Thermally decarboxylated sodium bicarbonate: Interactions with water vapour, calorimetric study
2013-12-23NtliVolkovHenriHnssonLennrtLjunggren
Ntli Volkov*, Henri Hnsson, Lennrt Ljunggren
aDepartment of Biomedical Science, Malm University, SE-205 06, Malm, Sweden
bGalenica AB, Medeon Science Park, SE-205 12, Malm, Sweden
cDepartment of Biotechnology, Center for Chemistry and Chemical Engineering, Lund University, P.O. Box 124,SE-221 00 Lund, Sweden
1. Introduction
Interactions between solid-state materials and water vapour may occur during different stages of manufacturing of pharmaceutical products [1]. Water vapour uptake will not only lead to possible decomposition of the active substance, but also to chemical and physical changes of the excipients [2-5]. This can cause further problems during the manufacturing process and have deleterious consequences for the final dosage form [6,7],including dissolution of the active component in the absorbed water layer. Furthermore the amount of water in intermediates and products is important not only for the stability but also for understanding the molecular basis of moisture effects, and eventual improvement of production procedures [8,9].
Sodium bicarbonate is one of the most important components in different pharmaceutical dosage forms, solid and liquid alike.It can degrade into sodium carbonate and carbon dioxide during sorption of moisture at low temperatures.The stability of effervescent products can be increased by introducing anhydrous Na2CO3, which acts as a desiccant[10,11]. This can be obtained either by adding carbonate physically to the effervescent mixture or by heat treatment(partly converting NaHCO3into Na2CO3prior to mixing).
Isothermal calorimetry (ITC) is one of the most useful methods to study physical changes occurring between pharmaceutical components [12-15]. In our previous study on pyrolytically decarboxylated NaHCO3[16] only a narrow range of decarboxylation degrees was investigated by ITC at two humidities: 54% and 100% RH.
The aim of the present study is to investigate the thermodynamic behaviour of the wide range of thermally converted NaHCO3at different humidities, ranging from 54% to 100%,in order to find optimal degree of conversion and stability conditions for the alkaline component of effervescent systems and to understand the mechanism of surface transformation caused by interaction with water vapour.
2. Materials and methods
2.1. Materials
Sodium bicarbonate (NaHCO3), from Codex Fein, Solvay,anhydrous sodium carbonate(Na2CO3)from Merck,and sodium sesquicarbonate(trona salt)from Sigma were used.Wegscheider's salt was synthesised in the laboratory according to Barrall and Rogers [17]. The water used was reagent grade Milli-Q water.
2.2. Sample preparation and characterisation
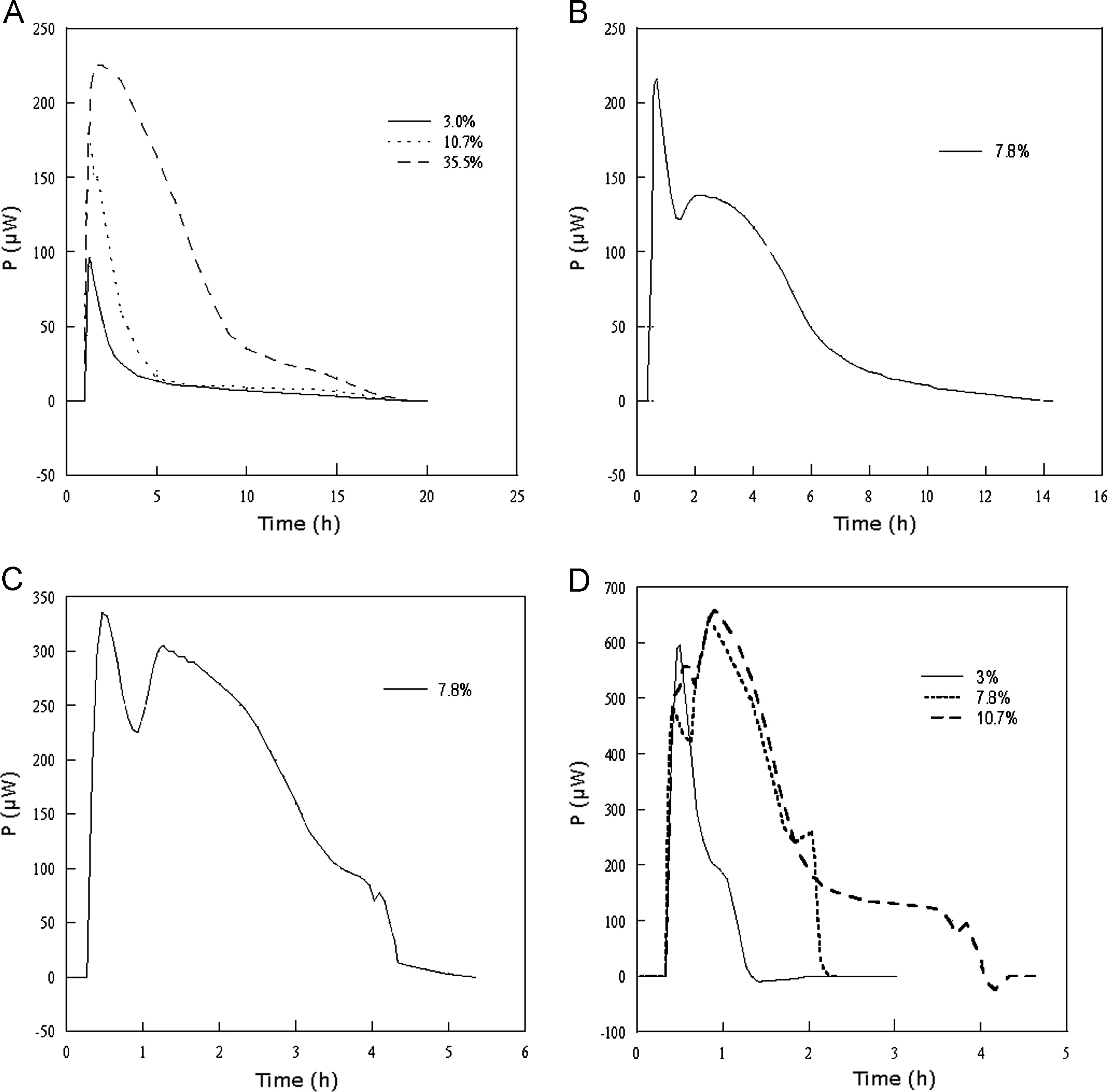
Fig.1 Experimental thermal power profiles obtained at different relative humidities for some of thermally decarboxylated NaHCO3 samples (the degree of decarboxylation is given on each figure): (A) 54% RH; (B) 75% RH; (C) 83% RH; and (D) 100% RH.
Decarboxylated samples of NaHCO3were prepared according to the procedure previously described in our previous publication [16]. The pH values for each sample were compared to standard mixtures of NaHCO3/Na2CO3to determine the degree of decarboxylation. Decarboxylation degrees obtained were varied from 3% to 35.5%.
2.3. Calorimetry
