Standardization and phytochemical investigation of antilithiatic polyphyto dispersible tablets
2014-03-22AbhishekBharadwajKumudUpadhayayaStheeshMadhav
Abhishek Bharadwaj, Kumud Upadhayaya, N.V. Stheesh Madhav
1Department of Pharmacognosy, Himachal Institute of Pharmacy, Poanta Sahib (HP), India
2Department of Pharmacognosy, Kumaun University, Nanital Bhimtal, (UK), India
3Department of Pharmaceutics, Dehradun Institute of Technology, Dehradun (UK), India
Standardization and phytochemical investigation of antilithiatic polyphyto dispersible tablets
Abhishek Bharadwaj1*, Kumud Upadhayaya2, N.V. Stheesh Madhav3
1Department of Pharmacognosy, Himachal Institute of Pharmacy, Poanta Sahib (HP), India
2Department of Pharmacognosy, Kumaun University, Nanital Bhimtal, (UK), India
3Department of Pharmaceutics, Dehradun Institute of Technology, Dehradun (UK), India
Objective: To deals with the characterization, phytochemical determination of antilithiatic polyphyto dispersible tablet. Methods: an attempt has been made to standardize polyphyto dispersible tablet by using macroscopy and microscopic characters, powder microscopy, fluorescence analysis, quantitative and physicochemical values. Results: The polyphyto combinations were subjected to macroscopical examination and observations were recorded. The proper examination of the polyphyto combinations was carried out under sun light and artificial source similar to day light. Conclusions: Data reveals that phytotherapeutic agents could be useful as either an alternative or a complementary therapy in the management of urolithiasis.
ARTICLE INFO
Article history:
Received 8 August 2013
Received in revised form 15 September 2013
Accepted 24 September 2013
Available online 20 June 2014
Microscopic characters
1. Introduction
Kidney stone affects up to 5% of the world population[1]. In normal conditions; Oxalate (Ox), an end product of metabolism is excreted in urine. However, under certain pathological conditions, Ox interacts with calcium within the renal tubular lumen to form calcium oxalate (CaOx). In addition, studies show that renal cells on exposure to Ox and/or CaOx crystals generate reactive oxygen species (ROS), develop oxidative stress (OS) and associated cellular injury. Stone formation in the kidney, also called Nephrolithiasis or kidney stones, the male-to-female incidence ratio is 4:1. Eighty percent of calculi are composed of calcium (either oxalate or phosphate), with others composed of struvite, uric acid, or cystine[2]. Approximately 1 million Americans develop a kidney stone each year and an estimated 12% of the population forms a stone some time during their life[3]. Many stones are asymptomatic until they begin to move down the ureter, causing pain due to obstruction. The pain often begins suddenly when a stone moves in the urinary tract, causing irritation or blockage. Typically, a person feels a sharp, cramping pain in the back and side in the area of the kidney or in the lower abdomen. Sometimes nausea and vomiting occur[4]. Lithotripsy may be an alternative to surgery. In this extra-corporeal shockwave lithotripsy, ultrasonic waves or shockwaves are used to break up stones so that they may be expelled in the urine or removed with an endoscope. The stones, once broken, pass out as fine sand particles. Stones are also removed using cystoscopy or ureteoscopy or percutaneous surgery. Nowadays, about 85% of all kidney stones contain calcium salts (calcium oxalate and/or calcium phosphate) as their main crystalline components. Because human urine is commonly supersaturated with respect to calcium salts as well as to uric acid, crystalluria is very common,i.e.healthy people excrete up to ten millions of microcrystals every day. Alternatively, crystal adhesion to urothelial surfaces may be enhanced in stone formers[5].
2. Material and method
The morphological studies were carried out for shape, size, colour, odour, taste and fracture of the polyphyto dispersible tablets.
2.1. Preparation of polyherbal formulation
Formulation was made by taking equal proportion of each powdered drugs. All the procured and authenticated individual drugs were dried in shade and cleaned by hand sorting. The individual drugs were then crushed using willing grinder and passed through mesh no. 40. The individual drugs were then weighed as per the quantity required. The drugs were mixed geometrically using a double cone blender. The mixed formulation was unloaded, weighed, and packed in labeled glass bottles[6].
2.2. Physicochemical standardization
The various physico-chemical values of polyphyto combination of such as ash value, extractive values, acid insoluble ash, loss on drying, bulk density and true density pH, angle of repose Foreign organic matter were determined according to the pharmacopoeial method[7,8].
