MgCl2 在LiCl-KCl熔 盐中的电化学特性
2013-09-17颜永得张密林张志俭杜卫超
唐 浩 颜永得,,* 张密林 薛 云 张志俭 杜卫超 何 辉
(1哈尔滨工程大学材料科学与化学工程学院,超轻材料与表面技术教育部重点实验室,哈尔滨150001;2哈尔滨工程大学核科学与技术学院,哈尔滨150001;3中国原子能科学研究院,北京102413)
1 Introduction
Magnesium,with a density of 1.74 g·cm−3,is the lightest structural metals used as engineering materials,known as“21st Century Green Engineering Materials”.Magnesium base materials have been widely used in the area of transportation,communication,electronic,aerospace,and sports industries,1-3because magnesium base materials exhibit a series of favorable properties,such as high specific strength and stiffness,superior machinability,and better electromagnetic shielding properties,etc.1,4Rare earth(RE)elements are often used as addition elements to improve alloys properties.The additions of small amount of RE elements to Mg alloys can improve castability,high temperature properties,mechanical properties,and corrosion resistance due to ageing,fine grain,dispersion,and solid solution strengthening.5-7In fact,high cost is the only one negative factor that limits the range of applications of Mg-RE alloys.
Electrolysis in molten chlorides and fluorides is still the main method for industrial production of pure Mg and RE metals.This is an energy demanding process.To date,Mg-RE master alloys are mainly prepared by mixing pure Mg and RE metals directly at temperatures above their melting points.These production processes result in high energy waste.In recent years,electrochemical co-deposition has been widely used to prepare RE alloys in different mediums.Iida et al.8investigated the electrochemical co-deposition of Sm-Co alloys from Li-Cl-KCl-SmCl3-CoCl2melts.Liand co-workers9prepared Sm-Co films on copper substrate by electrodeposition in urea-acetamide-NaBr-KBr melt at 353K.Li et al.10prepared Er-Bi films by co-deposition from ErCl3-Bi(NO3)3-LiCl-DMSO on copper substrate at 305 K.Massot et al.11-13have prepared Al-RE alloys by co-reduction of RE ions with Al ions to recover REs from molten fluorides.The extraction efficiency was greater than 95%.RE have been also reclaimed from molten LiCl-KCl melts by co-reduction of Al,RE,and Li ions to form Al-Li-RE alloys.14-16Hence,the method of electrochemical co-deposition is possible to provide a unique opportunity,which can improve the energy efficiencies and achieve cost-competitive production,for the preparation of Mg-RE master alloys in molten salts.
Electrolysis of magnesium in industry is a mature technology.In terms of production of pure magnesium metal,co-deposition process has adverse effect,which would introduce impurities to the products.To avoid the co-deposition reaction,the concentration of MgCl2in the electrolyte of industrial process is very high.However,this kind of electrolyte in industrial production is not suitable for preparation of Mg-RE alloys.Here,LiCl-KCl melts,which have low melting temperature,high conductivity,and low density,were selected to investigate the co-deposition process in our group.LiCl-KCl eutectic has the advantages of high conductivity,relatively low viscosity and operating temperature.The low operating temperature allows simpler design,with reduced energy consumption,salt volatility,and cell corrosion.Therefore,LiCl-KCl melts are promising molten salts for preparation of magnesium alloys at a temperature lower than the melting points of magnesium metal.Our group17-19already has abundant experience in preparation of Mg-Li-RE alloys.However,the content of REs is small in these materials prepared by electrolytic co-reduction.At present,our group wants to directly extract REs with the assistance of Mg ions in LiCl-KCl eutectic melts at a low temperature by fabrication of Mg-RE alloys which contain a large amount of REs.According to co-deposition theory,deposition potential and the ratio of the feeds determine the constitution of the products.Therefore,thorough understanding of the thermodynamic properties and electrochemical behavior of Mg ions in the melts is critical to their co-deposition process.Moreover,basic electrochemistry data can provide abundant information to design an effective deposition apparatus.