A four-channel heat conduction microcalorimeter,TAM 2277,Thermometric AB, Sweden was used. The calorimeter was electrically calibrated and measurements were conducted at 37°C in sealed 1.3 mL stainless steel vessels. The enthalpies(ΔH)of interaction between water vapour and the solid samples were measured. The constant relative humidity (RH) inside the measuring vessels was achieved by mounting small hygrostats filled with either distilled water (100% RH) or saturated salt solutions: NaBr (54% RH), KI (67% RH), NaCl (75% RH),KCl(83%RH)and KNO3(90%RH)[18,19].The weight of the samples was about 0.15 g. The experiments for each humidity were repeated at least three times. The obtained calorimetric signals were integrated. After each calorimetric run the water uptake of each sample was determined gravimetrically.
3. Results and discussion
The thermal power (P, μW) profiles obtained at different humidities are depicted in Fig.1(A-D). Fig.1(A) shows typical calorimetric traces for the sorption process at 54%RH (three decarboxylation degrees are given as examples).At this humidity all samples had similar pattern of the heat flow: at the beginning—a relatively steep initial exothermic peak, which slowly declines to the baseline.
The time needed to complete the sorption (about 20 h) was not solely dependent on the decarboxylation degree. The determined thermal power values were increased with decarboxylation degree and varied from 90 μW for 3% to 240 μW for 35.5% decarboxylation. Higher degree of decarboxylation also leads to the extension of the peak maximum. The pattern of calorimetric traces obtained at 67% RH were rather similar to those at 54% RH (data are not presented), but P values were almost double at maximum point for the corresponding decarboxylation degrees. No significant changes were observed in the pattern of calorimetric profiles at 75% RH, except for the decarboxylation degree of 7.8%(Fig.1(B)),this sample gave two exothermic peaks within four hours from the start of the sorption process. The second peak is less pronounced and much lower and wider than initial.The values of the heat power at 75% RH were between 80 μW and 650 μW.Experimental results obtained at higher humidities (83-100%) indicated the more complicated behaviour of studied system. Different peaks appeared on calorimetric profiles, depending on varied degrees of decarboxylation and humidity conditions. Fig.1(C) illustrates the profile at 83% RH for the same degree of carboxylation (7.8%) as Fig.1(B). Two major and one minor exothermic peaks were observed and theirs positions were shifted to shorter time; the total sorption process was completed within five hours, compared to 14 h at 75%RH. The samples with decarboxylation degrees 3%-10.7% showed similar behaviour at 90% RH.Samples with the higher decarboxylation degrees needed more time to complete the process at both humidities(83% and 90%)and the pattern of experimental calorimetric traces were different(data are not shown).At 100%RH the time needed to complete the process was less than 2 h for 3% decarboxylation,and up to 36 h for 35.5%. The process is quite complicated at this RH,which is reflected in the shape of the calorimetric traces.Fig.1(D) shows thermal power profiles at 100% RH for some of the decarboxylation degrees. All samples with the decarboxylation degrees higher than 3% exhibited three differently patterned exothermic peaks, which is in a perfect agreement with our previous observations [16]. The highest initial peak appeared within one hour from the start of the experiment and the heat power values at the maxima were very similar, varying from 600 μW for 3% to 880 μW for 35.5% (not shown)decarboxylation degree, compared to 54% RH, when the difference was almost a factor of 3. The other two peaks were changing the shape and the position with time and the degree of decarboxylation. At 100% RH a small endothermic signal was observed on all heat flow curves within one hour to the end of the sorption process. A similar observation was recorded by Angberg et al. [20], but for the material of different nature.