2.3. Evaluation of poly-herbal dispersible tablet
2.3.1. Thickness
The tablet thickness from each batch was measured using a digital vernier caliper[9].
2.3.2. Weight variation
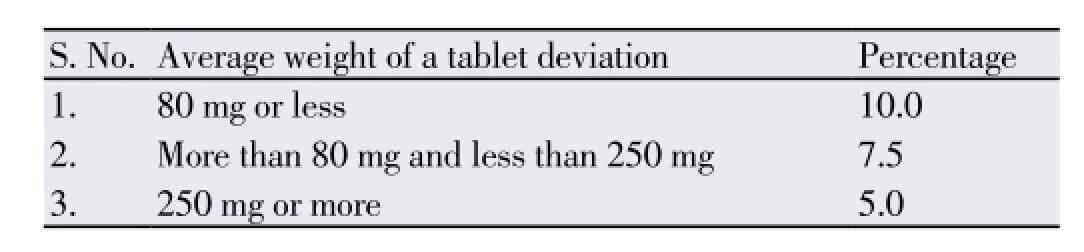
It is desirable that every individual tablet in a batch should be uniform in weight, but a small variation in the weight of the individual tablet is liable to occur. Therefore a litter variation is allowed in the weight of the tablet by the pharmacopoeia. The following percentage deviation in weight variation is allowed.
Weight 20 tablets selected at random and determine their average weight. Not more than 2 of the individual weights may deviate from the average weight by more than the percentage deviation given in the table and none should deviate by more than twice that percentage.
?
2.3.3. Dissolution test
The test is done for measuring the amount of time required for a given percentage of drug substance in a tablet to go into solution under specified conditionin vitro.The apparatus used for the test is as per specification given in I.P.
2.3.4. Method
Place 1 000 mL of water which should be free from dissolved air and previously warmed to 36.5 ℃ to 37.5 ℃into the vessel. Place the specified number of tablets in the dry basket. Set the apparatus. Start the moter and adjust the rotation speed to 100 rpm or as directed in the monograph. Withdraw the stated volume of solution from the vessel after 45 min or after the time specified in the monograph. Repeat the complete operation 4 times.
The tablets pass the test if for each of the 5 tablets; the amount of active ingredient is not less than 70 percent of the stated amount. In case were two or more tablets are directed to be placed together ion the basket for each test in the five replicate test, the amount of active ingredient in solution per tablet in each test should not be less than 70% of the stated amount or of the specified in the monograph. No re-testing is allowed[10].
2.3.5. Hardness
Hardness for six tablets from each formulation was determined using Monsanto hardness tester. The Monsanto chemical Co. Ltd had designed spring pressure device to test the hardness of a tablet. It has a graduated scale which gives the reading in kg/cm2. The tablet to be tested is placed between the spindle and the anvil. The desired pressure needed to hold the tablet in position is applied by moving the screw knob in clockwise direction. The scale is moved so that the indicator is fixed at zero. The pressure is then applied till the tablet breaks. The reading is noted, which indicates the pressure which is needed to break the tablet[11].
2.3.6. Friability test
Normally during the course of tablets a sufficient pressure is applied on the granules, so that the tablets can withstand the wear and tear during transportation and handling. But in spite of observing all the precautions, the tablets show considerable powdering after normal handling, giving an undesirable appearance. Friability test is performed to evaluate the ability of the tablet to withstand wear and tear in packing, handling and transporting. The apparatus used to perform this test is known as “friability.
The apparatus consists of a plastic chamber, which is divided into two parts and it revolves at a speed of 25 r.p.m. twenty tablets are weigh and placed in the plastic chamber. The chamber is rotated for 4 min or 100 revolutions. During each revolution the tablet falls from a distance of 6 inch. The tablets are removed for the chamber after 100 revolutions and weighed. Loss in weight indicates the friability. The tablets are considered to be of good quality if the loss in weight is less than 0.8%[12].
2.3.7. Bulk density
A powder is passed through a standard sieve No. 20. A weighed amount is introduced into a 100 mL graduated cylinder. The cylinder is fixed on the bulk density apparatus and the timer knob is set for 100 tappings. The volume occupied by the powder is noted. Further another 50 taps may be continued and the final volume is noted. For reproducible results, the process of tapings may be continued until concurrent volume is achieved. The final volume is the bulk volume. Then bulk density is calculated using equation[13].
Bulk Density = Weight of the sample / bulk volume
3. Result
3.1. Macroscopical evaluation
The polyphyto combinations were subjected to macroscopical examination and observations were recorded. The proper examination of the polyphyto combinations was carried out under sun light and artificial source similar to day light.
