Novel semicarbazide-sensitive amine oxidase inhibitor screening from anti-obesity drugs using HPLC-MS based technique
2013-11-05CHEBaoquan车宝泉WANGLin王琳ZHANGYongqian张永谦XIAOShengyuan肖盛元DENGYulin邓玉林
CHE Bao-quan(车宝泉), WANG Lin(王琳), ZHANG Yong-qian(张永谦),XIAO Sheng-yuan(肖盛元), DENG Yu-lin(邓玉林),✉
(1.School of Life Science,Beijing Institute of Technology,Beijing 100081,China;2.Beijing Institute for Drug Control,Beijing 100035,China)
Semicarbazide-sensitiveamine oxidase(SSAO)is an enzyme sensitive to semicarbazide and related hydrazine compounds[1],it is also identical to vascular adhesion protein-1(VAP-1)[2-3].The enzyme is localized on the outer surface of vascular endothelial cells,smooth muscle cells,and adipocytes[4-5]and catalyzes the deamination of methylamine and aminoacetone to produce toxic aldehydes,hydrogen peroxide and ammonia.SSAO activity increases in the serum of type 2 diabetic patients[6]and in the blood and kidney of streptozotocin-treated diabetic rats,but decreases in severe burn patients[7].SSAO activity is associated with a number of diseases including diabetes,inflammatory liver disease,and kidney transplant rejection[5,8].Several SSAO inhibitors such as semicarbazide,cyanide,hydrazide compounds,and 2-bromoethylamine(2-BrEA),have been discovered.Semicarbazide and cyanide depress the activities of other enzymes as well.Hydrazide compounds are inhibitors of monoamine oxidase(MAO)in a similar manner and 2-BrEA is a selective,suicide inhibitor of SSAO[9-10].However,none of the existing SSAO inhibitors have been approved for clinical use due to their toxicity.
SSAO has been found to be associated with the glucose transporter-4 and involved in the signaling of glucose uptake in adipocytes[11-12],with its activity observed to be high in adipose tissues and in obese patients[13-15].Adiposity is limited in both non-obese and obese rats by inhibiting MAO and SSAO activity[16]. Because over expression and accumulation of glucose and lipids are potential mechanisms for obesity,it is therefore logical to investigate the potential correlation between SSAO activity and obesity. Currently, “ anti-obesity drugs”act to depress the appetite,decrease absorption of nutrients,or increase the consumption of energy[17].To the best of our knowledge,there are no studies examining the inhibitory effect of anti-obesity drugs on SSAO activity.
The aim of the present study was to screen SSAO inhibitors with low toxicity from fifteen antiobesity drugs in vitro.A screening method was developed to evaluate alterations in SSAO activity in rabbit serum and rat adipose tissue from animals treated with anti-obesity drugs by detecting and calculating the concentration of formaldehyde formed when a specific substrate,methylamine,was used for the SSAO enzyme activity assay.
1 Materials and methods
1.1 Reagents
2-bromoethylaminehydrobromide, dopamine and methylamine were purchased from Sigma(St.Louis, MO, USA). Amfebutamone hydrochloride,caffeine,fenfluramine hydrochloride,fluoxetine hydrochloride,sertraline hydrochloride,sibutramine hydrochloride,orlistat,hydrochlorothiazide,metformin hydrochloride,phenformin hydrochloride,methylephedrine hydrochloride,phenolphthalein,bumetanide, indapamide, furosemide and isoproterenol standards were purchased from the National Institute for Drug Control(Beijing,China).Formaldehyde,ammonium formate,perchloric acid and other chemicals were purchased from Beijing Chemical Reagents Company(Beijing,China).HPLC grade acetonitrile was purchased from Fisher Scientific Canada(Edmonton,Canada).Milli-Q deionized water was used for all experiments.
1.2 Animal experiments for in vitro study
Rabbit serum:MaleNew Zealand rabbits(2.6 kg)were obtained from the Beijing Institute for Drug Control(Beijing,China),blood from the heart was collected and centrifuged at 1 500 g for 10 min to obtain serum and was stored at-70℃.
Rat adipose tissue:Male Wistar rats(160-180 g)were purchased from the Animal Center,Beijing University.The rat epididymal fat pad was collected,10 mmol/L phosphatebuffer(pH 6.8)was added to the fat at the proportion of 1∶3,and then homogenized and centrifuged at 17 000 g at 4℃for 10 min to obtain the adipose fraction,which was stored at-70℃.