The obtained calorimetric signals were integrated and the experimental enthalpies -ΔH (J/g) were calculated. They are all exothermic and show a positive linear correlation with decarboxylation degree (x) for each humidity studied. The following equations are given with regression coefficients (R):

These data are in a full agreement with our previous results[16],and may be used for estimation of decarboxylation degree of unknown samples with the help of ITC.
Ahlneck and Zografi [8] proposed three ways for water vapour interaction with solid surfaces: adsorption of water vapour to the solid-air interface; crystal hydrate formation;and deliquescence, a phase transformation whereby the adsorption of water vapour leads to dissolution of the solid.For some solids it is also possible that capillary condensation can take place even at low humidity [21]. Two of these processes, deliquescence and capillary condensation can lead to the formation of condensed or bulk water, which can dissolve water-soluble components on the surface. If crystal hydrates are formed then their stoichiometry, position of the water molecules and the strength of the interaction are determined by humidity conditions. The solubility of compounds present on the surface is one of the main issues to propose certain mechanism of water vapour interaction.For water-soluble solids the condensed water will dissolve the solid as long as a sufficiently high relative humidity is maintained.On the other hand under specific conditions,even small amounts of water below this point can cause some surface dissolution. The deliquescent materials are usually crystalline solids with high water solubility. The critical humidity(RHo)is a characteristic of the solid and is the point above which the adsorbed water assumes to have the character of bulk water [9]. However, the critical humidity is still unclearly defined and very few theoretical studies are reported[22]. Usually the critical humidity is determined in sorption experiments by means of classical static, or more modern dynamic water sorption (DVS) methods [23-25], but calorimetry also can be used to determine this characteristic [26].
The experimental data for each humidity were recalculated per mole of Na2CO3to obtain the mean -ΔH values (kJ/mol Na2CO3) and obtained enthalpies were plotted against RH.These results are presented in Fig.2, which shows the linear increase of the-ΔH from 54%to 75%RH followed by steady state part for the higher humidities. The observed inflection point at 75% RH can be classified as RHofor studied system.This observation is supported by the published data; i.e.Tabibi and Hollenbeck [27] reported 77.41% RHoat 25°C for sucrose; Xiang [22] and Zhan [23] determined the RHofor different drugs and inorganic salts and reported following values for some sodium salts: 75.3% for chloride, 76.15% for thiosulphate and 82.5% for salicylate. Salameh and Taylor
[28] studied the deliquescence process in binary mixtures used in the pharmaceutical industry and reported RHodata between 48% and 91%. Moisture sorption and stability study of sodium bicarbonate [29] gave 75-76% for the critical humidity value depending on the temperature.
The average enthalpy value at steady state was calculated as-26.9±0.6 kJ/mole Na2CO3and corresponds well to the heat of solution of Na2CO3(-26.7 kJ/mole) according to Berg and Vanderzee[30].This is in agreement with the conclusion in our previous publication, stating a complete dissolution of surface Na2CO3at 100% RH [16]. The results presented in Fig.2 support this mechanism and expand its application to the humidities above critical and wider range of decarboxylation degrees: 3.0-35.5% in comparison to 1.9-8.8% in the previous study.
