基于花菁的硫醇近红外比率荧光探针
2014-02-23朱东建
朱东建,江 华,2*
(1.北京分子科学国家实验室,中国科学院 化学研究所 光化学重点实验室,北京100190;2.北京师范大学 化学学院,北京100875)
生物体内的硫醇如半胱氨酸(Cys)、同型半胱氨酸(Hcy)、谷胱甘肽(GSH)在生理和病理过程中起至关重要的作用[1-2]。细胞内硫醇水平的改变与很多疾病密切相关。体内缺乏半胱氨酸(Cys)会导致多种病症,如儿童生长缓慢,肝损伤和皮肤损伤等[3-5]。血液中同型半胱氨酸(Hcy)的浓度增加会导致维生素B12的缺失和老年痴呆症[6-7]。谷 胱 甘 肽 (GSH)在 细 胞 内 含 量 在 1 mmol/L到15mmol/L 之间[8],是细胞内最富裕的硫醇,在维持细胞的氧化还原动态平衡中起着重要作用[9]。因此,检测生物体系中硫醇含量具有非常重要的意义。
目前,用于检测硫醇的方法有很多,如高效液相色谱法[10-11]、电化学法[12-13]、荧光法等。相比其他方法,荧光法由于具有选择性好、灵敏度高、快速简便等优点,因此,近年发展了许多检测硫醇的荧光探针[14-20]。由于生物样品基体的自身荧光波长一般小于600nm[21],而大多数探针的荧光发射波长与生物样品的背景荧光有重叠,因此极大地限制了这类荧光分析法灵敏度,而在近红外荧光(λem>600nm)光区,生物样品基体光吸收或荧光强度很小,因而背景干扰大大降低。因此,发展近红外硫醇探针是一项具有挑战性的工作。目前有少量近红外硫醇探针通过单个荧光信号强度的增加来达到检测目的[22-23],而荧光强度很容易受到其他因素(如样品环境条件、探针浓度等)的影响,因此,此类探针并不能提供足够的精度以进行定量的检测。具有自校正作用的、通过两个波长的变化指示识别过程的比率测量型荧光探针则能很好的实现定量检测[24]。
本工作根据我们以前设计的近红外比率荧光探针的原理[25],设计合成了以七甲川花菁为荧光发色团、二硫键为硫醇的特异性识别位点的硫醇近红外比率荧光探针分子CySS。
1 实验部分
1.1 仪器和主要试剂
UV-2500紫外-可见分光光度计(日本岛津公司),F-4600荧光分光光度计(日本日立公司),Bruker AVANCE-400核磁共振仪(美国 Bruker公司),APEXII型FT-ICR质谱仪(美国Bruker公司),FV-1000共聚焦显微镜(日本 Olympus)。
细胞实验中所用NIH 3T3细胞(鼠成纤维细胞),标准PBS缓冲液购于协和医科大学细胞中心;培养基DMEM(Hyclone,American),标准胎牛血清FBS(Hyclone,American),双抗(青霉素,链霉素;Hyclone,American)购于北京拜尔迪生物技术有限公司。培养基的配置:DMEM溶液,10%的FBS,1%的双抗,摇匀,4℃保存。
所有合成原料与溶剂均为市售分析纯。半胱氨酸,同型半胱氨酸,谷胱甘肽,二硫苏糖醇(DTT),2-氨基乙硫醇(2-AET),2-巯基乙酸(2-MEA),葡 萄 糖 (Glu),丙 氨 酸 (Ala),缬 氨 酸(Val),丝氨酸(Ser),组氨酸(His),亮氨酸(Leu),赖氨酸(Lys),2-氨基乙酸(2-AEA),KCl,NaCl,CaCl2,MgSO4溶解在超纯水中制得溶液。柱色谱硅胶为烟台化学工业研究所产品(200~300目)。
1.2 吸收光谱与荧光光谱测定方法
探针CySS溶于DMSO配制成浓度为2.0 mmol/L的溶液,并于4℃下保存;紫外可见及荧光光谱均在缓冲溶液(100mmol/L HEPES,100 mmol/L NaCl,pH =7.4,DMSO∶水=5∶95(体积比))中测定;荧光量子产率的测定以Cardiogreen在 DMSO(Φ=0.13)作为荧光标准物[26]。
1.3 细胞培养及成像方法
NIH 3T3细胞以配置好的DMEM 培养基(10%FBS,1% 双抗),在25cm3的细胞培养瓶中,置于37℃培养箱中培养,每两天换一次新鲜的培养基;将细胞培养在35mm适合倒置共聚焦荧光显微镜测试的表面皿中,孵化48h,细胞贴壁状态良好后,在做成像实验前更换为无血清、无双抗的DMEM培养基进行染色。
在共聚焦显微镜下进行细胞荧光成像实验前,吸出培养基并用PBS冲洗三次,再加入1mL PBS。激发波长为635nm,荧光收集窗口700~770nm和780~800nm。
1.4 CySS的合成
CySS的合成路线如图1所示。

图1 CySS的合成路线和可能的反应机理The synthetic route of CySS and the possible reaction mechanism for thiols
