The safety parameters of the study on intraductal cytotoxic agent delivery to the breast before mastectomy
2014-03-21BailinZhangSusanLoveGuojiChenJingWangJidongGaoXiaozhouXuZhongzhaoWangXiangWang
Bailin Zhang, Susan M. Love, Guoji Chen, Jing Wang, Jidong Gao, Xiaozhou Xu, Zhongzhao Wang, Xiang Wang
1Department of Breast Surgery, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China;2Dr. Susan Love Research Foundation, Santa Monica, CA 90403, USA
Correspondence to: Xiang Wang. Department of Breast Surgery, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China. Email: xiangw@vip.sina.com.
The safety parameters of the study on intraductal cytotoxic agent delivery to the breast before mastectomy
Bailin Zhang1, Susan M. Love2, Guoji Chen1, Jing Wang1, Jidong Gao1, Xiaozhou Xu1, Zhongzhao Wang1, Xiang Wang1
1Department of Breast Surgery, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China;2Dr. Susan Love Research Foundation, Santa Monica, CA 90403, USA
Correspondence to: Xiang Wang. Department of Breast Surgery, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China. Email: xiangw@vip.sina.com.
Background:Intraductal administration of cytotoxic agents has been shown to inhibit the development of breast cancer in animal models. The object of this study was to demonstrate the safety of intraductal delivery cytotoxic agents in patients prior to mastectomy. This method is hopeful to be developed as a chemoprevention approach in patients with pre-malignant or non-invasive ductal lesions to prevent breast cancer which will be further developed.
Methods:Two drugs, pegylated liposomal doxorubicin (PLD) and carboplatin were administered at three dose levels (PLD: 10, 20, 50 mg and carboplatin 60, 120, 300 mg). There were fve subjects in each group with 15 subjects treated with each drug once. Venous blood samples were obtained for pharmacokinetic analysis. The breast was removed surgically 2-5 days post administration and the treated ducts were marked to enable identifcation on pathological evaluation.
Results:Intraductal administration was generally well-tolerated with mild, transient breast discomfort. In the carboplatin arm, three women at the 300 mg dose experienced mild nausea and vomiting. In the PLD arm most women had mild erythema and swelling of the breast over the 72 hours following the drug administration. Patients receiving the 50 mg dose experienced local erythema until the time of surgery. Pharmacokinetic analysis showed that carboplatin rapidly entered systemic circulation with an early peak time (Tmax~30 min) with a corresponding plasma ultrafltrate area under the curve (AUC) consistent with the Calvert Formula using estimated glomerular fltration rate (GFR). Total plasma doxorubicin had delayed peak concentration times (Tmax>48 hours) with a linear dose response and peak concentrations substantially lower than expected from equivalent intravenous injection dosing. No doxorubicinol metabolite was detected in the plasma.
Conclusions:This study demonstrates that cytotoxic drugs can be safely administered into breast ducts with minimal toxicity.
Safety; intraductal therapy; cytotoxic agents; breast cancer
View this article at:http://dx.doi.org/10.3978/j.issn.1000-9604.2014.10.06
Introduction
Breast cancer is the most frequent cancer of women with an estimation of 1.38 million new cases globally in 2008 (1) and 29% (about 0.22 million) of all new cancer cases among women in the United States in 2012 (2). Because of the increased incidence in breast cancer, many studies have been done to identify those women at risk for breast cancer and ways to prevent it from occurring. Currently, several different statistical models exist which attempt to predict the relative risk of women to develop breast cancer. These models are based upon the patient’s medical history aswell as family history related to cancer, specifcally breast cancer (3-5). These models have been useful in identifying patients at increased risk for developing breast cancer, but there remain few viable methods to prevent breast cancer. The current available options for prevention are oophorectomy, bilateral mastectomy, or pharmaceutical therapy, such as tamoxifen (6-8), while many drugs have been researched with regards to prevention, aromatase inhibitors (AI) (9) and other selective estrogen receptor modulators (SERM) (10).