In order to propose more detailed mechanism of water vapour interaction with decarboxylated samples, especially at the humidities below RHo, the water uptake (m) was determined after each calorimetric experiment (Table 1), as a complement to the enthalpy data. The water uptake increases with the humidity as well as the degree of conversion which is to be expected and excessive water uptake occurs at 100%RH for all investigated samples. If the surface of the decarboxylated sample is formed by only two compounds: Na2CO3and NaHCO3, then the total water uptake can be calculated according to the given below simple additivity scheme
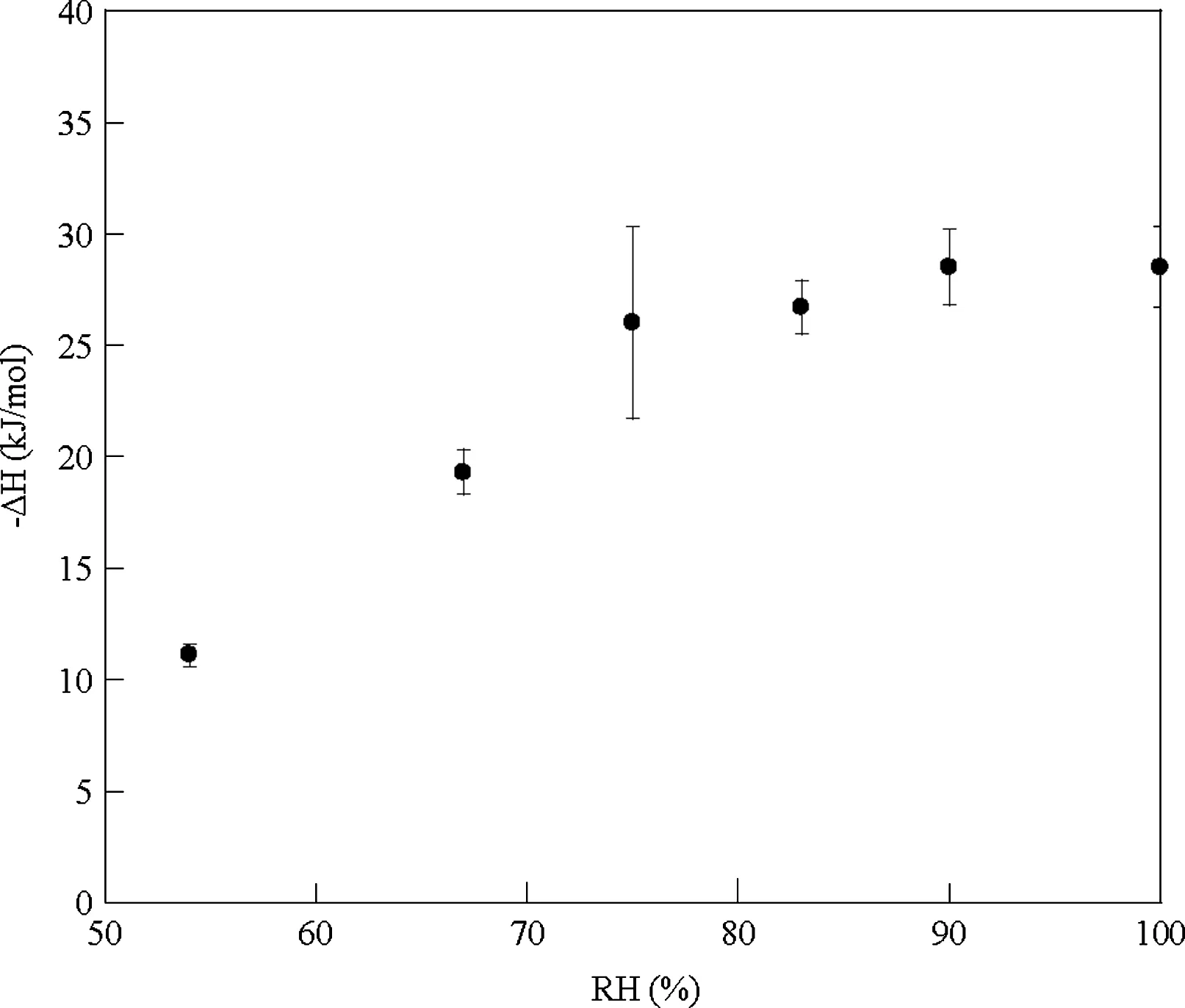
Fig.2 Calculated enthalpies of interaction(-ΔH kJ/mol Na2CO3)of thermally decarboxylated NaHCO3 samples against RH.

If the assumption is correct then for the humidities below RHo,the total water uptake values should be equal to the sum of the experimentally determined values for water uptake of the pure compounds multiplied by the percentage of them in a surface mixture. However, the results show that experimental and calculated data do not coincide (Table 1).
To exemplify, we used the 10.7% decarboxylation degree sample at 67%RH.The obtained experimental value of water uptake is 2.35 g/100 g. The decarboxylation degree of 10.7%implies that 10.7%of the bicarbonate surface is converted into Na2CO3and the rest (89.3%) remains unchanged (pure NaHCO3). The water uptake for pure Na2CO3at this humidity is 15.7 g/100 g and zero for pure NaHCO3; 10.7%of the value for pure Na2CO3equals 1.68 g/100 g (Table 1),which is lower than the experimental value, indicating the presence of other compounds on the surface of the sample.The difference between calculated and experimental data varies for different degrees of decarboxylation, showing the maximum discrepancy (calculated values are more than two times higher in comparison with experimental ones) for the sample with the lowest degree (3%). For the rest of the samples the difference decreases with the decarboxylation degree, and at 35.5% is within 6% of the total value, which leads to the conclusion that at high decarboxylation level the surface mainly consists of the pure Na2CO3and NaHCO3.
