血清ULBP-2、MIC-1联合检测诊断胰腺癌的价值
2013-10-19周郁芬黄李雅徐凌霄张帆国芳姚玮艳袁耀宗
周郁芬 黄李雅 徐凌霄 张帆 国芳 姚玮艳 袁耀宗
·论著·
血清ULBP-2、MIC-1联合检测诊断胰腺癌的价值
周郁芬 黄李雅 徐凌霄 张帆 国芳 姚玮艳 袁耀宗
目的探讨UL16结合蛋白2(ULBP-2)、巨噬细胞抑制因子-1(MIC-1)联合检测对胰腺癌诊断的价值。方法收集152例胰腺癌、20例胰腺癌前病变、91例慢性胰腺炎患者及96例健康对照者血清,应用酶联免疫吸附测定(ELISA)方法检测ULBP-2、MIC-1水平,并和CA19-9水平进行比较。采用受试者工作特征(ROC)曲线评估它们对胰腺癌的诊断价值。结果胰腺癌、胰腺癌前病变、慢性胰腺炎及健康对照者血清ULBP-2水平分别为(219.9±182.5)、(62.6±11.4)、(68.4±36.8)、(76.5±40.9)μg/L;MIC-1水平分别为(3521.3±3903.4)、(973.6±589.0)、(959.6±879.0)、(427.6±317.0)μg/L;CA19-9水平分别为(1448.8±3707.0)、(12.0±9.3)、(38.2±139.0)、(7.7±5.0)kU/L。胰腺癌患者均显著高于癌前病变、慢性胰腺炎患者及健康对照者(χ2值分别为40.628、71.662、45.505,15.827、36.433、63.494,26.264、73.427、49.088,P值均<0.01)。ULBP-2、MIC-1、CA19-9诊断胰腺癌的曲线下面积(AUC)分别为0.909、0.818、0.864,三者联合诊断的AUC为0.982,单指标诊断以ULBP-2为佳,三者联合诊断效能最高。对胰腺癌早期病变(胰腺癌前病变+胰腺癌ⅠA期病变)的诊断,ULBP-2、MIC-1、CA19-9的AUC分别为0.506、0.837、0.684,单指标以MIC-1为佳,而MIC-1联合CA19-9的诊断效能最高(AUC为0.897)。结论ULBP-2、MIC-1在胰腺癌患者血清中含量升高,二者联合CA19-9检测可提高对胰腺癌的诊断价值。
胰腺肿瘤; UL16结合蛋白2; 巨噬细胞抑制因子-1; 生物学标记
虽然临床上常用糖链抗原19-9(CA19-9)诊断和指导治疗胰腺癌,但其敏感性和特异性均不高[1]。UL16结合蛋白2(UL-16 binding proteins 2,ULBP-2)属于经典MHC Ⅰ类相关分子,可与NK细胞激活受体NKG2D/DAP10结合,使NK细胞启动对靶细胞(肿瘤细胞、感染细胞)的杀伤作用,通过基质金属蛋白酶类(MMPs)的作用,成为血清中的游离分子[2]。巨噬细胞抑制因子-1(macrophage inhibitory cytokine-1,MIC-1)属于转化生长因子β(TGF-β)超家族成员,在生理状态下仅胎盘组织有高表达,但在炎症、肿瘤组织中可以出现高表达[3]。ULBP-2、MIC-1与肿瘤的发生、发展密切相关。本研究旨在探讨ULBP-2、MIC-1联合检测对胰腺癌的早期诊断价值。
材料与方法
一、病例资料
