(1):19-24.
[13] Wang SJ, Omori N, Li F, et al. Functional improvement by electro-acupuncture after transient middle cerebral artery occlusion in rats. Neurol Res. 2003;25(5):516-521.
[14] Kim M, Chung YC, Jung HC, et al. Scalp electroacupuncture at the Baihui acupoint (DU 20) improves functional recovery in rats with cerebral ischemia Association with increased expression of vascular endothelial growth factors. Neural Regen Res. 2011;6(36):2822-2828.
[15] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defi ning multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
[16] Yang ZX, Ma XM, Yu HB, et al. Effects of electroacupuncturing Ren Vessel on protein and mRNA expression of bFGF after focal cerebral ischemia-reperfusion in rats. Zhonghua Zhongyiyao Xuekan. 2009;27(8):1580-1583.
[17] Ma XM, Yang ZX, Yu HB, et al. Effects of electroacupuncturing Ren Vessel on expression of EGF after focal cerebral ischemia-reperfusion in rats. Zhonghua Zhongyiyao Xuekan. 2011;29(7):1602-1605.
[18] Wang Q, Wang F, Li X, et al. Electroacupuncture pretreatment attenuates cerebral ischemic injury through 7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J Neuroin fl ammation. 2012;9:24.
[19] Wang Q, Peng Y, Chen S, et al. Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke. 2009;40(6):2157-2164.
[20] Diao LH, Yang ZX, Yu HB. Exploration on Ren Meridian correlating with brain. Liaoning Zhongyi Zazhi. 2006;33(4):398-399.
[21] Luo WS, Rao XD, Zeng CG, et al. Theoretical study of acupuncture therapy on Du Vessel for ischemic cerebrovascular disease. Zhongguo Xiandai Yisheng. 2011;49(3):14, 19.
[22] Yang FX, Yang ZX, Yu HB, et al. Regulating and unblocking acupuncture therapy in the treatment of patients with convalescent cerebral infarction. Zhenjiu Linchuang Zazhi. 2011;27(4):48-50.
[23] Yancopoulos GD, Davis S, Gale NW, et al. Vascular-speci fi c growth factors and blood vessel formation. Nature. 2000;407(6801):242-248.
[24] Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19(14):5731-5740.
[25]Fredduzzi S, Mariucci G, Tantucci M, et al. Nitro-aspirin (NCX4016) reduces brain damage induced by focal cerebral ischemia in the rat. Neurosci Lett. 2001;302(2-3):121-124.
[26] Harris SR, Schoeffner DJ, Yoshiji H, et al. Tumor growth enhancing effects of vascular endothelial growth factor are associated with increased nitric oxide synthase activity and inhibition of apoptosis in human breast carcinoma xenografts. Cancer Lett. 2002;179(1):95-101.
[27] Louissaint A Jr, Rao S, Leventhal C, et al. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945-960.
[28] Chen J, Zhang C, Jiang H, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25(2):281-290.
[29] Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92(6):692-699.
[30]Chen J, Li Y, Wang L, et al. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189(1-2):49-57.
[31] Wechsler LR. Stem cell transplantation for stroke. Cleve Clin J Med. 2004;71 Suppl 1:S40-41.
[32] Bliss T, Guzman R, Daadi M, et al. Cell transplantation therapy for stroke. Stroke. 2007;38(2 Suppl):817-826.
[33] Wang M, Zhang W, Crisostomo P, et al. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol. 2007;42(6):1009-1015.
[34] Yang ZX, Chen PD, Yu HB, et al. Thinkings on mechanisms of conception and governor vessel acupuncture underlying MSC transformation and differentiation into neurons after transplantation for cerebral ischemia. Liaoning Zhongyi Zazhi. 2012;39(9):1663-1666.
[35] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[36] Lim JY, Jeong CH, Jun JA, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2(5):38.
[37] Yang Z, Yu H, Rao X, et al. Effects of electroacupuncture at the conception vessel on proliferation and differentiation of nerve stem cells in the inferior zone of the lateral ventricle in cerebral ischemia rats. J Tradit Chin Med. 2008;28(1):58-63.
[38] Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20(9):1311-1319.
[39] Li ZR. Experimental Acupuncture. Beijing: China Press of Traditional Chinese Medicine, China. 2003.
[40] Chen Y, Constantini S, Trembovler V, et al. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive de fi cits. J Neurotrauma. 1996;13(10):557-568.
[41] Schallert T, Kozlowski DA, Humm JL, et al. Use-dependent structural events in recovery of function. Adv Neurol. 1997;73:229-238.
Copyedited by McGowan D, Zhang DS, Wang XM, Wang J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M
10.4103/1673-5374.125334
Zhuoxin Yang, M.D., Affiliated Shenzhen Maternity & Child Healthcare Hospital, Southern Medical University, Shenzhen 518028, Guangdong Province, China, dalexisu@126.com.
http://www.nrronline.org/
Accepted: 2013-11-25
杂志排行
中国神经再生研究(英文版)的其它文章
- Critical illness polyneuropathy and myopathy: a systematic review
- Lipid rafts participate in aberrant degradative autophagic-lysosomal pathway of amyloid-beta peptide in Alzheimer’s disease
- Hippocampal gene expression in a rat model of depression after electroacupuncture at the Baihui and Yintang acupoints
- A non-invasive, rapid method to genotype late-onset Alzheimer’s disease-related apolipoprotein E gene polymorphisms
- Mechanism underlying the protective effect of Kaixin Jieyu Fang on vascular depression following cerebral white matter damage
- Changes in brain functional network connectivity after stroke