The presence of different peaks on the thermal power curves and the discrepancy between experimental and calculated data of water uptake for thermally decarboxylated samples indicate that the surface is not physically or chemically homogenous[20],and that the mechanism of water vapour interaction with samples is more complicated than a simple adsorptiondissolution process. Both salts initially present on the surface,NaHCO3and Na2CO3, are soluble in water and can form different intermediates during the interaction with the water vapour. Already in 1927 Hill and Bacon [31] published the results from the investigation of the ternary system: Na2CO3-NaHCO3-liquid H2O and reported the formation of several stable intermediates, including mono, hepta and decahydrates and Na2CO3·NaHCO3·2H2O (i.e. trona salt) at three different temperatures: 25, 30, and 50°C. The formation of stable hydrates and other compounds is temperature dependent and very sensitive to any changes in the system[32-34].The double salt of carbonate and bicarbonate: Wegscheider's salt(Na2CO3·3NaHCO3), can be formed as an intermediate during thermal decomposition of sodium bicarbonate and trona [35-37]. Water uptake for the pure compounds:Na2CO3, NaHCO3, trona salt, and Wegscheider's salt was determined in separated experiments; these data are also shown in Table 1. At the humidities below 90% only two compounds can gain water: sodium carbonate and Wegscheider's salt,both sodium bicarbonate and trona salt are resistant to water vapour under these conditions. The calorimetric cell used for the measurements can be considered as a closed system, containing three components: sodium bicarbonatesodium carbonate-water. Several reactions can take place in this system at atmospheric pressure: Wegscheider's salt can be formed,but the presence of water can convert it into trona salt and bicarbonate. These chemical reactions are given below
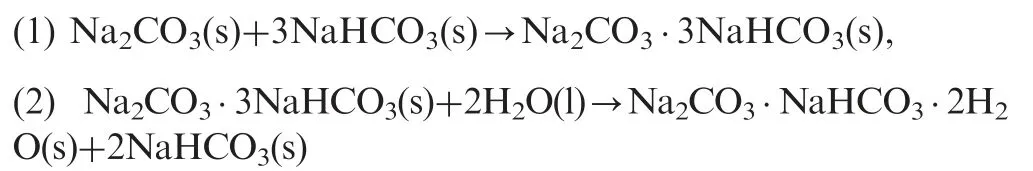
The energy change for the first reaction(Eq.(1))is-8 kJ/mole and for the second (Eq.(2)) -24.32 kJ/mol, calculated upon the heats of formation of the components involved [38].
These two equations can be summarised giving the following Eq. (3):

If such type of transformation is taking place, then it will increase the decarboxylation degree of the surface by the factor of two. We will use the previous example, i.e. 10.7%decarboxylation degree at 67%RH,for calculations according to this proposal. If the Wegscheider's salt is formed on the surface and then converted into trona(Eqs.(1)and(2)),it will mean that 10.7% of free NaHCO3is coupled to Na2CO3; the remaining pure NaHCO3will be only 78.6% (89.3-10.7) and the new decarboxylation degree will be 21.4%, instead of 10.7%. The modified calculated value for water uptake then will become 3.36 g/100 g, which is rather closed to the experimental value for pure Wegscheider's salt (3.20 g/100 g),given in Table 1. Unfortunately, the real proportions of the different compounds on the surface in the new mixture are unknown. This can explain the discrepancy between experimental and calculated water uptake values, which are generally lower than that for the pure Na2CO3, but higher than for the pure Wegscheider's salt. A probable reason for this observation could be that the inner core consists of pure NaHCO3, and an intermediate layer is formed by solid state rearrangements of different salts, including trona, Wegscheider's and pure Na2CO3. The set of additional experiments performed on the pre-humidified samples(data are not shown)shows the enormous decrease in the heat effects when analysed in the humidity range from 54% to 75% and is indirect evidence for the presence of trona salt (unchanged by water vapour) on the surface.
The number of water molecules involved in the interaction process is a limiting factor for the formation of trona salt.The molar ratio of mole H2O/mole Na2CO3was calculated and the data are presented in Table 2.The number of water molecules per molecule of sodium carbonate at the humidities below or equal to critical is lower than two, which is an indication that the formation of trona salt is not completed,implying that the surface composition is complicated. This observation is in agreement with Robey et al. [39], who patented a method for the conversion of anhydrous sodium carbonate to Wegschei-der's salt and stated that with humidity changes from 63% to 79% the ratio between sodium carbonate, Wegscheider's and trona is changed from 5:85:10 to 20:0:80.