Although, there is considerable work being done systemically with pharmaceuticals such as SERMs and AIs to prevent breast cancer, little work with regards of stopping carcinomatous changes has been done locally in the breast. It is recognized that the majority of breast cancer begins in the lining of the duct (11). Atypical lesions, such as atypical ductal hyperplasia (ADH) are thought to progress to ductal carcinoma in situ (DCIS) and to invasive cancer (12,13). Therefore it makes considerable sense to attempt to identify these changes and stop them from ever developing into carcinoma. Providing local intervention methods into the ducts could reduce the morbidity associated with prevention while targeting the potential carcinoma cell. Initial research shows much promise with regard to intraductal infusion in order to reduce the risk of breast cancer. Researchers have recently demonstrated the feasibility of intraductal delivery of epirubicin and paclitaxel (14,15). These intraductal methods did not produce severe toxic side effects and significantly reduced tumor burden. Additionally, some researchers also tested the efficacy of intraductal administration of the anti-cancer agents 4-hydroxy tamoxifen (4-OHT) and pegylated liposomal doxorubicin (PLD), in the prevention of breast cancer using the rat models of breast cancer (16). Carboplatin, an analog of cisplatin, is an alkylating agent used in the treatment of many cancers. PLD is doxorubicin encapsulated in long-circulating STEALTH®liposomes, which improve stability of the drug in the circulation. This pattern of drug distribution leads to enhanced localization of doxorubicin in tumor tissues and malignant tumor exudates.
Based on preclinical and clinical data, the intraductal method is being developed to eliminate precancerous cells inside mammary ductus. The main goal of this method is focus on “chemical stopping” of canceration. This clinical study represents a discreet step to assess the safety of intraductal administrating cytotoxic agents’ methods. In this paper, we describe and discuss the safety characters of this method.
Subjects, materials and methods
Subjects
This study was approved by the Institutional Review Board (IRB) of the Cancer Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College. A total of 31 subjects with a median age of 51 years (range, 25-75 years) were evaluated in this study. All patients were pathological diagnosed of invasive carcinoma of breast and scheduled to receive chemotherapy after mastectomy. The subjects had normal hematopoietic, cardiac, pulmonary, renal and hepatic functions and signed informed consents prior to entering this study. One subject in the PLD arm withdrew from study because research drug cannot be injected in.
Cytotoxic agent administration and pathological examination
After the studied nipple was cleaned, an injection of 1 mL of lidocaine mixed with 0.1 mL blue dye was made into the base of the nipple. At this point, patients were asked to assess their pain using a visual analog scale (VAS). Investigator inserted catheters into studied ducts and instilled 1ml of radio-opaque dye into the ducts. Then, 0.5-6 mL (depending on the number of ducts identified, the drug used and the group) of the chemotherapy drug were slowly injected into each duct (doses of drug are listed in Table 1). The total procedure took 30 minutes to one and half hours. The operation of mastectomy was performed at least 48 hours to five days after this procedure. After the intraductal administration of the cytotoxic agents was complete, subjects were assessed for local and systemic side effects. Subjects completed a pain assessment at completion of procedure, then at 0.5, 1, 4, 12 h and then daily after study related procedures were completed. Subjects were assessed for pain; adverse events (AE), ECOG performance scale, blood drawn per drug schedule and vital signs (blood pressure, respiratory rate, pulse, temperature) recorded.
Pathological examination was performed after the operation. All sections were reviewed by two pathologists without specific knowledge of dose levels of each drug treatment arm. Inflammatory response and ductal epithelial cell change were graded as no change, mild, moderate, or severe change. The detailed methods and results of cytotoxic agents’ administration and pathological examination have been described by Dr. Love previously (17).
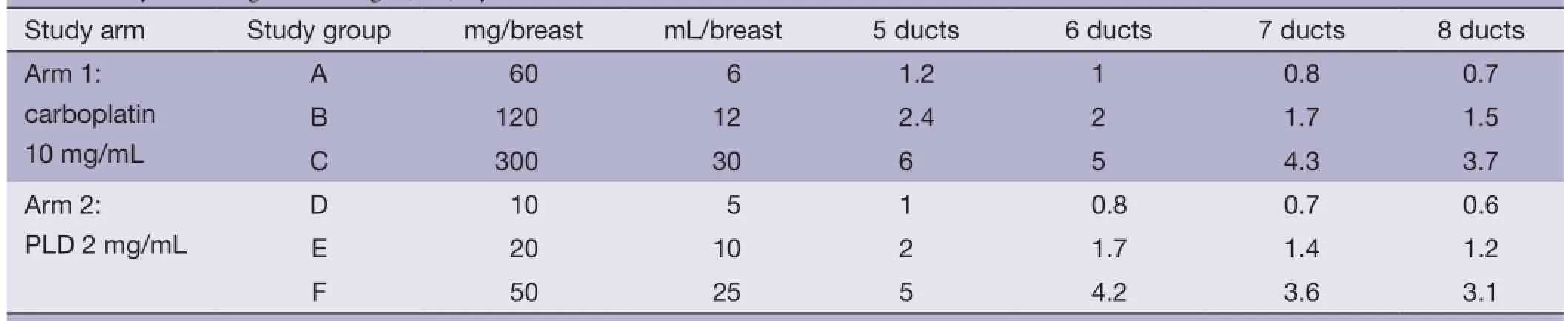
Table 1 Cytotoxic agents’ dosage (mL) by ducts

Table 2 Venous draw schedule
Pain and systemic effects assessment post intraductal administration
After the intraductal administration of the cytotoxic agents was complete, subjects were assessed for pain, local and systemic AE. These assessments were performed at completion of procedure, 0.5, 1, 4, 12, 24 h after and then daily after study related procedures were completed.