1.3 Animal experiments for in vivo study
Male Wistar rats(160-180 g)were purchased from the Animal Center,Beijing University,and were housed in hanging wire cages with free access to water and standard food in 12 h light/dark cycle at 25±1℃.All animals used in the current study were handled and treated in accordance with the guidelines of the Beijing Municipal Science&Technology Commission for experimental care and use of animals.
Following one week of acclimatization,the rats were randomized into three groups—one was treated with normal saline(1 mL/100 g/day,i.p.)as the control group,one was treated with 2-BrEA(50 mg/kg/day,i.p.)and one was treated with caffeine(50 mg/kg/day,i.p.).Duration of the experiment was 10 days.At the end of the protocol,the rats were sacrificed by decapitation.Blood was immediately collected and centrifuged at 2 500 g for 20 min to obtain serum,which was then stored at-70℃.
1.4 SSAO activity assay for in vitro study
A 10 μL aliquot of rabbit serum or rat adipose tissue was preincubated with 300 μL of 10 mmol/L phosphate buffer(pH 6.8)at room temperature for 20 min in the presence or absence of various anti-obesity drug solutions(50 μL of 10 mmol/L drugs).20 μL of 25 mmol/L methylamine and 20 μL of 2 mmol/L dopamine were then added and the reaction mixture was incubated at 37℃for 30 min.The reaction was stopped by the addi-tion of 0 .01 mmol/L isoproterenol(20 μL)followed by addition of 1 mmol/L perchloric acid solution(80 μL).The sample was centrifuged at 17 000 g for 10 min and the supernatant was filtered through 0.2 μm nylon filters.A 20 μL aliquot of supernatant was injected into the HPLC-MS.
SSAO activity was determined by a HPLCESI-MS method[18]. The HPLC-MS conditions were as follows:An Agilent(Agilent Technologies)1 100 series HPLC system equipped with a degasser,a quaternary pump,an autosampler,a column compartment and a mass spectrometry detector(Trap SL,Agilent Technologies)with an electrospray ionization(ESI)source mass spectrometer was used.Chromatographic separation was performed at ambient temperature on a pentafluorophenylpropyl bonded silica column(HSF5,Discovery,USA)with a particle size of 5 μm,a length of 250 mm and an inner diameter of 4.6 mm.The mobile phase,including 15%(v/v)acetonitrile and 10 mmol/L ammonium formate,with the final pH value adjusted to 4.0,was maintained at a flow rate of 0.8 mL/min.Injection volume was 20 μL.
The HPLC eluent was delivered to the ESI source in a splitting ratio of 1∶2.The mass spectrometer was operated in the positive ionization mode.The interface and MS parameters were as follows:nebulizer pressure,25.0 psi(N2);dry gas,8.0 L/min(N2);dry gas temperature,310℃;spray capillary voltage,3 500 V;skimmer voltage,40.0 V;ion transfer capillary exit,100 V;scan range,160 - 220 m/z.All data were processed using Agilent Chemstation software.Quantitation was based on the detector response in the extracted ion chromatogram of specific ions,such as norsalsolinol(166 m/z)and isoproterenol ion(212 m/z).Analyses were each performed in triplicate.
1.5 SSAO activity assay for in vivo study
The procedure was performed as described under SSAO activity assay for in vitro study with the exception that the anti-obesity drug solutions were not added to the mixture of rabbit serum and 300 μL of 10 mmol/L phosphate buffer,while the rabbit serum was prepared from the in vivo study.
1.6 Screening method
Methylamine was used as a specific substrate for the enzyme activity assay of SSAO[19].Anti-obesity drugs were added separately to rabbit serum and rat adipose tissue,representing two types of SSAO,2 -BrEA was used as a positive control,and blank samples were used as negative controls. Formaldehyde, produced from the SSAO-mediated deamination of methylamine,was derivatized with dopamine,the reaction of formaldehyde and dopamine could produce norsalsolinol(1,2,3,4-tetrahydroisoquinoline-6,7-diol)and 1,2,3,4-tetrahydroisoquinoline-7 ,8 -diol(isomer of norsalsolinol)mediated by a Schiff’s base,which was formed by the process of dehydration.To determine these two isomers,ESI-MS detection of formaldehyde-dopamine adducts was used to quantitate the SSAO activity,and isoproterenol was used as an internal standard.SSAO activity was defined as the amount of formaldehyde(μmol)formed per ml of serum per min.The inhibitory effect of anti-obesity drugs on SSAO activity was calculated and compared to the level of formaldehyde produced in drug-treated samples with the negative controls.If SSAO activity was decreased and the difference was significant,the drug was then assumed to be an effective inhibitor.