在三口瓶中加入1.59g(15mmol)无水碳酸钠,高温除水后冷却到室温,抽换气三次后,在氩气保护下加入5mL无水甲苯,冰浴至0℃左右,滴加888.72mg(3mmol)三光气的甲苯溶液,滴加完搅拌15min,滴加294mg(1.5mmol)1[27]的甲苯溶液,滴加完缓慢升至室温反应3h后,用氩气赶走剩余的光气后,过滤抽干后,将其用10 mL无水二氯甲烷溶解,低温缓慢滴加到170mg(0.25mmol)CyN[25]和0.75mL (4.5mmol)N,N′-二异丙基乙胺(DIEA)的二氯甲烷溶液中,加完缓慢升至室温反应过夜,TLC检测有产物生成。反应完毕后用1mol/L盐酸洗两次,饱和氯化钠液洗一次,有机相用无水硫酸钠干燥,过滤旋干后,用硅胶色谱柱提纯得到绿色目标化合物CySS 45mg。产率20%。1HNMR (400MHz,CD2Cl2):7.42~7.37(m,6H),7.35~7.31(t,J=7.2Hz,2H),7.29~7.27(d,J=7.6Hz,2H),7.25~7.22(t,J=7.2Hz,3H),7.19~7.17(d,J=8.0 Hz,2H),6.10~6.06(d,J=14.0Hz,2H),4.76(s,2H),4.27~4.24(t,J=6.4Hz,2H),4.19~4.16(t,J=6.4Hz,2H),3.60(s,6H),2.81~2.76(m,5H),2.59~2.53(m,3H),2.07~2.04(m,2H),1.96(s,3H),1.26(s,12H)。13CNMR(400MHz,CD2Cl2):172.63,170.69,155.84,154.67,143.21,142.43,141.13,136.97,130.81,129.14,128.94,128.69,128.31,125.36,122.30,111.05,101.54,64.24,62.35,55.75,49.25,37.83,37.59,32.08,28.18,27.72,25.35,21.12,20.85。HRMS(ESI)for C46H54N3O4S2+([M-I]+):calcd:776.35503,found:776.35385。
2 结果与讨论
2.1 探针CySS对半胱氨酸的识别

图2 CySS加入半胱氨酸的紫外(a)和荧光(b)光谱(a)Absorption spectra of CySS(1μmol/L)0,15,30,45,60,90and 120min after addition of Cys(2mmol/L).(b)Fluorescence spectra of CySS(2μmol/L)every 5min within 2hafter addition of Cys(3mmol/L),λex= 676nm
如图 2(a)所示,1μmol/L CySS在 HEPES缓冲液中的最大吸收峰大约在785nm(ε=1.81×105L·mol-1·cm-1),向该缓冲溶液中加入2 mmol/L的半胱氨酸后,我们可以观察到,随着时间的变化785nm处的吸收强度减弱,与之同时在645nm处一个新的吸收峰不断增强,与CyN在HEPES缓冲液中的最大吸收峰一样[25],而且两者之间的吸收波长相差达到140nm。这些数据表明,当半胱氨酸加入到该反应体系后,探针分子CySS上面的二硫键与半胱氨酸反应,并且经过分子内进攻反应使酰胺键断裂释放出产物分子CyN,与我们提出的反应机理一致(如图1所示)。同时,我们也研究了荧光发射光谱的变化情况,如图2(b)所示,用676nm 激发,2mol/L CySS在HEPES缓冲液中的最大荧光发射峰大约在808 nm (Φ=0.035),向该缓冲溶液中加入3mmol/L的半光氨酸后,我们可以观察到,随着时间的变化808nm处的荧光发射强度逐渐减弱,与之同时在747nm处一个新的荧光发射峰不断增强,与CyN在HEPES缓冲液中的最大荧光发射峰相同[25],而且两者之间的吸收波长相差达到61nm,并且在793nm处产生一个等发射点。荧光发射光谱的变化同样证明了上述反应机理。
2.2 探针CySS对硫醇的选择性识别
CySS对硫醇有选择性识别的效果。我们向2 μmol/L CySS的HEPES缓冲溶液中分别加入3 mmol/L各种巯基化合物,包括半胱氨酸、同型半胱氨酸、谷胱甘肽、二硫苏糖醇、2-氨基乙硫醇、2-巯基乙酸,2h后对其荧光光谱进行测试,结果表明,747nm和808nm处的荧光发射强度之间的比率(F747nm/F808nm)发生了明显变化,反应前后比率(F747nm/F808nm)从0.06变化到2.9~4.3,分别由图3(a)灰黑条表示。而其它不含巯基的氨基酸(丙氨酸、缬氨酸、丝氨酸、组氨酸、亮氨酸、赖氨酸)、葡萄糖、2-氨基乙酸和常见的金属离子(K+、Na+、Ca2+、Mg2+)3mmol/L,反应2h都没有引起明显的荧光发射强度比率(F747nm/F808nm)上的改变,如图3(b)灰条所示。然后再当向其中加入3mmol/L半胱氨酸反应2h,荧光发射强度比率(F747nm/F808nm)仍可以看到明显变化,并且与直接加入3mmol/L半胱氨酸的比率值接近,如图3(b)黑条所示,说明上述化合物对检测硫醇没有影响。上述结果说明,探针分子CySS在近红外区域对硫醇具有极好的化学选择性。