Pharmacokinetic analysis
Subjects had blood drawn several times as described in Table 2. Humam plasma samples were analyzed at the Chinese Center for Disease Control and Prevention, National Institute of Occupational Health and Poison Control (Beijing, China). The assay of concentration level of carboplatin was developed and validated in human plasma ultrafiltrate that was obtained from stored frozen plasma using fameless atomic absorption spectrometry for diction of platinum. Total plasma doxorubicin was assayed by highperformance liquid chromatograph-mass spectrometry (HPLC/MS).
Statistical methods
The pharmacokinetic parameters of each agent were calculated by WinNonlin. SAS version 9.1.3 was used for all statistical analysis. Descriptive statistics were used in this study and were tabulated by treatment group and overall: descriptive statistics (N, Mean, SD, Min, Median, and Max) for continuous variables and counts and percentages for categorical variables. Due to the low number of subjects in each treatment group, the statistic SD and percentages were for reference only.
Results
Pain assessment
During study drug instillation, pain was measured from 0 to 10. The mean of pain was 1.2 in carboplatin arm and 1.5 in PLD arm. A total of 26 subjects experienced pain, 13 subjects in each arm. Pain assessment was performed at 0, 0.5, 1, 4, 12, 24 h and daily after intraductal administration, the mean of pain ranged from 0.2 to 1.2 in the carboplatin arm and ranged from 0.4 to 1.3 in the PLD arm. No subjects felt pain at 72 and 96 h after intraductal administration (Table 3 and Figure 1). The types of pain were sharp pain, dull pain, stabbing pain, burning pain.
Systemic effects after intraductal administration
Two subjects experienced nausea at 72 h, one subject at 96 and 120 h in the carboplatin arm of dosage 60 mg. Two subjects experienced nausea at 48, 72 and 96 h in the carboplatin arm of dosage 120 mg. Two subjects experienced nausea at 12 h in the carboplatin arm of dosage 300 mg. One subject experienced nausea at 0.5 and 1 h in the PLDarm of dosage 20 mg. One subject experienced vomiting at 72, 96 and 120 h in the carboplatin arm of dosage 60 mg. three subjects experienced vomiting at 12 h in the carboplatin arm of dosage 300 mg. No subjects experienced diarrhea, rash and itching after intraductal administration. One subject experienced headache at 12 and 24 h in the PLD arm of dosage 20 mg. Two subjects experienced fatigue at 12, 24 and 48 h in the carboplatin arm of dosage 300 mg. No subjects experienced diarrhea and hair loss and rash and itching after intraductal administration. One subject experienced headache at 12 and 24 h in the PLD arm of dosage 20 mg. Two subjects experienced fatigue at 12, 24 and 48 h in the carboplatin arm of dosage 300 mg.