1.7 Statistical analyses
Results were assessed using SPSS 11.0 analytical software followed by multiple comparisons(Newman-Keuls).In general,the null hypothesis used for all analyses was that the factor had no influence on the measured variable and significance was accepted at the >95%confidence level.
2 Results and discussion
2.1 HPLC-MS analysis
Chromatograms obtained from the formalde-hyde-dopamine adducts are shown in Fig.1.Baseline separation by a HPLC equipped with sensitive mass spectrometry detection was achieved,as seen in the extracted ion chromatogram of a blank serum sample(Fig.1a)and a blank serum sample spiked with 2 μmol/L formaldehyde(Fig.1b).Comparison of the chromatograms shown in Fig.1c and Fig.1d suggests the concentration dependence of formaldehyde-dopamine adduct formation.Peak I is the principal product,norsalsolinol,peak II is 1,2,3,4-tetrahydroisoquinoline-7,8-diol,and peak III is isoproterenol(internal standard).The structure and mass scan spectra of norsalsolinol and isomer of norsalsolinol are shown in Fig.1 e and Fig.1 f.
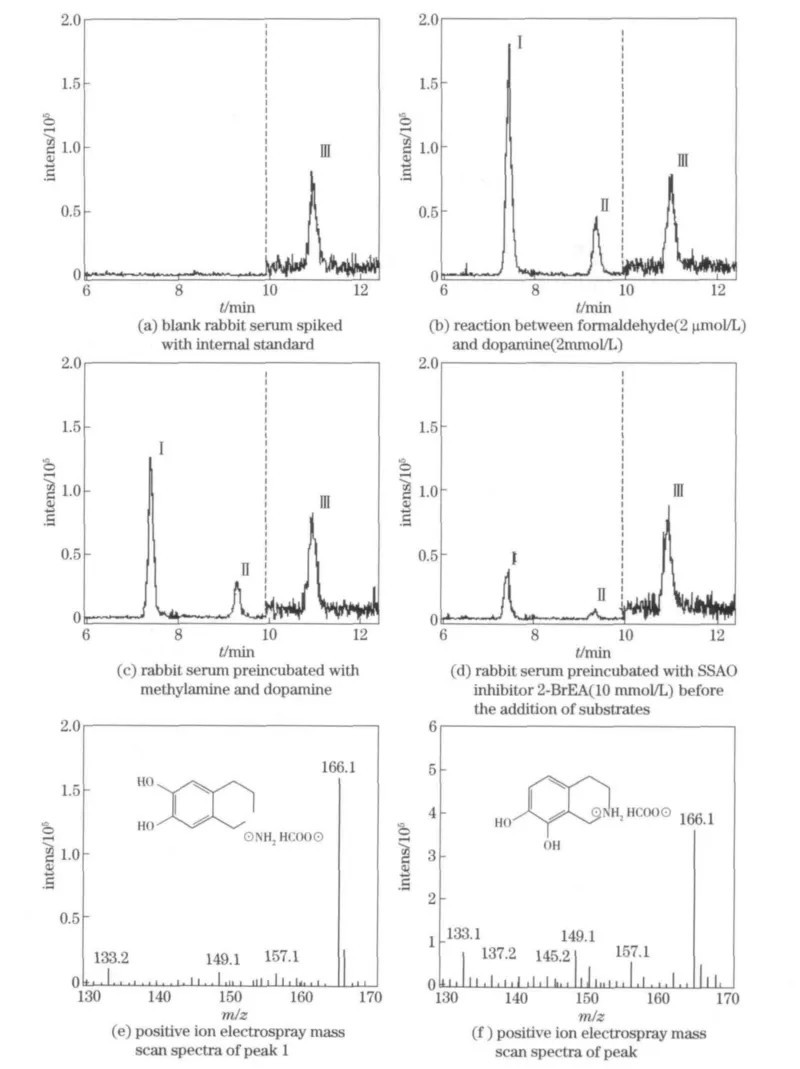
Fig.1 Chromatograms of formaldehyde-dopamine derivatives
Good linearity in the calibration curves was obtained between concentration and the area of the formaldehyde-dopamine adducts.The regression equation was y=0.004 2x- 0.113 5,and the correlation coefficient was 0.999 2,at a linear range of concentrations of 0.32 to 2.0 μmol/L.The inter-day and intra-day precisions ranged from 2.2%to 7.9%and 4.4%to 9.2%respectively for each quality control sample of formaldehyde at 0.06,0.50 and 2.00 μM.The accuracy of both inter and intra-day ranged from 96.0% to 111.5% .The limit of detection(LOD)for serum SSAO activity was 1 nmol/h/mg protein.
