大果榕茎的化学成分
2013-12-23邵泰明宋小平韩长日陈光英陈文豪戴春燕宋鑫明
邵泰明,宋小平 ,韩长日,陈光英,陈文豪,戴春燕,宋鑫明
海南师范大学教育部热带药用植物化学省部共建重点实验室;海南师范大学化学化工学院,海口571158
Introduction
The genus of Ficus comprises about 1000 species in the world,among which about 120 are distributed in China[1]. F. auriculata distributed in Ledong,Dongfang,Baoting,Lingao of Hainan province China[2]. It has been used as Chinese folk medicine for the treatment of hyperactivity cough,nocturnal emission[3]. The leaves of F.auriculata have antioxidant,antiinflammatory,antidiabetic and hepatoprotective activity,and fruit has a strong antibacterial activity[4-6].In order to investigate the constituents of this plant,eleven compounds were isolated from the 95% ethanol extracts and identified as genistein(1),wighteone(2),5,7,4',-trihydroxy-6-(2-hydroxy-3-methyl-3-butenyl)isoflavone(3),5,7,4'-trihydroxy-3'-(2-hydroxy-3-methyl-3-butenyl )isoflavone(4),alpinumisoflavone(5),derrone(6),(3R)-5-formylmellein(7),3β-(1-Hydroxyethyl)-7-hydroxy-1-isobenzofuranone(8),trans-p-phydroxycinnamic acid ethyl ester(9),β-sitosteral(10),and β-daucosterin(11).Compounds 4,7,8,9 were reported from the genus Ficus for the first time.Compounds 1-10 were isolated from this plant material for the first time.
Experimental
Apparatus and reagents
Melting points were determined on a WRX-4 micromelting-point apparatus(uncorrected).NMR spectra were measured on a Bruker AV-400 instrument with TMS as the internal standard. Silica gel used for column chromatography(CC)was supplied by Qingdao Marine Chemical Factory(Qingdao,China). Sephadex LH-20 was used(Pharmacia,Sweden). TLC and preparative TLC were purchased from Yantai Chemical Industry Institute(Yantai,China).All solvents were purchased from XiLong Chemical Reagent Factory(Shan-Tou,China). The optical density was measured by an enzyme-labeled detector(Elx800,BioTek Instruments,Inc).
Plant materials
The sterm of F. auriculata were collected in Jian feng ling National Forest Park,Hainan Province China,in 2010,and authenticated by associate Prof. Zhong Qiong-Xin,College of Life Science,Hainan Normal University.A voucher specimen was deposited in the Key Laboratory of Tropical Medicinal Plant Chemistry of Ministry of Education,Hainan Normal University.
Extraction and isolation
The dried sterm of F. auriculata(13.5 kg)were powdered and extracted with 95% aqueous ethanol(v/v)three times at room temperature(3 ×5 d).The extract was suspended in 2.0 L of water and then partitioned with petroleum ether,CHCl3,EtOAc successively. The petroleum ether soluble fraction(240 g)was chromatographed on a silica gel column eluted with petroleum ether/EtOAc(100∶1 to 1∶100,v/v)to afford major fractions 1-20.Fraction 4(3.45g)purified by recrystallization to yield compounds 10(750. 3mg);Fraction 6(5.40 g)was subjected to CC(silica gel,petroleum ether/EtOAc,10 ∶1 to 0 ∶1)and purified by CC(Sephadex LH-20,CHCl3/MeOH,2 ∶3)to provide compounds 5(45.4mg),6(23.5mg),and 7(13.0 mg);Fraction 7(2.40 g)was further chromatographed over Sephadex LH-20(CHCl3/MeOH,2 ∶3),followed by PTLC to give compounds 8(13.3 mg),9(8.7 mg);Fraction 10(4.70 g)was further chromatographed over Sephadex LH-20(CHCl3/MeOH,2 ∶3),followed by PTLC to give compounds 1(20.1 mg),2(13.5 mg),3(24.4 mg)and 4(13.2 mg).The ethyl acetate fraction(23g)was subjected to silica gel CC(CHCl3/MeOH 100∶1→2∶1)to yield 11(14.4 mg).