收集瑞金医院消化科、外科2008年12月至2012年1月期间收治的152例经手术病理、内镜超声(EUS)细针穿刺活检、ERCP胰管刷检证实的胰腺癌患者。另选取胰腺癌前病变(手术病理确诊为不典型增生者)20例、慢性胰腺炎患者91例、健康志愿者96例作为对照。收集患者一般情况、临床表现及实验室检查结果等资料。
二、方法
收集各组患者及健康对照者的血清,采用酶联免疫吸附测定(ELISA)方法检测血清ULBP-2、MIC-1及CA19-9的水平。ELISA试剂盒购于R&D公司,按试剂盒说明书操作[4-5]。
三、统计学处理
结 果
一、各组血清ULBP-2、MIC-1、CA19-9水平
胰腺癌、胰腺癌前病变、慢性胰腺炎患者及健康对照者血清ULBP-2水平分别为(219.9±182.5)、(62.6±11.4)、(68.4±36.8)、(76.5±40.9)μg/L;MIC-1水平分别为(3521.3±3903.4)、(973.6±589.0)、(959.6±879.0)、(427.6±317.0)μg/L;CA19-9水平分别为(1448.8±3707.0)、(12.0±9.3)、(38.2±139.0)、(7.7±5.0)kU/L。胰腺癌患者均较癌前病变、慢性胰腺炎患者及健康对照者显著升高(χ2值分别为40.628、71.662、45.505,15.827、36.433、63.494,26.264、73.427、49.088,P值均<0.01)。胰腺癌前病变患者仅血清MIC-1水平较健康对照者显著升高(χ2=12.274,P<0.01)。慢性胰腺炎患者的血清MCI-1及CA19-9水平均较健康对照者升高。
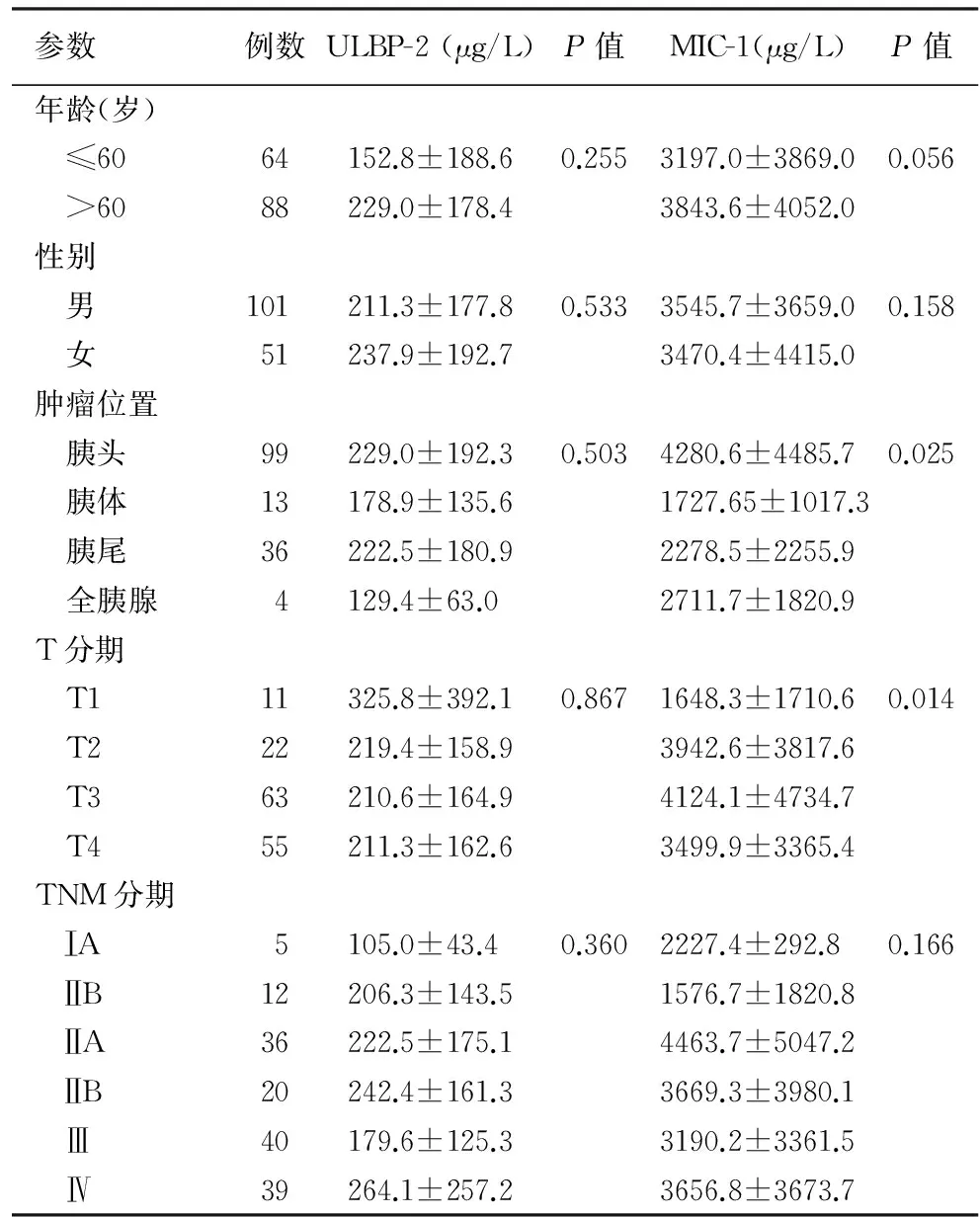
二、胰腺癌患者血清ULBP-2、MIC-1水平与肿瘤临床病理特征间的关系
胰腺癌患者血清ULBP-2水平与患者的性别、年龄及肿瘤部位、T分期、TNM分期均无关;MIC-1水平与肿瘤的部位、T分期有关,而与患者的性别、年龄及肿瘤TNM分期无关(表1)。
三、ULBP-2、MIC-1、CA19-9诊断胰腺癌的效能
ULBP-2、MIC-1、CA19-9诊断胰腺癌的AUC分别为0.909、0.818、0.864(图1),ULBP-2>CA19-9>MIC-1。ULBP-2与MIC-1、ULBP-2与CA19-9、MIC-1与CA19-9联合诊断的AUC分别为0.953、0.977、0.932。三者联合诊断的AUC为0.982。以三者联合诊断的效能最佳。

参数例数ULBP-2(μg/L)P值MIC-1(μg/L)P值年龄(岁) ≤6064152.8±188.60.2553197.0±3869.00.056 >6088229.0±178.43843.6±4052.0性别 男101211.3±177.80.5333545.7±3659.00.158 女51237.9±192.73470.4±4415.0肿瘤位置 胰头99229.0±192.30.5034280.6±4485.70.025 胰体13178.9±135.61727.65±1017.3 胰尾36222.5±180.92278.5±2255.9 