Table 3 Pain assessment after intraductal administration
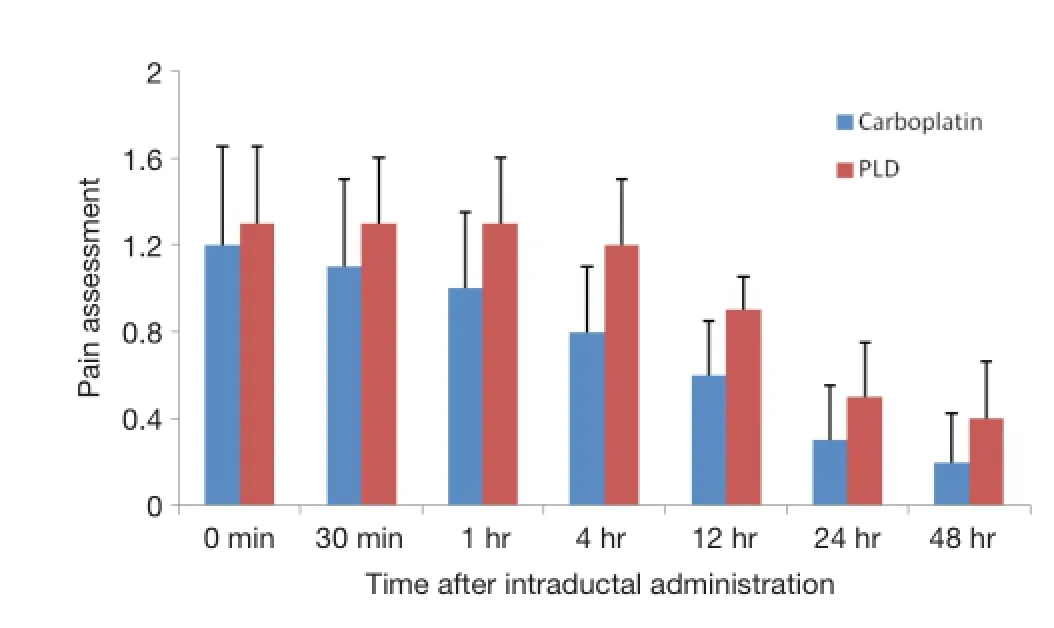
Figure 1 Pain assessment after intraductal administration of carboplatin and PLD. PLD, pegylated liposomal doxorubicin.
Systemic doxorubicin exposure was erratic in each dosing group. AUC was poorly characterized from the time points collected. Total plasma doxorubicin had delayed peak concentration times (Tmax>48 h) with a linear dose response and peak concentrations substantially lower than expected from equivalent intravenous injection dosing. No doxorubicinol metabolite was detected in the plasma and no systemic toxicity was observed.
Safety analysis
All 30 subjects experienced a total of 123 AE. Fifteen subjects in the carboplatin arm experienced 61 AE (17 AE in the dosage 60 mg and dosage 120 mg, 27 AE in the dosage 300 mg) and 15 subjects in the PLD arm experienced 62 AE (18 AE in the dosage 10 mg, 24 AE in the dosage 20 mg, 20 AE in the dosage 50 mg). All of the AE were mild to moderate.
Of the 30 subjects, 21 subjects (70%) experienced a total of 62 drug related AE. Six subjects in the carboplatin arm experienced 19 AE (1 subject in the dosage 120 mg experienced 1 AE and 5 subjects in the dosage 300 mg experienced 18 AE), and 15 subjects in the PLD arm experienced 43 AE (14 AE in the dosage 10 mg and 16 AE in the dosage 20 mg and 13 AE in the dosage 50 mg). There was no severe related AE in the two arms (Table 4 and Figure 2). No serious AE was reported during this study and no AE withdrew from this study prematurely. No subject in each arm had newly occurred abnormality with clinical signifcance pre- and post-mastectomy. The liver and renal function of all patients was normal. No clinically relevant shifts from baseline for the most of biochemistry parameters in each arm. Only one subject in the PLD arm of dosage 10 mg and one subject of 50 mg had a newly occurred abnormality of the parameter creatine kinase with clinicalsignificance post-mastectomy. There were no clinically relevant changes from baseline were observed in the vital signs parameters. ECOG performance statuses of all 30 subjects were asymptomatic at baseline and pre-mastectomy.


Figure 2 Adverse events and types related to body system in different dosage of carboplatin and PLD. PLD, pegylated liposomal doxorubicin.
After more than 7 years of following up, 28 subjects is still living without locally adverse effect. Two subjects died of metastasis of breast cancer.
Pharmacokinetic analysis
Pharmacokinetic evaluation demonstrated that there were detectable levels of both drugs in the systemic circulation. Using Compartmental model, the mean of AUC was 33,107.9 h×μg/L, and the mean of Cmax(Maximum observed concentration) was 2,352.7 μg/L, and the mean of Tmax(observed time at which Cmaxoccurred) was 0.5 h in the carboplatin arm of dosage 60 mg. The mean of AUC was 35,768.7 h× noncompartmental g/L, and the mean of Cmaxwas 4,714.1 μg/L, and the mean of Tmaxwas 0.5 h in the carboplatin arm of dosage 120 mg. The mean of AUC was 253,039.2 h×μg/L, and the mean of Cmaxwas 9,380.4 μg/L, and the mean of Tmaxwas 0.8 h in the carboplatin arm of dosage 300 mg.