3.2. Description
a) Colour: Light brown
b) Odour: Characterstics
c) Taste: Bitter
3.3. Physico-chemical parameters
a) Loss on drying at 105 ℃: 6 % w/w
b) Total-ash: 9 g
c) Acid insoluble ash: 32% w/w
d) Alcohol soluble extractive: 10.6% w/w
e) Water soluble extractive: 50.4% w/w
f) Chloroform soluble extractive: 11.6% w/w
g) pH: 7
h) Bulk density: 2 g/mL
i) True density: 0.001 g/mL
j) Angle of repose: tan-1(0.32)
k) Foreign organic matter: 62.9 % w/w

Table 1 Phytochemical screening.

Table 2 Quantitative evaluation of tablet from of drugs.
4. Discussion
The use of the herbals as medicine is an age old tradition which will serve as a source of alternative medicine in future also and help to overcome the toxic effects of the synthetic drugs. The results have come out to be very motivating and further pharmacological study could be carried out on the samples to reveal an effective drug for the urolithiasis. Data reveals that phytotherapeutic agents could be useful as either an alternative or a complementary therapy in the management of urolithiasis.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgment
The authors are thankful to the chairman, director and principal of the Himachal Institute of pharmacy, Paonta Sahib for providing necessary facilities to carry out the research work.
Reference
[1] Malvinder S Parmar. Kidney stones. BMJ 2004; 328(7453): 1420-1424.
[2] Integrative Medicine. Quick access professional guide to conditions, herbs and supplements. 1st Edition. New York: Integrative medicine communications; 2000.
[3] Gerstenbluth RE, Resnick ML. Medical management of calcium oxalate urolithiasis. Med Clin North Am 2004; 88: 431-442.
[4] Kidney Stones in Adults national kidney and urologic disease information clearing house (NKL a service of the national institute of diabeties and digestive and kidney disease Page: 1-14.
[5] Hess B. Pathophysiology, diagnosis and conservative therapy in calcium kidney calculi. Ther Umsch 2003; 60(2): 79-87.
[6] Anonymous. The Ayurvedic Formulary of India. 2nd ed. New Delhi: Government of India, Ministry of Health and Family Welfare; 2003, p. 113.
[7] Ananymous. Quality control methods for medicinal plant materials. Geneva: World Health Organisation; 1998, p. 25-28.
[8] Ajay Kr Meena, MM Rao, P Panda, Kiran, Ajay Yadav, Uttam Singh, et al. Standardisation of ayurvedic polyherbal formulation, Pancasama Churna. Int J Pharma Phytochem Res 2010; 1: 11-14.
[9] Leon Lachman, Herbert A. Lieberman, Joseph L. Kanig. The Theory and Practice of Industrial Pharmacy, 3rd Edn, Bombay: Varghese Publishing House; 1987.
[10] British Pharmacopoeia Commission. British Pharmacopoeia 2007, Vol III. London: The stationery office; 2276.
[11] Anoop Kumar Singh, Panner Selvam R, Sivakumar T. Isolation, characterisation and formulation properties of a new plant gum obtained from mangifera indica. Int J Pharm Biomed Res 2010; 1: 35.
[12] British Pharmacopoeia Commission. British Pharmacopeia 2007, Vol IV. London: The stationery office, Appendix XVII: HA397.
[13] Subrahmanyan CBS. Text Book of Physical Pharmacy 2nd edition. 2008, p. 215-217.
ment heading
10.1016/S2221-6189(14)60032-9
*Corresponding author: Abhishek Bharadwaj, Department of Pharmacognosy, Himachal Institute of Pharmacy, Poanta Sahib (HP), India.
Tel: +91-9736439021
E-mail: abhishekbbhardwaj@gmail.com
Fluorescence analysis
Physico-chemical
Standardization
杂志排行
Journal of Acute Disease的其它文章
- Submersion and acute respiratory failure
- A review on some poisonous plants and their medicinal values
- Laboratory diagnosis, clinical manifestations, epidemiological situation and public health importance of cutaneous leishmaniasis in Shushtar County, Southwestern Iran
- Antihyperglycemic and antihyperlipidemic properties of aqueous root extract of Icacina senegalensis in alloxan induced diabetic rats
- Neuroprotective and antioxidant role of Phoenix dactylifera in permanent bilateral common carotid occlusion in rats
- The ameliorative effects of Averroha carambola on humoral response to sheep erythrocytes in non-treated and cyclophosphamideimmunocompromised mice