Identification
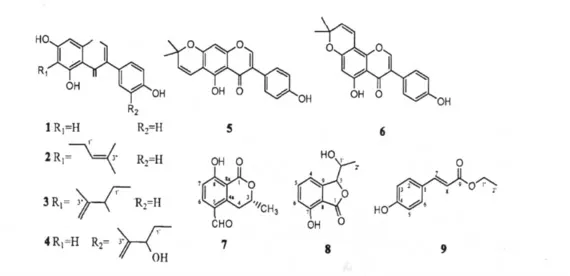
Fig.1 Structures of compounds 1-9
Compound 1White powder,C15H10O5,1H NMR(Acetone,400 MHz),δ:13.04(1H,s,OH-5),8.18(1H,s,H-2),7.46(2H,d,J = 7.6 Hz,H-2',6'),6.91(2H,d,J =7.6 Hz,H-3',5'),6.42(1H,s,H-8),6.29 (1H,s,H-6);13C NMR (Acetone,100 MHz),δ:182.6(C-4),166.1(C-7),164.9(C-5),160.0(C-8a),159.5(C-4'),155.3(C-2),132.2(C-2',6'),125.0(C-3),124.0(C-1'),117.0(C-3',5'),107.1(C-4a),100.8(C-6),95.5(C-8). It was identified as genistein by comparison of the physical and spectral data with the reported data[7].
Compound 2 Yellow powder,C20H18O5,1H NMR(MeOD,400 MHz);δ:13.01(1H,s,OH-5),8.01(1H,s,H-2),7.36(2H,d,J = 8.0 Hz,H-2',6'),6.84(2H,d,J =8.0 Hz,H-3',5'),6.37(1H,s,H-8),5.23(1H,t,J=7.2 Hz,H-2″),3.31(2H,d,overlapped,H-2″),1.78(3H,s,H-4″),1.66(3H,s,H-5″);13C NMR(MeOD,100 MHz)δ:182.3(C-4),163.7(C-7),160.5(C-5),158.8(C-4'),157.6(C-8a),154.6(C-2),132.1(C-3″),131.5(C-2',6'),124.6(C-3),123.5(C-1'),123.4(C-2″),116.3(C-3',5'),113.1(C-6),106.1(C-4a),93.9(C-8),26.0(C-5″),22.3(C-1″),18.0(C-4″). It was identified as wighteone by comparison of the physical and spectral data with the reported data[8].
Compound 3 Yellow powder,C20H18O6,1H NMR(MeOD,400 MHz),δ:13.16(1H,s,OH-5),8.03(1H,s,H-2),7.36(2H,d,J = 8.0 Hz,H-2',6'),6.84(2H,d,J =8.0 Hz,H-3',5'),6.38(1H,s,H-8),4.76,4.70(1H each,s,=CH2),4.41(1H,t,J =6.4 Hz,H-2″),3.01(1H,dd,J = 6.4 Hz,J = 13.6 Hz,Ha-1″),2.90(1H,dd,J =7.2 Hz,J =13.6 Hz,Hb-1″),1.83(3H,s,H-4″);13C NMR(MeOD,100 MHz),δ:182.3(C-4),164.6(C-7),161.3(C-5),158.8(C-4'),158.0(C-8a),154.7(C-2),148.7(C-3″),131.5(C-2',6'),124.7(C-3),123.5(C-1'),116.3(C-3',5'),111.1 (= CH2),110.5 (C-6),106.1(C-4a),94.4(C-8),76.3(C-2″)29.8(C-1″),17.8(C-4″). It was identified as 5,7,4',2-hydroxy-3-methyl-3-butenyl isoflavone by comparison of the physical and spectral data with the reported data[9].
Compound 4White powder,C20H18O6,1H NMR(Acetone,400 MHz),δ:13.06(1H,s,OH-5),8.15(1H,s,H-2),7.37(1H,s,H-2'),7.34(1H,d,J =8.0 Hz,H-6'),6.87(1H,d,J =8.0 Hz,H-5'),6.41(1H,s,H-8),6.28(1H,s,H-6),4.98,4.78(1H each,s,=CH2),4.42(1H,t,J =6.0 Hz,H-2″),2.91(2H,m,overlapped,H-1″),1.81(3H,s,H-4″);13C NMR(Acetone,100 MHz),δ:182.6(C-4),166.2(C-7),164.9(C-5),160.0(C-8a),158.1(C-4'),155.3(C-2),149.5(C-3″),134.0(C-2'),130.4(C-6'),127.8(C-3'),125.1(C-3),124.1(C-1'),117.7(C-5'),111.6(= CH2),107.1(C-4a),100.8(C-6),95.5(C-8),78.0(C-2″),39.8(C-1″),19.3(C-4″). It was identified as 5,7,4'-trihydroxy-3'-(2-hydroxy-3-methyl-3-butenyl)isoflavone by comparison of the physical and spectral data with the reported data[10].