In recent years,electrochemical behavior of rare earths in LiCl-KCl melts has been intensively studied because of pyro-metallurgical reprocessing technology.20These basic data give us abundant information to study how to extract REs from molten salts via electrolytic reduction.The electrochemical behavior of magnesium and the electrodeposition of pure magnesium have been studied in LiCl-KCl melts.21,22Cyclic voltammetry and chronopotentiometry have been employed to determine the diffusion coefficients of Mg(II)species(DMg(II))in LiCl-KCl eutectic melts in the temperature range of 673-773 K.The effect of the temperature on the value of DMg(II)has also been determined by plotting the lnD versus T-1.The activation energy for this diffusion process is 33.1 kJ·mol-1.Hamer et al.23have calculated the theoretical electromotive forces of Mg/MgCl2/Cl2according to the thermodynamic data.Laitinen and Liu24have measured Mg/MgCl2system in LiCl-KCl eutectic melts at 723 K.However,based on our knowledge,the standard apparent potentials of Mg(II)/Mg(0)system and the activity coefficients of Mg(II)ions in a wide temperature range have not been investigated in LiCl-KCl eutectic melts so far.On the basis of these backgrounds,we propose to systematacially investigate the electrochemical behavior and thermodynamic properties of Mg ions in LiCl-KCl eutectic melts.The results will help us to further study the co-deposition of Mg-RE alloys which contain large quantity of RE.
2 Experimental
2.1 Preparation and purification of the melts
All operations of the salts were carried out in a glove box under a high purity argon gas(99.999%)previously dehydrated by heating in a vacuum.Purchased anhydrous lithium chloride(99.0%)and potassium chloride(99.5%)were first dried under high vacuum for more than 72 h at 473 K to remove possible physical moisture.And then the mixture of eutectic LiCl-KCl(nLiCl:nKCl=58.8:41.2)was introduced to an alumina crucible placed in a cylindrical quartz cell located in an electric furnace.The electrolyte was melted under the dry argon gas and the temperature of the melts was monitored with a nickel-chromium thermocouple sheathed with an alumina tube.Magnesium element was introduced into the bath in the form of anhydrous MgCl2(99.0%)powder.
2.2 Electrochemical apparatus and electrodes
All electrochemical measurements were performed using Im6eX electrochemical workstation(Zahner Co.,Ltd.)with the THALES Z1.31 software package.The reference electrode was fabricated by a silver wire(d=1 mm,99.99%purity)dipped into a solution of AgCl(1%,mass fraction)in the LiCl-KCl eutectic contained in a Pyrex tube.All potentials were referred to this Ag/Ag+couple.The counter electrode was a spectral pure graphite rod(d=6 mm).The working electrodes were tungsten(W)wires(d=1 mm,99.99%purity),which were polished thoroughly using SiC paper,and then cleaned ultrasonically with ethanol prior to use.Between each measurement the W working electrode was cleaned by applying an anodic polarization.The active electrode surface area was determined after each experiment by measuring the immersion depth of the electrode in the molten salts.
3 Results and discussion
3.1 Cyclic voltammetry
Fig.1 illustrates the cyclic voltammograms obtained on tungsten electrodes in LiCl-KCl eutectic melts before and after the addition of MgCl2at 723 K.The cyclic voltammogram of the purified melts is the dashed line in Fig.1.The sharp increase of cathodic current from about-2.44 V(vs Ag/Ag+)and the corresponding anodic current are considered to be the reduction of lithium metal and the reverse reaction,respectively.In the positive potential region,the quick rise of anodic current is observed from about 1.20 V,which is considered as the oxidation of chloride ions to chlorine gas.There are no other additional peaks within the electrochemical window,which indicates that the melts are suitable for investigations.The solid line in Fig.1 shows the cyclic voltammogram containing MgCl2in the LiCl-KCl eutectic melts.The reduction peak A,at approximately-1.90 V,shows a stepped rise and gradual decay associated with Mg(II)reduction,characteristic of deposition of a new insoluble phase on the inert electrode limited by diffusion.25The reverse anodic scan shows an oxidation peak Aʹof the depletion of the deposited Mg metal.The cathodic peak B at about-2.36 V is attributed to the underpotential deposition of Li on pre-deposited Mg film to form Mg-Li alloys,and the corresponding anodic peak Bʹis found at around-2.31 V.