2.2 Inhibitory effect of anti-obesity drugs in vitro
A screening method,based on a HPLC-MS technique,was described and used to assess the in vitro inhibition of SSAO activity of fifteen antiobesity drugs.The method was validated for accuracy and reproducibility using 2 -BrEA as a positive control.The extent of inhibition by 2-BrEA was about 80%(Fig.2),which agreed with previously reported results[20],demonstrating the accuracy and reproducibility of the method used in this study.The level of SSAO activity in rat serum was low,while it was relatively high in rat adipose tissue and rabbit serum,so the inhibitory effects of anti-obesity drugs were conducted in two types of SSAO matrices,rabbit serum and rat adipose tissue.Results showed high degrees of accuracy and precision.
Fifteen anti-obesity drugs were assessed for inhibitory effects on SSAO activity.Among these,nine drugs were water-soluble.Inhibition of SSAO activity in rabbit serum is shown in Tab.1;Compared with control,caffeine,amfebutamone,fenfluramine,fluoxetine,sertraline,phenformin and sibutramine showed effective depression of SSAO activity at the same molar concentration.The inhibition of SSAO activity in rat adipose tissue is shown in Tab.1. Similarly,caffeine,methylephedrine, amfebutamone, fenfluramine and phenformin effectively inhibited SSAO activity at the same molar concentration.The inhibition efficiency of 1.4 mmol/L caffeine was the highest among the nine water-soluble anti-obesity drugs,which was 31.9%in rabbit serum.The extent of inhibition by 1.4 mmol/L caffeine was 18.8%in rat adipose tissue.
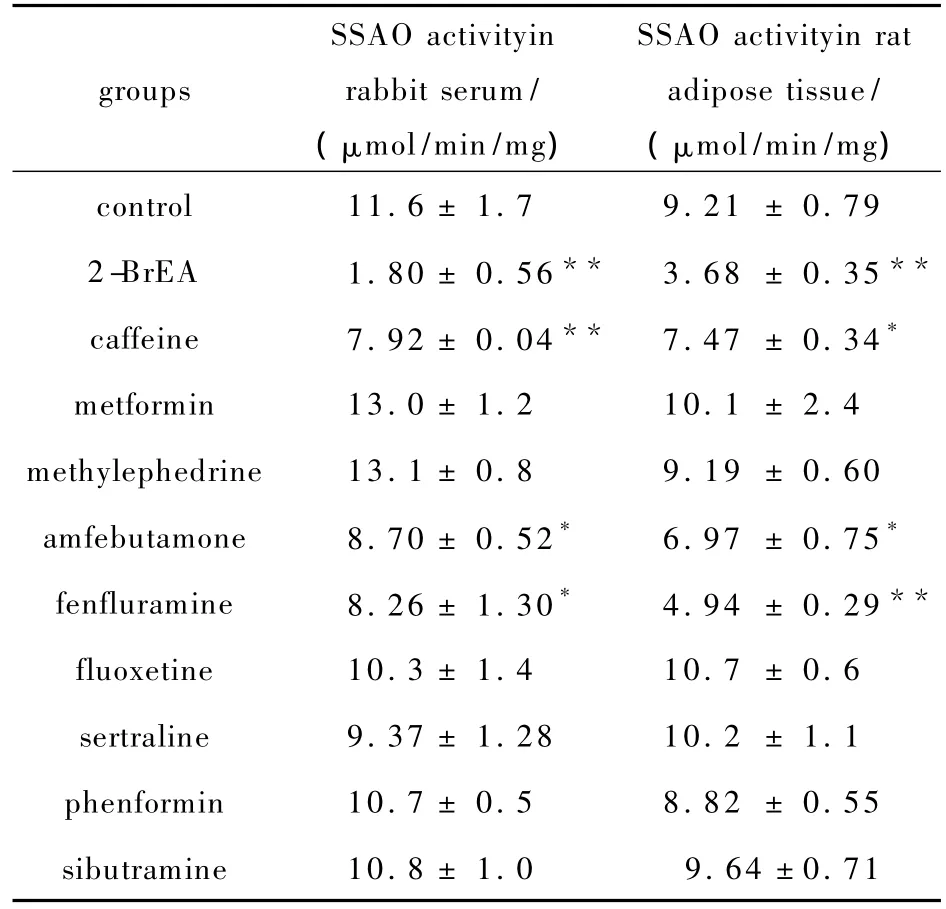
Tab.1 Inhibition by water-soluble anti-obesity drugs on rabbit serum and rat adipose tissue SSAO activity in vitro
Among the fifteen drugs,six were not soluble in water at a concentration of 10 mmol/L,so 8% dimethyl sulfoxide solution was added to help dissolve the substances.Inhibition of SSAO activity in rabbit serum and that in rat adipose tissue are shown in Tab.2.Compared with control,bumetanide showed significant inhibition of SSAO activity in both rabbit serum and rat adipose tissue at the same molar concentration.