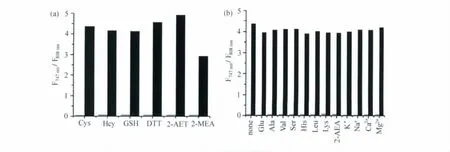
图3 CySS的化学选择性(a)Fluorescence responses of CySS toward various thiols.The gray and black bars represent emission intensity ratios F747nm/F808nmof CySS(2μmol/L)before and 120min after addition of the thiols(3mmol/L),respectively.(b)Fluorescence responses of CySS toward other analytes.The gray and black bars represent emission intensity ratios F747nm/F808nm of CySS(2μmol/L)120min after addition of various analytes(3mmol/L)and further 120min after addition of Cys(3mmol/L),respectively.λex=676nm
2.3 pH对CySS和CyN的影响
在生理pH范围6~8内,CySS在808nm处的荧光发射强度和CyN在747nm处的荧光发射强度几乎不受pH的影响,如图4(a)所示;它们的比率(F747nm/F808nm)也同样都不受pH的影响,如图4(b)所示。也就是说,在生理范围内,CySS对硫醇的比率测量型检测并不受到酸碱环境改变的影响。
2.4 CySS在细胞成像中的应用
对CySS在水溶液中的应用做了上述研究之后,我们接下来将它应用到活细胞成像中。如图5所示,将 NIH 3T3细胞用10μmol/L CySS在37℃下培养30min后,我们发现,CySS具有良好的细胞膜穿透性,已经渗透到细胞内部,并且700~770nm荧光发射窗口和长波窗口780~800nm处都有荧光(图5上部);当NIH 3T3细胞用500 μmol/L硫 辛 酸 培 养 一 天 后,再 用 10μmol/L CySS在37℃下培养30min后,进行成像实验,在窗口700~770nm处观察到荧光有明显的增强(图5中部);这与以前文献报道的硫辛酸会使细胞内谷胱甘肽浓度增加结果一致[28];当NIH 3T3细胞用100μmol/L N-乙基马来酰亚胺(NEM)培养30min后,再用10μmol/L CySS在37℃下培养30min进行成像实验,在窗口700~770nm处观察到荧光明显减弱(图5底部),这是因为NEM作为硫醇的清除剂[29],使细胞内硫醇浓度降低所致。这说明CySS荧光发射的变化确实为细胞内的硫醇浓度变化所引起,而且通过对两个监测窗口做比率图像,我们可以更加直观、灵敏地检测细胞内硫醇浓度的变化。因此,CySS可以成功应用于检测细胞内的硫醇浓度的变化,具有很好的应用前景。
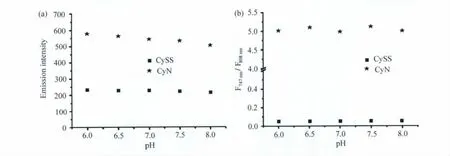
图4 pH对CySS和CyN两个波长荧光强度(a)和比率(b)的影响(a)Effect of pH on fluorescence intensity at 747nm for CyN and 808nm for CySS.(b)Effect of pH on fluorescence intensity ratio of F747nm/F808nmfor CyN and CySS.λex= 676nm

图5 将CySS用于NIH 3T3细胞中硫醇成像实验(a)明场图像;(b)700~770nm荧光发射窗口的荧光信号;(c)780~800nm荧光发射窗口的荧光信号;(d)图(b)与(c)的比率图像Confocal fluorescence images of intracellular thiols in NIH 3T3cells with CySS(a)Bright-field transmission images.(b)Fluorescence images with emission collected at 700-770nm.(c)Fluorescence images with emission collected at 780-800nm.(d)Ratiometric images generated from (b)and(c)