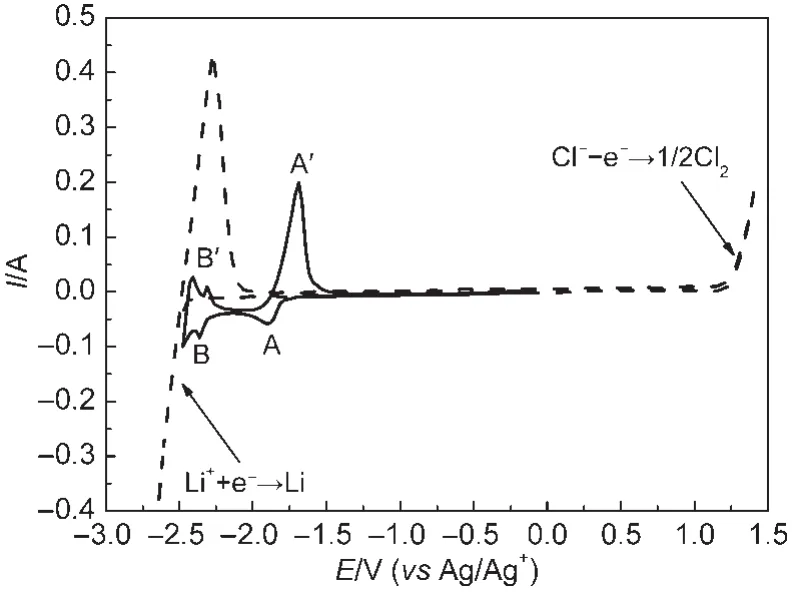
Fig.1 Cyclic voltammograms obtained on W electrodes(S=0.322 cm2)in the LiCl-KCl eutectic melts before and after the addition of 1.18%(mass fraction)MgCl2
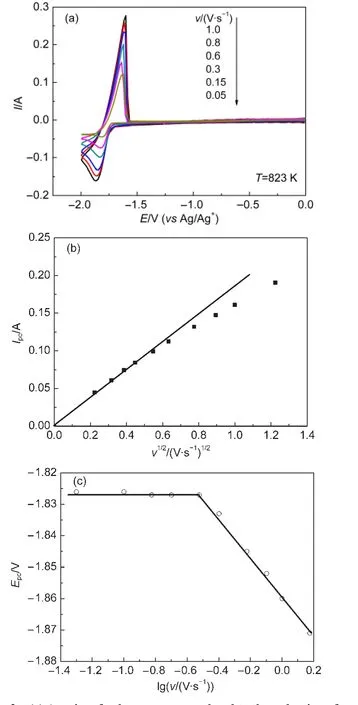
Fig.2 (a)Aseries of voltammograms related to the reduction of Mg(II)on a W electrode(S=0.322 cm2)in the LiCl-KCl eutectic melts containing 1.18%of MgCl2;(b)variation of the cathodic peak current with the square root of the scan rate;(c)variation of the cathodic peak potential with the logarithm of the scan rate
The reversibility of Mg(II)/Mg(0)system was evaluated from 723 to 908 K and the results were similar.Fig.2(a)shows the voltammograms obtained upon deposition and dissolution of Mg(II)/Mg(0)system at different sweep rates at 823 K.In excess of about 0.30 V·s-1,peak potentials clearly shift toward more negative potentials with the increase of scan rate.This is characteristic of a reaction limited by electron transfer.Further-more,a further analysis of the recorded voltammograms is based on the measurement of the variation of peak potentials and peak currents as scan rates change,according to the methodology proposed in the literature.25-27Fig.2(b)illustrates that the plot of Ipcvs v1/2(Ipcis the cathodic peak current and v is the scan rate)is a line from the origin at low scan rates.Moreover,the peak potentials are almost constant until scan rate is greater than 0.30 V·s-1(Fig.2c).The behaviors revealed from Figs.2a to 2c are characteristic of a quasi-reversible system where the electrochemical reaction is controlled by the mass transfer rate at low scan rates.Nevertheless,for scan rates greater than 0.30 V·s-1,the electron transfer rate predominates the electrochemical reaction.
3.2 Square wave voltammetry
Square wave voltammetry has been carried out to calculate the number of electrons involved in the magnesium reduction process.According to the literature,28,29for a reversible system,the mathematical analysis of a square wave voltammogram yields a simple equation relating the width of the half peak(W1/2)and the number of exchanged electrons:

where R is the universal gas constant,T is the absolute temperature,n is the number of exchanged electrons,and F is the Faradayʹs constant.
Eq.(1)also can be utilized to estimate the number of electrons involved in the reaction of Mg(II)/Mg when we apply this technique at low frequencies and small step potentials.Fig.3 shows a square wave voltammogram obtained at a step potential of 1 mV and frequency of 10 Hz in the LiCl-KCl-MgCl2system for the reduction of Mg(II)on a tungsten electrode at 823 K,in which the reaction was in the reversible range.A single peak at-1.79 V was observed and the computed value of n is 1.92±0.10,close to two electrons,which is related to the reaction of Mg(II)/Mg.