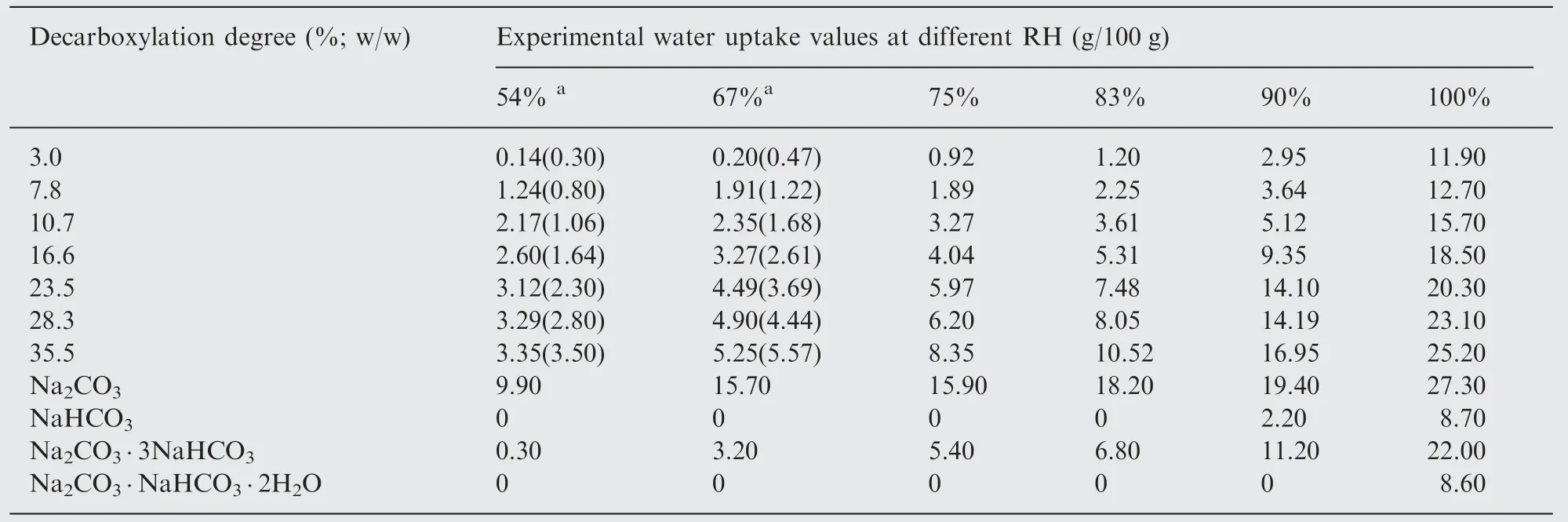
Table 1 Experimental water uptake values (g/100 g sample), determined after calorimetric measurements and the comparison between experimental and calculated (simple additivity scheme) water uptake values (g/100 g sample) for relative humidities 54%and 67%.
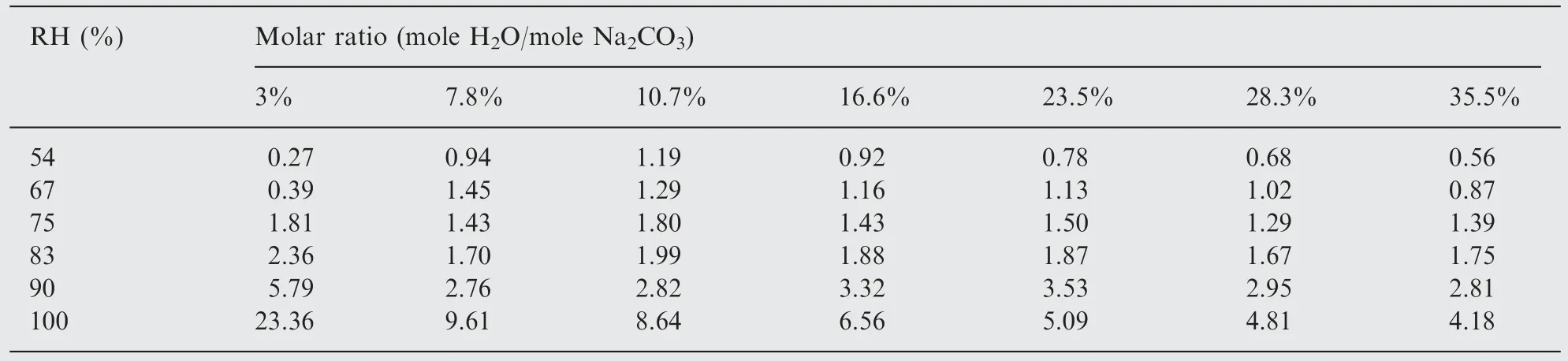
Table 2 The calculated molar ratios (mole H2O/ mole Na2CO3) at different humidities.
Above critical humidity, 75%, the heat effects were independent upon the storage at low humidity but were related to the dissolution of Na2CO3, as previously stated. At this condition the presence of Wegscheider's salt on the surface is unimportant; the enthalpy values, calculated per mole of Wegscheider's salt will give its heat of solution, instead of the heat of solution of sodium carbonate.