3 结论
本文基于硫醇与二硫键的选择性反应,设计合成了以七甲川花菁为荧光发色团的硫醇近红外比率荧光探针CySS,并对其性质和应用做了详细深入的研究。探针分子CySS具有灵敏度高,选择性好,且不受pH影响等优点,被成功应用到细胞内硫醇的检测。
[1] Wood Z A,Schröder E,Harris J R,Poole L B.Structure,mechanism and regulation of peroxiredoxins[J].Trends in Biochemical Sciences,2003,28(1):32-40.
[2] Dalton T P,Shertzer H G,Puga A.Regulation of gene expression by reactive oxygen[J].Annual Review of Pharmacology and Toxicology,1999,39:67-101.
[3] Saetre R,Rabenstein D L.Determination of cysteine in plasma and urine and homocysteine in plasma by high-pressure liquid chromatography[J].Analytical Biochemistry,1978,90(2):684-692.
[4] Lash L H,Jones D P.Distribution of oxidized and reduced forms of glutathione and cysteine in rat plasma[J].Archives of Biochemistry and Biophysics,1985,240(2):583-592.
[5] Shahrokhian S.Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode[J].Analytical Chemistry,2001,73(24):5972-5978.
[6] Seshadri S,Beiser A,Selhub J,et al.Plasma homocysteine as a risk factor for dementia and Alzheimer's disease[J].The New England Journal of Medicine,2002,346(7):476-483.
[7] Savage D G,Lindenbaum J,Stabler S P,et al.Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies[J].The American Journal of Medicine,1994,96(3):239-246.
[8] Anna P,Fiorella P,Mattia L,et al.Determination of blood total,reduced,and oxidized glutathione in paediatric subjects[J].Clinical Chemistry,2003,47:1467-1469.
[9] Mar M,Morales A,Colell A,et al.Mitochondrial glutathi-one,a key survival antioxidant[J].Antioxidants & Redox Signaling,2009,11(11):2685-2700.
[10] Ivanov A R,Nazimov I V,Baratova L.Determination of biologically active low-molecular-mass thiols in human blood:I.Fast qualitative and quantitative,gradient and isocratic reversed-phase high-performance liquid chromatography with photometric and fluorescence detection[J].Journal of Chromatography A,2000,895(1-2):157-166.
[11] Amarnath K,Amarnath V,Amarnath K,et al.A specific HPLC-UV method for the determination of cysteine and related aminothiols in biological samples[J].Talanta,2003,60(6):1229-1238.