全胰腺4129.4±63.02711.7±1820.9T分期 T111325.8±392.10.8671648.3±1710.60.014 T222219.4±158.93942.6±3817.6 T363210.6±164.94124.1±4734.7 T455211.3±162.63499.9±3365.4TNM分期 ⅠA5105.0±43.40.3602227.4±292.80.166 ⅡB12206.3±143.51576.7±1820.8 ⅡA36222.5±175.14463.7±5047.2 ⅡB20242.4±161.33669.3±3980.1 Ⅲ40179.6±125.33190.2±3361.5 Ⅳ39264.1±257.23656.8±3673.7
ULBP-2、MIC-1、CA19-9诊断胰腺癌早期病变(胰腺癌前病变+胰腺癌ⅠA期病变)的AUC分别为0.506、0.837、0.684,MIC-1>CA19-9> ULBP-2。ULBP-2与MIC-1、ULBP-2与CA19-9、MIC-1与CA19-9联合诊断的AUC分别为0.861、0.689、0.897。三者联合诊断的AUC为0.894。以MIC-1和CA19-9联合诊断的效能最佳(图2)。

图1ULBP-2、MIC-1、CA19-9诊断胰腺癌的ROC曲线图2MIC-1、CA19-9联合诊断胰腺癌早期病变的ROC曲线
讨 论
Li等[6]报道,ULBP-2基因中有p53的反应单元,这一单元的去甲基化使得p53与之结合,导致ULBP-2表达上调,从而激发抗肿瘤的固有免疫机制。台湾学者Chang等[4]检测了胰腺癌患者、健康对照者的血清ULBP-2水平,发现胰腺癌患者的血清ULBP-2在癌早期即明显升高,对胰腺癌的诊断价值优于CA19-9,若两者联合诊断可提高诊断的敏感性和特异性。但ULBP-2在其他消化系肿瘤中只有低水平表达,在其他系统肿瘤的血清中尚未发现表达增高。Yang等[7]报道,MIC-1是p53活化的新的生物学标记物,MIC-1在野生型p53活化时表达增高,其他转录因子如Egr-1、NF-kB均可促进MIC-1的促凋亡作用[8]。Koopmann等[5]检测胰腺癌、慢性胰腺炎及健康对照者血清MIC-1水平,发现MIC-1对胰腺癌和正常人鉴别诊断较CA19-9更有效。但对胰腺癌和慢性胰腺炎的鉴别并未表现出优势。
本研究结果显示,胰腺癌患者血清ULBP-2、MIC-1水平显著高于胰腺癌前病变、慢性胰腺炎患者及健康对照者。MIC-1水平随T分期增大而增高。因此MIC-1可能和胰腺癌的进展相关。在胰腺癌诊断方面,单独应用的效能是ULBP-2>CA19-9>MIC-1,联合应用可以提高胰腺癌的诊断效能。在诊断胰腺癌早期病变时,单个指标MIC-1的诊断效能最佳,联合应用中以MIC-1联合CA19-9的诊断效能为最佳。
[1] Goggins M, Canto M, Hruban R. Can we screen high-risk individuals to detect early pancreatic carcinoma? J Surg Oncol, 2000, 74:243-248.