4. Conclusions
This study by means of ITC reveals the complicated behaviour of investigated system regarding the carbonate chemistry and interaction with water vapour. The critical humidity, 75%, was determined as the inflection point on a plot of the mean-ΔH kJ/mole Na2CO3against relative humidity.The incubation at low humidities is beneficial with respect to the stability of the samples. For the storage purposes the humidity should not exceed RHo. The humidities above the critical can lead to the dissolution of Na2CO3on the surface of NaHCO3.The possible surface composition,including trona and Wegscheider's salt was suggested. The latter has to be elucidated in further studies.
[1] S. Airaksinen, M. Karjalainen, A. Shevchenko, et al., Role of water in the physical stability of solid dosage formulations, J.Pharm. Sci. 94 (2005) 2147-2165.
[3] A. Mihranyan, A.P. Llagostera, R. Karmhag, et al., Moisture sorption by cellulose powders of varying crystallinity, Int. J.Pharm. 269 (2004) 433-442.
[4] P. Hedenus, M. StrømmeMattsson, G.A. Niklasson, et al.,Characterisation of instantaneous water absorption properties of pharmaceutical excipients, Int. J. Pharm. 202 (2000) 141-149.
[5] T. Sebhatu, C. Ahlneck, G. Alderborn, The effect of moisture content on the compression and bond-formation properties of amorphous lactose particles, Int. J. Pharm. 146 (1997) 101-114.
[6] C.R. Dalton, B.C. Hancock, Processing and storage effects on water vapour sorption by some model pharmaceutical solid dosage formulations, Int. J. Pharm. 156 (1997) 143-151.
[7] S. Malamataris, P. Goidas, A. Dimitriou, Moisture sorption and tensile strength of some tableted direct compression excipients,Int. J. Pharm. 68 (1991) 51-60.
[8] C. Ahlneck, G. Zografi, The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state,Int. J. Pharm. 62 (1990) 87-95.
[9] L. VanCampen, G.L. Amidon, G. Zografi, Moisture sorption kinetics for water-soluble substances. I. Theoretical considerations of heat transfer control,J.Pharm.Sci.72(1983)1381-1388.
[10] B. White, Stable Effervescent Compositions and Methods of Preparing Same, (1963). US patent, 3.105.792.
[11] F. Usui, J.T. Carstensen, Interactions in the solid state 1:interactions of sodium bicarbonate and tartaric acid under compessed conditions, J. Pharm. Sci. 74 (1985) 1293-1297.
[12] M.A.A. O'Neill, S. Gaisford, Application and use of isothermal calorimetry in pharmaceutical development, Int. J. Pharm. 417(2011) 83-93.
[13] L.A.E.Sousa,N.Alem,A.E.Beezer,et al.,Quantitative analysis of solid-state processes studied with isothermal microcalorimetry, J.Phys. Chem. B 114 (2010) 13173-13178.
[14] C.V. Skaria, S. Gaisford, M.A.A. O'Neill, et al., Stability assessment of pharmaceuticals by isothermal calorimetry: two component systems, Int. J. Pharm. 292 (2005) 127-135.
[15] A.E. Beezer, M.A.A. O'Neill, K. Urakami, et al., Pharmaceutical microcalorimetry: recent advances in the study of solid state materials, Thermochim. Acta 420 (2004) 19-22.
[16] L.Ljunggren,N.Volkova,H.Hansson,Calorimetry a method to be used to characterise pyrolyticallydecarboxylated bicarbonate and assess its stability at elevated humidities, Int. J. Pharm. 202 (2000)71-77.
[17] E.M. Barrall, L.B. Rogers, Differential thermal analysis of the decomposition of sodium bicarbonate and its simple double salts,J. Inorg. Nucl. Chem. 28 (1966) 41-51.
[18] H. Nyqvist, Saturated salt solutions for maintaining specified relative humidities, Int. J. Pharm. Tech. Prod. Mfr. 4 (1983) 47-48.
[19] M. Angberg, C. Nystrm, S. Castensson, Evaluation of heatconduction microcalorimetry in pharmaceutical stability studies.V. A new approach for continuous measurements in abundant water vapour, Int. J. Pharm. 81 (1992) 153-167.
[20] M. Angberg, C. Nystrm, S. Castensson, Evaluation of heatconduction microcalorimetry in pharmaceutical stability studies.VI. Continuous monitoring of the interaction of water vapour with powders and powder mixtures at various relative humidities,Int. J. Pharm. 83 (1992) 11-23.
[21] M. El-Sabaawi, D.C.T. Pei, Moisture isotherms of hygroscopic porous solids, Ind. Eng. Chem. Fundam. 16 (1977) 321-326.
[22] C. Xiang, Y. Wang, X. Zhan, et al., Calculating critical relative humidity from solubility according to Pitzer ion interaction model, Chem. Pharm. Bull. 58 (2010) 1366-1368.