Using noncompartmental model, the mean of AUC(0-∞) was 34,883.2 h×μg/L, and the mean of AUC(0-t) was 20,727.8 h×μg/L, and the mean of Cmaxwas 2,375.9 μg/L, and the mean of Tmaxwas 0.5 h in the carboplatin arm of dosage 60 mg. The mean of AUC(0-∞) was 39,343.5 h×μg/L, and the mean of AUC(0-t) was 31,730.3 h×μg/L, and the mean of Cmaxwas 4,546.6 μg/L, and the mean of Tmaxwas 0.5 h in the carboplatin arm of dosage 120 mg. The mean of AUC(0-∞) was 138,496.5 h×μg/L, and the mean of AUC(0-t) was 111,869 h×μg/L, and the mean of Cmaxwas 11,740.9 μg/L, and the mean of Tmaxwas 0.5 h in the carboplatin arm of dosage 300 mg (Table 5 and Figure 3). The parameters of PLD were not available due to the low plasma level, and the summary statistics couldn’t be calculated.
Discussion
Investigation of the role that the intraductal route may play in the treatment and prevention of breast cancer is in its infancy (18). Currently, cancer chemotherapy is administered predominantly through the systemic route orally or by intravenous injection. Although systemic administration is the most efficient route of delivery to cancers in many organs, it also exposes all healthy tissues to the delivered drugs, frequently resulting in harmful side effects. The goal of this study is to exploring a local, minimally invasive method which will be used in the prevention of breast cancer.

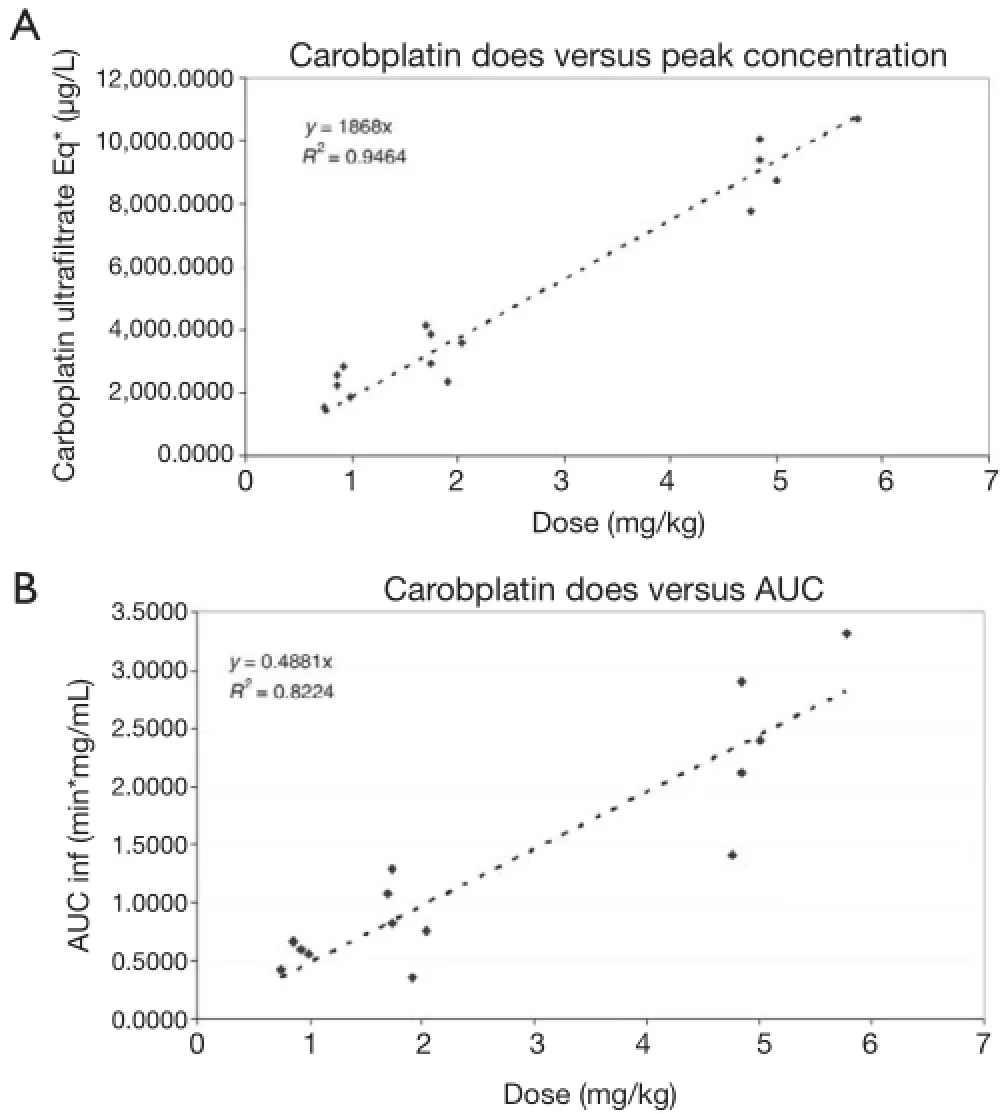
Figure 3 Pharmacokinetics of intraductal administration of carboplatin. (A) Dose versus peak concentration; (B) dose versus AUC. This fgure has been previously published by Dr. Love (17). AUC, area under the curve.
This trial was designed as a mono-centre, uncontrolled observational dose escalation study, to evaluate the feasibility and safety of intraductal administration of cytotoxic agents (carboplatin and PLD) in women with breast cancer prior to mastectomy and to study the pathological effects on breast cancer cells. We demonstrated in this study that intraductal administrated with carboplatin or PLD can be easily and safely performed with acceptable advised effects in patients with breast cancer. There was a dose-response increase in mild to moderate inflammatory response and ductal epithelial cell changes in subjects in carboplatin groups. Furthermore, we also found dose-response increase in the ducal epithelium cell changes suggestive of cellular degeneration in patients treated with carboplatin. For PLD, there was a trend of increased inflammatory response in nipple, dye stained ducts and stromal tissue. Compared with ducts without blue dye, epithelial response to PLD in ducts with dye was significant increased with all dose levels (17). The safety of PLD has been reported by Serarns who performed a neoadjuvant intraductal trial in 17 women scheduled for mastectomy for invasive carcinoma (19). No serious AE as a result of the treatment were reported in that study.