Compound 5Yellow crystal,C20H16O5,1H NMR(CDCl3,400 MHz)δ:13.08(1H,s,OH-5),7.81(1H,s,H-2),7.33(2H,d,J = 8.4 Hz,H-2',6'),6.83(2H,d,J =8.4 Hz,H-3',5'),6.72(1H,d,J =10.4 Hz,H-4″),6.34(1H,s,H-8),5.62(1H,d,J =10.4 Hz,H-3″),1.47(6H,s,CH3-5″,6″);13C NMR(CDCl3,100 MHz)δ:181.1(C-4),159.7(C-7),157.4(C-5),156.9(C-8a),156.1(C-4'),152.7(C-2),130.4(C-2',6'),128.3(C-3″),123.7(C-3),122.9(C-1'),115.7(C-3',5'),115.5(C-4″),106.1(C-4a),105.7(C-6),95.0(C-8),78.2(C-2″),28.4(C-5″,6″). It was identified as alpinumisoflavone by comparison of the physical and spectral data with the reported data[11].
Compound 6Yellow crystal,C20H16O5,1H NMR(CDCl3,400 MHz)δ:12.92(1H,s,OH-5),7.89(1H,s,H-2),7.40(2H,d,J = 7.6 Hz,H-2',6'),6.91(2H,d,J =7.6 Hz,H-3',5'),6.68(1H,d,J =10.0 Hz,H-4″),6.29(1H,s,H-6),5.59(1H,d,J =10.0 Hz,H-3″),1.47(6H,s,CH3-5″,6″);13C NMR(CDCl3,100 MHz)δ:180.9(C-4),162.3(C-5),159.6(C-7),155.8(C-4'),152.3(C-2),152.2(C-8a),130.4(C-2',6'),127.5(C-3″),123.6(C-3),123.1(C-1'),115.5(C-3',5'),114.6(C-4″),106.0(C-4a),101.1(C-8),100.4(C-6),78.1(C-2″),28.2(C-5″,6″). It was identified as derrone by comparison of the physical and spectral data with the reported data[12].
Compound 7 Pale yellow oil C11H10O4,1H NMR(DMSO,400 MHz),δ:11.81(1H,br s,OH-8),10.07(1H,s,-CHO),8.05(1H,d,J =8.8 Hz,H-6),7.07(1H,d,J =8.8 Hz,H-7),4.82(1H,m,H-3),3.87(1H,dd,J =17.6 Hz,2.8 Hz,Ha-4),3.06 (1H,dd,J=15.2 Hz,12.0 Hz,Hb-4),1.46(3H,d,J=6.4 Hz,-CH3);13C NMR(DMSO,100 MHz),δ:190.7(-CHO),168.8(C-1),165.1(C-8),144.2(C-4a),138.6(C-6),124.3(C-5),116.1(C-7),108.9(C-8a),75.2(C-3),29.7(C-4),20.2(-CH3). It was identified as(3R)-5-formylmellein by comparison of the physical and spectral data with the reported data[13].
Compound 8Yellow oil C10H10O4,1H NMR(CDCl3,400 MHz),δ:11.0(1H,s,OH-7),7.55(1H,t,J=8.0 Hz,H-5),7.02(2H,t,J =8.0 Hz,H-4,H-6),4.62(1H,m,H-3),4.61(1H,m,H-1'),1.53(3H,d,J=5.6 Hz,H-2');13C NMR(CDCl3,100 MHz),δ:168.4(C-1),162.0(C-7),141.1(C-9),136.9(C-5),117.9(C-6),116.1(C-4),106.6(C-8),79.9(C-1'),69.2(C-3),17.9(C-2'). It was identified as 3β-(1β-hydroxyethyl)-7-hydroxy-1-isobenzofuranone by comparison of the physical and spectral data with the reported data[14].