The eleven drugs that did not show an obvious inhibitory effect on SSAO activity were excluded from further study,while the extent of inhibition of the remaining four drugs was calculated.As shown in Fig.2,caffeine,fenfluramine,bumetanide and amfebutamone were effective in inhibiting the SSAO activity,with the extent of inhibition being 31.9%,29.0%,40.3%and 25.2%in rabbit serum and 18.8%,46.4% ,54.3%and
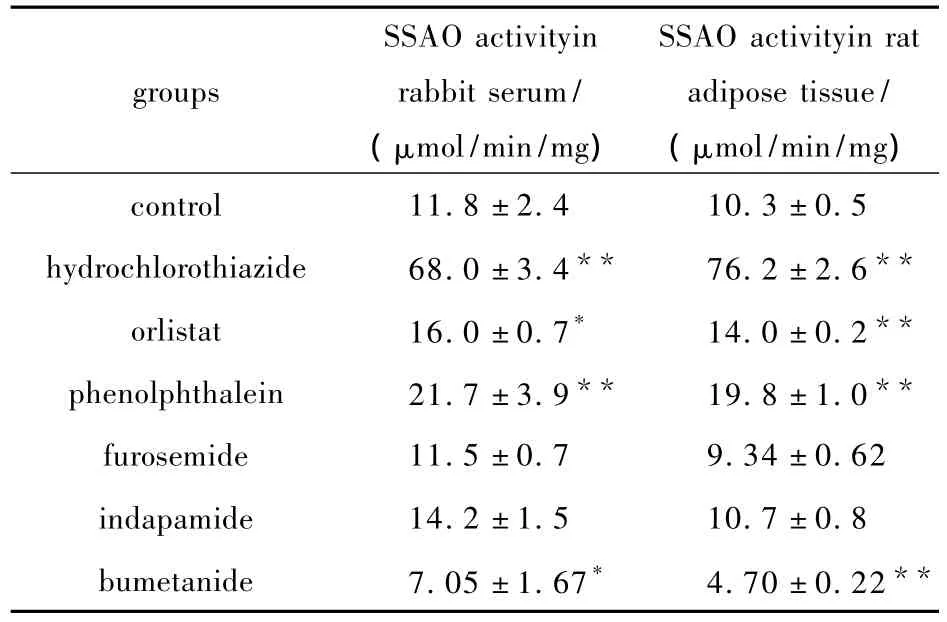
Tab.2 Inhibition by lipid-soluble anti-obesity drugs on rabbit serum and rat adipose tissue SSAO activity in vitro
24.3 %in rat adipose tissue,respectively.SSAO activity was significantly suppressed,therefore caffeine,fenfluramine,bumetanide and amfebutamone were concluded to be effective inhibitors whereas the other eleven drugs were regarded as ineffective.