The pharmacokinetic population included all subjects who received the study drug through intraductal administration and provided results that could be evaluated. It was demonstrated that plasma drug concentrations were lower and drug concentrations in the breast were higherin women who received intraductal PLD compared with those who received intravenous PLD (19). This result also support our hypothesis that duct administration of chemotherapeutic drugs offers the potential of providing prolonged drug concentrations at the site of disease and minimal systemic exposure to these toxic agents. This method has been found success in treatment of bladder cancer and intraperitoneal treatment of ovarian cancer (20,21). In breast cancer, local therapy could enable both anatomical and molecular targeted therapy, possibly eliminating the need for surgery altogether by providing a pharmacological method for eradicating premalignant tissue in the breast. This “chemical mastectomy” could be used as a strategy for breast cancer prevention.
Safety parameters of all 30 subjects were acceptable. For most of the laboratory parameters, no subject in each arm had newly occurred abnormality with clinical signifcance at pre- and post-mastectomy, just the biochemistry parameter creatine kinase of two subjects had newly occurred clinically significant abnormalities at post-mastectomy. Vital signs and ECOG performance statuses of all 30 subjects showed no clinically signifcant abnormalities.
Conclusions
It can be concluded from this study that it is feasible and safe for the intraductal administration of cytotoxic agents (carboplatin and PLD) in women with breast cancer prior to mastectomy. All the drug dosage groups were well tolerated by the subjects. Our analysis results suggest that the intraductal method has the potential of being used as chemoprevention in patients with precancerous lesions. Further studies should be conducted to prove this probability.
Acknowledgements
This research was sponsored by Windy Hill Medical, Inc., a privately held company, which is no longer in existence. We thank the Cancer Institute of the Chinese Academy of Medical Sciences (CICAMS) with providing their expertise in the development of this study. We also thank Windy Hill Medical, Inc. for assisting us with completing this project successfully.