Compound 9White crystal C11H12O3,1H NMR(CDCl3,400 MHz),δ:7.63(1H,d,J =16.0 Hz,H-7),7.42(2H,d,J =8.0 Hz,H-2,H-6),6.84(2H,d,J=8.0 Hz,H-3,H-5),6.30(1H,d,J =16.0 Hz,H-8),5.50(1H,br s,OH-3),4.25(2H,q,J =7.2 Hz,H-1'),1.33(3H,t,J = 7.2 Hz,H-2');13C NMR(CDCl3,100 MHz),δ:167.5(C-9),157.6(C-4),144.3(C-7),129.9(C-2,C-6),127.3(C-1),115.8(C-3,C-5),115.7(C-8),60.4(C-1'),14.3(C-2'). It was identified as trans-p-hydroxycinnamic acid ethyl ester by comparison of the physical and spectral data with the reported data[15].
Compound 10 White needles crystal;It was characterized by comparing it with authentic sample on TLC. It was identified as β-sitosterol[16].
Compound 11White amorphous powder;It was characterized by comparing it with authentic sample on TLC. It was identified as β-daucosterol[17].
1 Hou KZ.A dictionary of the families and genera of Chinese seed plants. second edition. Beijing:Sciences Press,1982.195.
2 Wu DL.A checklist of flowering plants of islands and reefs of Hainan and Guangdong province. Beijing:Sciences Press,1994.119.
3 Institute of medicinal plant development Hainan branch.Species List of South China Medicinal Plant Garden. Beijing:China Agriculture Press,2007.150.
4 Yin XS,You KX,Hua BH,et al. Preliminary assessment of antioxidant activity of young edible leaves of seven Ficus species in the ethnic diet in Xishuangbanna,Southwest China.Food Chem,2011,128:889-894.
5 Ahlam EF,Rawia Z,Sherif A.Phytochemical and pharmacological studies of Ficus auriculata Lour.J Nat Prod(Gorakhpur,India),2011,4:184-195.
6 Sarla S,Subhash C.In vitro antimicrobial activity,nutritional profile and phytochemical screening of wild edible fruit of Garhwal Himalaya(Ficus auriculata). Int. J Pharm Sci Rev Res,2012,12:61-64.
7 Mamoalosi AS,Siegfried ED. Total synthesis of the pyranoisoflavone kraussianone 1 and related isoflavones. J Nat Prod,2010,73:1680-1685.
8 Li XL,Wang NL,Zhang X. NMR characteristics of prenyl isoflavones.J Shenyang Pharm Univ,2005,22:189-192.
9 Giuliano DM,Rosalba S,Alberto V. Two isoflavones and a flavone from the fruits of Maclura pomifera. Phytochemistry,1994,37:893-898.
10 Li XC,Alpana SJ,Elsohly NH,et al,Fatty acid synthase inhibitors from plants:Isolation,structure elucidation,and SAR studies.J Nat Prod,2002,65:1909-1914.
11 Li XL,Wang NL,YAO XS.Chemical constituents from stem bark of Erythrina variegate. Chin Tradit Herb Drugs,2005,36:975-978.
12 Mizuo M,Toshiyuki T,Nobuyasu M,et al. Flavonoids in the roots of Euchresta horsfieldii in Thailand. Phytochemistry,1990,29:2663-2665.
13 Mark WS,Eva P,Barbara AB,et al. Characterization of polyketide metabolites from foliar endophytes of Picea glauca.J Nat Prod,2008,71:1393-1398.
14 Rahman MM,Gray AI. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity.Phytochemistry,2005,66:1601-1606.
15 Xiao MT,Ye J,Hong BB,et al.Study on Chemical Constituents of Artemisia lactiflora.Chin Pham J,2011,46:414-417.
16 Lin LP,Qu W,Liang JY. Chemical constituents from the stems of Ilex pubescens var.glabra.Chin J Nat Med,2011,9:176-179.
17 Wang Y,Zhang HN,Wang WJ,et al. Chemical constituents from barks of Ailanthus altissima. Chin Tradit Herb Drugs,2012,43:649-65.