[23] X. Zhan, Y. Wang, L. Cao, et al., Determining critical relative humidity by measuring air humidity in equilibrium directly, Eur.J. Pharm. Sci. 41 (2010) 383-387.
[24] Q.E. Li, S.J. Schmidt, Use of ramping and equilibrium water vapor sorption methods to determine the critical relative humidity at which the glassy to rubbery transition occurs in polydextrose, J. Food Sci. 76 (2011) E149-E157.
[25] X. Yuan, B.P. Carter, S.J. Schmidt, Determining the critical relative humidity at which the glassy to rubbery transition occurs in polydextrose using an automatic water vapor sorption instrument, J. Food Sci. 76 (2011) E78-E89.
[26] L.D. Hansen, J.W. Crawford, D.R. Keiser, et al., Calorimetric method for rapid determination of critical water vapor pressure and kinetics of water sorption on hygroscopic compounds,Int.J.Pharm. 135 (1996) 31-42.
[27] S.E. Tabibi, R.G. Hollenbeck, Interaction of water vapor and compressible sugar, Int. J. Pharm. 18 (1984) 169-183.
[28] A.K. Salameh, L.S. Taylor, Role of deliquescence lowering in enhancing chemical reactivity in physical mixtures, J. Phys.Chem. B 110 (2006) 10190-10196.
[29] W.-Y.Kuu,R. Chilamkurti,C. Chen,Effect of relative humidity and temperature on moisture sorption and stability of sodium bicarbonate powder, Int. J. Pharm. 166 (1998) 167-175.
[30] R.L. Berg, C.E. Vanderzee, Thermodynamics of carbon dioxide and carbonic acid: (a) the standard enthalpies of solution of Na2CO3(s),NaHCO3(s),and CO2(g)in water at 298.15 K;(b)the standard enthalpies of formation, standard Gibbs energies of formation, and standard entropies of CO2(aq), HCO3-(aq),CO(aq), NaHCO3(s), Na2CO3(s), Na2CO3·H2O(s) and Na2CO3·10H2O(s), J. Chem. Thermodyn. 10 (1978) 1113-1136.
[31] A.E. Hill, L.R. Bacon, Ternary systems. VI. Sodium carbonate,sodium bicarbonate and water, J. Am. Chem. Soc. 49 (1927)2487-2495.
[32] C.G. Waterfield, R.G. Linford, B.B. Goalby, et al., Thermodynamic investigation of disorder in the hydrates of sodium carbonate, J. Trans. Faraday Soc. 64 (1968) 868-874.
[33] O.Ozcan,J.D.Miller,Flotation of sodium carbonate and sodium bicarbonate salts from their saturated brines, Miner. Eng. 15(2002) 577-584.

[35] C.E. Vanderzee, Thermodynamic relations and equilibria in(Na2CO3+NaHCO3+H2O): standard Gibbs energies of formation and other properties of sodium hydrogen carbonate,sodium carbonate heptahydrate, sodium carbonate decahydrate, trona:(Na2CO3·NaHCO3·2H2O) and Wegscheider's salt: (Na2CO3·3NaHCO3), J. Chem. Thermodyn. 14 (1982) 219-238.
[36] W.F.Waldeck,G.Lynn,A.E.Hill,Aqueous solubility of salts at high temperatures. 11. The ternary system Na2CO3-NaHCO3-H2O from 100 to 200°, J. Am. Chem. Soc. 56 (1934) 43-47.
[37] M.C. Ball, R.A. Clarke, A.N. Strachan, Investigation of the formation of wegscheiderite, Na2CO3·NaHCO3, J. Chem. Soc.Faraday Trans. 87 (1991) 3683-3686.
[38] C.E. Vanderzee, D.A. Wigg, The standard enthalpies of solution and formation of Wegscheider's salt: Na2CO3·3NaHCO3(s) and of trona: Na2CO3·NaHCO3·2H2O (s) at 298.15 K, J. Chem.Thermodyn. 13 (1981) 573-583.
[39] R.J. Robey, W. Mass, J. Capozzolo, Method for the Conversion of Anhydrous Sodium Carbonate to Wegscheider's salt, (1979),US Patent, 4.151.266.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Development of a validated UPLC-qTOF-MS/MS method for determination of bioactive constituent from Glycyrrhiza glabra
- Determination of sildenafil by preconcentration on surfactant coated polymeric resin followed by spectrofluorimetry
- Development and validation of a rapid chromatographic method for the analysis of flunarizine and its main production impurities
- Volatile components of Rhizoma Alpiniae Officinarum using three different extraction methods combined with gas chromatography-mass spectrometry
- Determination and stress studies on YK-1101,a potential histone deacetylase, by HPLC-UV and HPLC-TOF/MS methods
- Accurate quantitation standards of glutathione via traceable sulfur measurement by inductively coupled plasma optical emission spectrometry and ion chromatography