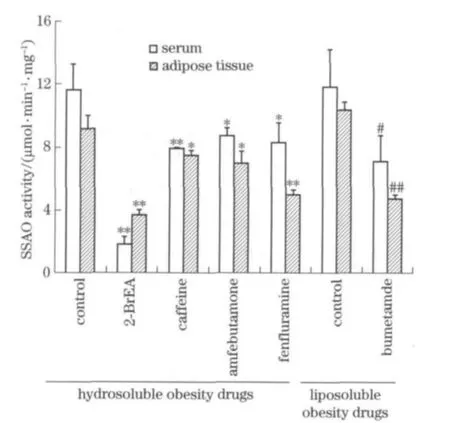
Fig.2 Effect of positive anti-obesity drugs on rabbit serum and rat adipose tissue SSAO activity in vitro
Thirteen of 15 anti-obesity drugs that were investigated contain an aza group,and eight contain an amino group.The chemical structure of metformin and phenformin is similar to hydrazide compounds,therefore it is rational to predict that some anti-obesity drugswould have inhibitory effects on SSAO activity.In our previous study,eleven anti-obesity drugs were assessed for their inhibitory effect on SSAO activity in vitro using HPLC[21], and caffeine wasfound to inhibit SSAO activity.In the present study,common anti-obesity drugs including caffeine,fenfluramine,bumetanide and amfebutamone showed an inhibitory effect on SSAO activity.Interestingly,four drugs were found to suppress SSAO activity in rat adipose tissue or in rabbit serum to different degrees.This may be due to the presence of various forms of SSAO.It is believed that only soluble forms of SSAO present in the serum,while both tissue-bound and soluble forms of SSAO are present in adipose tissue.
2.3 Effect of caffeine on rat serum SSAO in vivo
Because bumetanide is insoluble in water,it is difficultto perform furtheranimalsexperiments.Amfebutamone is used in treating mental disorders.Furthermore, fenfluramine shows severe side effects in clinical studies.Therefore,fenfluramine,bumetanide and amfebutamone are not suitable for long-term clinical usage.Caffeine,on the other hand,is a naturally occurring substance in many dietary products and its toxicity has been reported as very low,so it is prospective to conduct further study.
The level of SSAO activity in the serum was compared among the control group,2-BrEA group and caffeine group(Fig.3).Caffeine inhibited rat serum SSAO activities in vivo.SSAO activity in serum was suppresses by 2 -BrEA and caffeine,with the extent of inhibition being 11.2%with 2-BrEA and 15.6%with caffeine.
The inhibitory effect in vivo of caffeine was even stronger than that of 2-BrEA.In comparison,caffeine was less effective in vitro than 2-BrEA.This difference may be due to the use of enzymes from different sources.In this study,rabbit SSAO was used for in vitro experiments while rat SSAO was used for in vivo measure-ments.

Fig.3 Effect of 2-BrEA and caffeine on rat serum SSAO activity in vivo
It has been reported that SSAO is sensitive to hydrazine compounds containing a carboxyl group and the inhibition is irreversible.2-BrEA is often described as a typical suicide inhibitor[22].In this experiment,2 -BrEA was used as a potent and selective SSAO inhibitor as a positive control.It showed strong inhibitory effects against SSAO activity,even at a low dose.It has been noted that 2-BrEA may potentially damage some organs. It is highly desirable,therefore,to identify a natural product that is highly effective and a selective inhibitor of SSAO as a substitute for 2-BrEA.The present study showed that caffeine,although it does not contain hydrazine or bromo groups like the other inhibitors,showed a strong inhibitory effect on SSAO activity.The results showed that caffeine was a substrate for SSAO and could combine with the enzyme,thus inhibited SSAO activity by reducing the combination of methylamine with SSAO.Further investigations on the mechanism of inhibition of SSAO activity by caffeine are being conducted.
3 Conclusion
An in vitro SSAO activity-based method was developed to screen inhibitors from 15 anti-obesity drugs. Caffeine, fenfluramine, bumetanide and amfebutamone inhibited SSAO activity,and caffeine was the most effective one.The inhibition of SSAO activity by caffeine was confirmed in both an in vitro and an in vivo study.Caffeine provides a natural source of inhibitor of SSAO activity and may,therefore,be a promising inhibitor for the study for the role of SSAO in various diseases.
[1] Obata T.Diabetes and semicarbazide-sensitive amine oxidase(SSAO)activity:a review [J].Life Sci,2006,79(5):417-422.
[2] Jaakkola K,Kaunismaki K,Tohka S,et al.Human vascular adhesion protein-1 in smooth muscle cells[J].Am J Pathol,1999,155(6):1953 -1965.
[3] Smith D J,Salmi M,Bono P,et al.Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule[J].J Exp Med,1998,188(1):17-27.
