Separation and determination of D,L-cloperastine fendizoate enantiomer by chiral high-performance liquid chromatography
2013-10-11DUANHaibaoGAOLijingXIAOGuomin
DUAN Hai-bao,GAO Li-jing,XIAO Guo-min*
(1.School of Chemistry and Chemical Engineering,Southeast University,Nanjing211189,Jiangsu,China;2.Changzhou Science &Information Center,Changzhou213001,Jiangsu,China)
1-{2-[(p-Chloro-α-phenylbenzyl)oxy]ethyl}piperidine fendizoate (Fig.1),also called cloperastine fendizoate,is a antitussive and anti-histamine drug[1].It can be safely used in a wide section of population(children,adolescents and adults)[2],andL-cloperastine fendizoate is thought to be more active and effective[3].Although the conformation of cloperastine fendizoate[4]and the genotoxic impurities in cloperastine fendizoate are available[5],few analytical methods are currently available for the determination of the enantiomers of cloperastine fendizoate.In this paper we report a chiral high-performance liquid chromatographic(HPLC)technique for the determination ofD,L-cloperastine fendizoate.This method is convenient and accurate for the analysis ofD,L-cloperastine fendizoate.
1 Experimental
1.1 Apparatus
HPLC analysis was conducted with a Shimadzu 20AT unit equipped with a CLASS-VP software and fitted with chiral HPLC column (Chiralcel OD-H,dimension 250mm×4.6mm (Daicel Chemical Industries,Japan)).Optical rotations were measured with a JASCO P2000polarimeter.
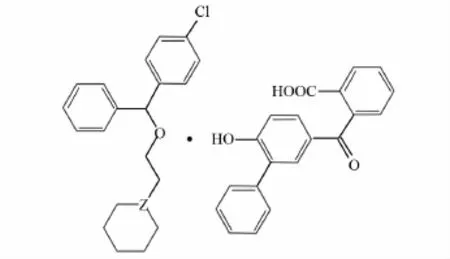
Fig.1 Structures of title compounds
1.2 Reagents
Chromatographic grade diethylamine,hexane and isopropyl alcohol were provided as gifts from Hanbon Science & Technology Company Ltd.
1.3 Sample preparation
Cloperastine fendizoate was synthesized by our laboratory.The sample was dissolved in in mobile phase and filtered by a filter film with a pore diameter of 0.45μm.
1.4 Analytical conditions
The optimised conditions for chiral HPLC analysis are as follows:column chiralcel OD-H,250mm×4.6mm;mobile phase 2%isopropyl alcohol in hexane containing 0.1%diethylamine;flow rate 0.3mL/min,and detection wavelength 254nm.The retention time ofD-cloperastine fendizoate andL-cloperastine fendizoate under the optimized condition is 15.06min and 16.65min,respectively.The configurations and retention time are assigned on the basis of the injection of enantiomerically pureD-cloperastine fendizoate.
2 Results and discussion
Chiral HPLC is convenient and accurate for chiral resolution.The optimum chromatographic separation conditions can be obtained by adjusting the mobile phase composition,flow rate,and column temperature.A mixed solvent consisting ofn-hexane/2-propanol solvent mixture(volume ratio 90∶10)was used as the shipping solvent of the chiralcel OD-H columns.To-be-tested cloperastine fendizoate sample has a low polarity,and hence lowering mobile phase polarity is favourable for acquiring a better separation of the sample.The baseline separation(Fig.2)of the sample was obtained while the volume ratio ofn-hexane/2-propanol mixed solvent was kept as 99∶1.
The best separation result as shown in Fig.3is obtained after a series of optimization.It is seen from Fig.3that bothDandLcloperastine fendizoates can be well separated with Chiralcel OD-H column;and the resolution for the HPLC analysis is 3.3.

Fig.2 Chromatogram of baseline separation of D,L-cloperastine fendizoate
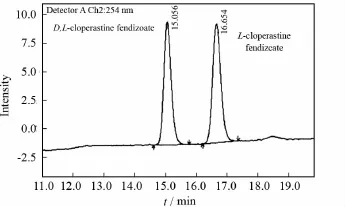
Fig.3 Chromatogram of Dand L-cloperastine fendizoate on under optimized condition
The chromatogram ofD-cloperastine fendizoate under the same condition is shown in Fig.4.In order to check calibration and accuracy,we resolved 2-200μg of to-be-tested sample with the concentration of 0.1-10g/L by injection under the optimum conditions.The regression coefficient is determined to be 0.996-0.997(n=10).
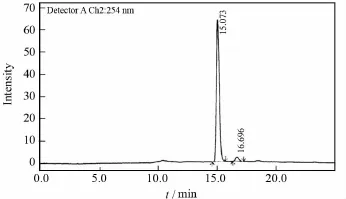
Fig.4 Chromatographic of D-cloperastine fendizoate
3 Conclusions
A convenient and accurate chiral HPLC method is established to determineD,L-cloperastine fendizoate.The optimal condition for the chiral HPLC analysis of title compound has been established.It has been found that the established HPLC method can be well adopted to separate the two enantiomers of cloperastine fendizoate and determine its spectrometric purity as well.In one word,the analysis method is applicable for the quality control of manufacturers.
[1]CANTI G,FRANCO P,NICOLIN A,et al.Studio sull’attivita farmacologica della 1-[2-(p-cloro-α-fenilbenzilossi)etil]-piperidina(cloperastina)[J].Boll Chim Farm,1983,122:384-392.
[2]CATANIA M A,CUZZOCREA S.Pharmacological and clinical overview of cloperastine in treatment of cough[J].Risk Manage,2011,7:83-92.
[3]ALIPRANDI P,CIMA L,CARRARA M.Therapeutic use of levocloperastine as an antitussive agent:an overview of preclinical data and clinical trials in adults and children[J].Drug Invest,2002,22:209-220.
[4]MARIBAYASHI N,FUJII I,HIRAYAMA N.Crystal structure of 1-{2-[(p-chloro-α-phenylbenzyl)oxy]ethyl}piperidine fendizoate(cloperastine fendizoate)[J].Analytical Sci,1999,15:813-814.
[5]GARCÍA A,RUPÉREZ F J,CEPPA F,et al.Development of chromatographic methods for the determination of genotoxic impurities in cloperastine fendizoate[J].J Pharmac Biomed Anal,2012,61:230-236.