Author contributions: Conception/design: Xiang Wang and Susan M. Love; Provision of study material or patients: Bailin Zhang, Jing Wang, Ji-Dong Gao, and Guo-Ji Chen; Collection and/or assembly of data: Bailin Zhang, Xiaozhou Xu, and Zhongzhao Wang; Data analysis and interpretation: Bailin Zhang and Susan M. Love; Manuscript writing: Bailin Zhang and Susan M. Love; Final approval of manuscript: Bailin Zhang, Susan M. Love, Jing Wang, Jidong Gao, Guoji Chen, Xiaozhou Xu, Zhongzhao Wang, and Xiang Wang.
Disclosure: The authors declare no confict of interest.
1 Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2 Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29.
3 Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst 1999;91:1541-8.
4 Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994;73:643-51.
5 Jones JL, Hughes KS, Kopans DB, et al. Evaluation of hereditary risk in a mammography population. Clin Breast Cancer 2005;6:38-44.
6 Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol 2006;7:223-9.
7 Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol 2005;23:7491-6.
8 Fisher B, Costantino JP, Wickerham DL, et al.Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study.J Natl Cancer Inst. 2005;97:1652-62.
9 Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011;364:2381-91.
10 Riggs BL, Hartmann LC. Selective estrogen-receptor modulators -- mechanisms of action and application to clinical practice. N Engl J Med 2003;348:618-29.
11 Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst 1975;55:231-73.
12 Holland R, Hendriks JH, Vebeek AL, et al. Extent, distribution, and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet 1990;335:519-22.
13 Mai KT, Yazdi HM, Burns BF, et al. Pattern of distribution of intraductal and infltrating ductal carcinoma: a threedimensional study using serial coronal giant sections of the breast. Hum Pathol 2000;31:464-74.
14 Goulet RJ, Badve S, Brannon-Peppas L, et al. Pilot trial assessing the feasibility of intra-ductal delivery of epirubicin (epi)-containing nanoparticles (NP) via InDuct ® Microcatheter (IDBM). J Clin Oncol 2004:22,828.
15 Okugawa H, Yamamoto D, Uemura Y, et al. Effect of perductal paclitaxel exposure on the development of MNU-induced mammary carcinoma in female S-D rats. Breast Cancer Res Treat. 2005;91:29-34.
16 Murata S, Kominsky SL, Vali M, et al. Ductal access for prevention and therapy of mammary tumors. Cancer Res 2006;66:638-45.
17 Love SM, Zhang W, Gordon EJ, et al. A feasibility study of the intraductal administration of chemotherapy. Cancer Prev Res (Phila) 2013;6:51-8.
18 Okugawa H, Yamamoto D, Uemura Y, et al. Effect of perductal paclitaxel exposure on the development of MNU-induced mammary carcinoma in female S-D rats. Breast Cancer Res Treat. 2005;91:29-34.
19 Love SM, Zhang W, Gordon EJ, et al. A feasibility study of the intraductal administration of chemotherapy. Cancer Prev Res (Phila) 2013;6:51-8.
20 Shen Z, Shen T, Wientjes MG, et al. Intravesical treatments of bladder cancer: review. Pharm Res 2008;25:1500-10.
21 Echarri Gonzalez MJ, Green R, Muggia FM. Intraperitoneal drug delivery for ovarian cancer: why, how, who, what, and when? Oncology (Williston Park) 2011;25:156-65, 170.
Cite this article as:Zhang B, Love SM, Chen G, Wang J, Gao J, Xu X, Wang Z, Wang X. The safety parameters of the study on intraductal cytotoxic agent delivery to the breast before mastectomy. Chin J Cancer Res 2014;26(5):579-587. doi: 10.3978/j.issn.1000-9604.2014.10.06
10.3978/j.issn.1000-9604.2014.10.06
Submitted Aug 13, 2014. Accepted for publication Sep 09, 2014.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Clinical significance of stanniocalcin expression in tissue and serum of gastric cancer patients
- Inhibition of non-small-cell lung cancer cell proliferation by Pbx1
- Experimental study on enhancement of the metastatic potential of portal vein tumor thrombus-originated hepatocellular carcinoma cells using portal vein serum
- Prognostic prediction in gastric cancer patients without serosal invasion: comparative study between UICC 7thedition and JCGS 13thedition N-classification systems
- Value of18F-FDG PET/CT and MRI in diagnosing primary endometrial small cell carcinoma
- Dermatosis as the initial presentation of gastric cancer: two cases