[4] Buffoni F.Semicarbazide-sensitive amine oxidases:some biochemical properties and general considerations[J].Prog Brain Res,1995,106:323-331.
[5] Mátyus P,Dajka-Halász B,Földi A,et al.Semicarbazide-sensitive amine oxidase(SSAO):current status and perspectives[J].Curr Med Chem,2004,11(10):1285-1298.
[6] Nunes S F,Figueiredo I V,Soares P J,et al.Semicarbazide-sensitive amine oxidase activity and total nitrite and nitrate concentrations in serum:novel biochemical markers for type 2 diabetes[J].Acta Diabetol,2009,46(2):135-140.
[7] Coleman D K.Obese and diabetes:two mutant genes causing diabetes-obesity syndromes in mice [J].Diabetologia,1978,14(3):141 -148.
[8] Nunes S F,Figueiredo I V,Pereira J S,et al.Changes in the activities of semicarbazide-sensitive amine oxidase in inferior mesenteric artery segments and in serum of patients with type 2 diabetes[J].Acta Diabetol,2010,47(2):179-182.
[9] Lu S H,Zhao Y M.The etiology and pharmacologic treatment of obesity[J].Chin Pharmacol Bull,2001,17(6):614-616.
[10] Lizcano J M,Unzeta M,Tipton K F.A spectrophotometric method for determining the oxidative deamination of methylamine by the amine oxidases[J].Anal Biochem,2000,286(1):75-79.
[11] Peter H Y,Wang M,Fan H,et al.Involvement of SSAO mediated deamination in adipose glucose transport and weight gain in obese diabetic KKAy mice[J].Am J Physiol Endocrinol Metab,2004,286(4):E634-641.
[12] Hadri K,Moldes M,Mercier N,et al.Semicarbazide-sensitive amine oxidase in vascular smooth muscle cells:differentiation-dependent expression and role in glucose uptake[J]. Arterioscler Thromb Vasc Biol,2002,22:89 - 94.
[13] Tarancón G E,Marti L,Morin N,et al.Role of semicarbazide-sensitive amine oxidase on glucose transport and GLUT4 recruitment to the cell surface in adipose cells[J].J Biol Chem,1998,273(14):8025-8032.
[14] Kobayashi N,Takahashi K,Takayanagi K,et al.Molecular characteristics of tissue-bound semicarbazide-sensitive amine oxidase(SSAO)in guinea pig tissue[J].Life Sci,2002,70(13):1519 -1531.
[15] Smith I,Goulder M.Randomized placebo-controlled trial of long-term treatment with sibutramine in mild to moderate obesity[J].J Family Pract,2001,50(6):505-512.
[16] Carpéné C,Abello V,Iffiú-Soltész Z,et al.Limitation of adipose tissue enlargement in rats chronically treated with semicarbazide-sensitive amine oxidase and monoamine oxidase inhibitors[J].Pharmacol Res,2008,57(6):426-434.
[17] Kandulska K,Szkudelski T,Nogowski L.Lipolysis induced by alloxan in rat adipocytes is not inhibited by insulin [J].Physiol Res,1999,48(2):113-117.
[18] Wang L,Zhang Y Q,Xiao S Y,et al.LC-MS method for determining the activity of semicarbazide-sensitive amine oxidase in rodents[J].Anal Methods,2012,4:1383-1388.
[19] Peter H Y.Oxidative deamination of aliphatic amines by rat aorta semicarbazide-sensitive amine oxidase[J].Pharm Pharmacol,1990,42(12):882-884.
[20] Peter H Y,Davis B A,Deng Y L.2-Bromoethylamine as a potent selective suicide inhibitor for semicarbazide-sensitive amine oxidase [J]. Biochem Pharmacol,2001,61(6):741 - 748.
[21] Che B Q,Wang L,Xiao S Y,et al.In vitro selection of semicarbazide-sensitive amine oxidase inhibitors in antiobesitic drugs by HPLC[C]∥Proceedings of the 13th Amine Oxidase and Related Diseases Workshop-AO2008,Oct 11 - 15.Beijing:[s.l.],2008:54.
[22] Kinemuchi H,Jinbo M,Tabata A,et al.2-Bromoethylamine,a suicide inhibitor of tissue-bound semicarbazide-sensitive amine oxidase[J].Jpn J Pharmacol,2000,83(2):164 - 166.