[2] Cosman D, Müllberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity, 2001, 14: 123-133.
[3] Bootcov MR,Bauskin AR,Valenzuela SM,et al.MIC-1,a novel macrophage inhibitory cytokine,is a divergent member of the TGF-beta superfamily.Proc Natl Acad Sci USA,1997,94:11514-11519.
[4] Chang YT, Wu CC, Shyr YM, et al. Secretome-based identification of ULBP2 as a novel serum marker for pancreatic cancer detection. PLoS One, 2011, 6: e20029.
[5] Koopmann J,Rosenzweig CN,Zhang Z,et al.Serum markers in patients with resectable pancreatic adenocarcinoma:macrophage inhibitory cytokine 1 versus CA19-9.Clin Cancer Res,2006,12:442-446.
[6] Li H, Lakshmikanth T, Garofalo C, et al. Pharmacological activation of p53 triggers anticancer innate immune response through induction of ULBP2. Cell Cycle, 2011, 10: 3346-3358.
[7] Yang H, Filipovic Z, Brown D, et al. Macrophage inhibitory cytokine-1: a novel biomarker for p53 pathway activation. Mol Cancer Ther, 2003, 2: 1023-1029.
[8] Baek SJ, Kim JS, Moore SM, et al. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol, 2005, 67: 356-364.
DiagnosticvalueofcombinedmeasurementofserumULBP-2andMIC-1forpancreaticcancer
ZHOUYu-fen,HUANGLi-ya,XULing-xiao,ZHANGFan,GUOFang,YAOWei-yan,YUANYao-zong.
DepartmentofGastroenterology,RuijinHospital,ShanghaiJiaotongUniversitySchoolofMedicine,Shanghai200025,China
Correspondingauthor:YUANYao-zong,Email:yyz28@medmail.com.cn
ObjectiveTo investigate the diagnostic value of UL-16 binding protein 2(ULBP-2), macrophage inhibitory cytokine-1(MIC-1) for pancreatic cancer.MethodsThe serum samples of 152 pancreatic cancer patients, 20 precursors of pancreatic cancer, 91 chronic pancreatitis patients and 96 age/sex-matched healthy persons were collected. The serum ULBP-2 and MIC-1 levels were determined by using the ELISA kit and were compared with level of CA19-9. A receiver operating characteristic (ROC) curve was constructed to evaluate their diagnostic values for pancreatic cancer.ResultsThe serum levels of ULBP-2 in patients with pancreatic cancer, precursors of pancreatic cancer, chronic pancreatitis and healthy persons were (219.9±182.5), (62.6±11.4), (68.4±36.8), (76.5±40.9)μg/L, the corresponding values of MIC 1 were (3521.3±3903.4), (973.6±589.0), (959.6±879.0), (427.6±317.0) μg/L, while the corresponding values of CA19-9 were (1448.8±3707.0), (12.0±9.3), (38.2±139.0), (7.7±5.0)kU/L. The parameters in pancreatic cancer patients were significantly higher than those in control group (χ2=40.628,71.662,45.505,15.827,36.433,63.494,26.264,73.427,49.088,P<0.01). The area under ROC curves(AUC) of ULBP-2, MIC-1, CA19-9 were 0.909, 0.864, 0.818, and ULBP-2 was superior to CA19-9 and MIC-1, however the combined measurement of three markers produced the highest diagnostic yield(AUC=0.982). For early stage pancreatic diseases (precursors to pancreatic cancer and IA stage pancreatic cancer), AUC of ULBP-2, MIC-1, CA19-9 were 0.506,0.837,0.684,MIC-1 was superior to ULBP-2 and CA19-9, however the combined measurement of MIC-1 and CA19-9 produced the highest diagnostic yield(AUC=0.897).ConclusionsSerum ULBP-2, MIC-1 levels are significantly elevated in pancreatic cancer patients. The combined measurement of ULBP-2, MIC-1 and CA 19-9 can increase the diagnostic yield for pancreatic cancer.
Pancreatic neoplasms; UL-16 binding proteins 2; Macrophage inhibitory cytokine-1; Biological markers
2012-10-29)
(本文编辑:屠振兴)
10.3760/cma.j.issn.1674-1935.2013.02.003
200025 上海,上海交通大学附属瑞金医院消化内科(周郁芬、徐凌霄、张帆、国芳、姚玮艳、袁耀宗);宁夏医科大学总医院消化内科(黄李雅)
袁耀宗,Email: yyz28@medmail.com.cn