[12] Jiang H,Ju H.Electrochemiluminescence sensors for scavengers of hydroxyl radical based on its annihilation in CdSe quantumdots film/peroxide system[J].Analytical Chemistry,2007,79(17):6690-6696.
[13] Wang W,Li L,Liu S,et al.Determination of physiological thiols by electrochemical detection with piazselenole and its application in rat breast cancer cells 4T-1[J].Journal of the American Chemical Society,2008,130(33):10846-10847.
[14] Chen X,Zhou Y,Peng X,et al.Fluorescent and colorimetric probes for detection of thiols[J].Chemical Society Reviews,2010,39(6):2120-2135.
[15] Sreejith S,Divya K P,Ajayaghosh A.A near-infrared squaraine dye as a latent ratiometric fluorophore for the detection of aminothiol content in blood plasma[J].Angewandte Chemie International Edition,2008,47(41):7883-7887.
[16] Zhang M,Yu M,Li F,et al.A highly selective fluorescence turn-on sensor for cysteine/homocysteine and its application in bioimaging[J].Journal of the American Chemical Society,2007,129(34):10322-10323.
[17] Jung H S,Han J H,Pradhan T,et al.A cysteine-selective fluorescent probe for the cellular detection of cysteine[J].Biomaterials,2012,33(3):945-953.
[18] Chen Y,Zhao J,Guo H,Xie L.Geometry relaxation-induced large stokes shift in red-emitting borondipyrromethenes(BODIPY)and applications in fluorescent thiol probes[J].The Journal of Organic Chemistry,2012,77(5):2192-2206.
[19] Zhang M,Wu Y,Zhang S,et al.A nitroolefin functionalized BODIPY chemodosimeter for biothiols driven by an unexpected conjugated addition mechanism [J].Chemical Communications,2012,48(71):8925-8927.
[20] Zhou X,Jin X,Sun G,et al.A cysteine probe with high selectivity and sensitivity promoted by response-assisted electrostatic attraction[J].Chemical Communications,2012,48(70):8793-8795.
[21] Patonay G,Antoine M D.Near-infrared fluorogenic labels:new approach to an old problem[J].Analytical Chemistry,1991,63(16):321A-327A.
[22] Yuan L,Lin W,Zhao S,et al.A unique approach to development of near-Infrared fluorescent sensors for in vivo imaging[J].Journal of the American Chemical Society,2012,134(32):13510-13523.
[23] Wang R,Chen L,Liu P,et al.Sensitive near-infrared fluorescent probes for thiols based on SeN bond cleavage:imaging in living cells and tissues[J].Chemistry-A European Journal,2012,18(36):11343-11349.
[24] Tsien R Y,Poenie M.Fluorescence ratio imaging:a new window into intracellular ionic signaling[J].Trends in Biochemical Sciences,1986,11(11):450-455.
[25] Zhu D,Li G,Xue L,et al.Development of ratiometric near-infrared fluorescent probes using analyte-specific cleavage of carbamate[J].Organic & Biomolecular Chemistry,2013,11(28):4577-4580.
[26] Licha K,Riefke B,Ntziachristos V,et al.Hydrophilic cyanine dyes as contrast agents for near-infrared tumor imaging:synthesis,photophysical properties and spectroscopic in vivo characterization[J].Photochemistry and Photobiology,2000,72(3):392-398.
[27] Li C,Wu T,Hong C,et al.A general strategy to construct fluorogenic probes from charge-generation polymers(CGPs)and AIE-active fluorogens through triggered complexation[J].Angewandte Chemie International Edition,2012,51(2):455-459.
[28] Hultberg B,Andersson A,Isaksson A.Lipoic acid increases glutathione production and enhances the effect of mercury in human cell lines[J].Toxicology,2002,175(1-3):103-110.
[29] Yellaturu C R,Bhanoori M,Neeli I,Rao G N.N-Ethylmaleimide inhibits platelet-derived growth factor BB-stimulated Akt phosphorylation via activation of protein phosphatase 2A[J].Journal of Biological Chemistry,2002,277:40148-40155.