3.3 Diffusion coefficient
Diffusion coefficient of Mg(II)in LiCl-KCl eutectic was determined in the temperature range of 723-908 K by cyclic voltammetry.For Mg(II)/Mg(0)system,at low scan rates,the Berzins and Delahay equation30can be used to estimate the diffusion coefficient from the following formula:

Fig.3 Net-current square wave voltammogram for the reduction of Mg(II)at a W electrode(0.322 cm2)at 823 K
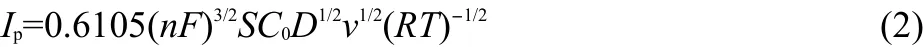
where Ipis the peak cathodic current(A),S is the electrode surface area(cm2),C0is the bulk concentration of Mg ion(mol·cm-3),D is the diffusion coeffcient(cm2·s-1),v is the potential sweep rate(V·s-1).
The obtained diffusion coefficient values at different temperatures are listed in Table 1.The influence of the temperature on the value of D was determined by plotting the lgD versus T-1,as shown in Fig.4.The trend shown in Fig.4 is described by

The results obey theArrheniusʹs law expressed as

where D0is the coefficient of the Arrhenius equation and EAthe activation energy for diffusion.From the relationship(3),the value of the activation energy for diffusion of Mg(II),was computed to be(39.0±2.5)kJ·mol-1.It is slightly higher than the value obtained by Støre22who found EAto be 33.1 kJ·mol-1.This discrepancy may be attributed to the different temperature ranges.
3.4 Equilibrium potential and apparent standard potential
EMF measurements were performed to determine equilibrium potential for calculating the apparent standard potential.31For each temperature,the tungsten electrode was coated with Mg film by applying a cathodic potential for 60 s,and the open-circuit potential(OCP)was recorded versus the Ag/Ag+reference electrode(Fig.5).A very stable plateau was obtained each time,allowing the measurement of the corresponding equilibrium potential Eeq.The measured potential for a metal in equilibrium with its metal chloride,in this case the Mg(II)/Mg(0)couple,is determined by the Nernst relationship.
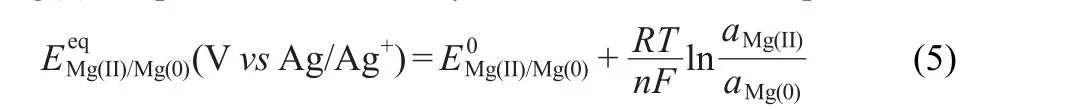
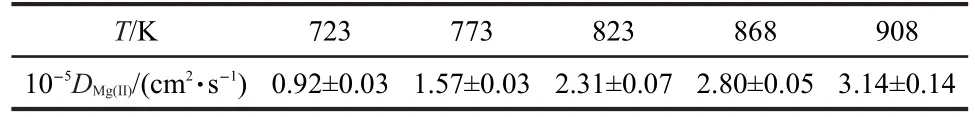
Table 1 Diffusion coefficient of Mg(II)ions in a molten LiCl-KCl eutectic at different temperatures
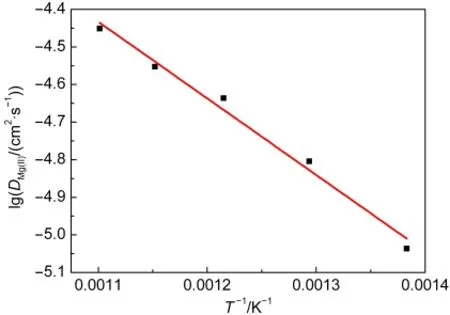
Fig.4 Variation of diffusion coefficient of Mg(II)with temperature in the LiCl-KCl eutectic melts
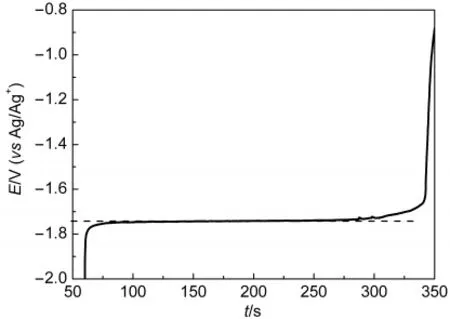
Fig.5 Typical open-circuit potential transient curve for a Welectrode after electrodepositing at-2.00 V(vsAg/Ag+)for 60 s in the LiCl-KCl eutectic melts containing 1.18%MgCl2at 823 K
The apparent standard potential,E*0Mg(II)/Mg(0)of the Mg redox couple is defined as
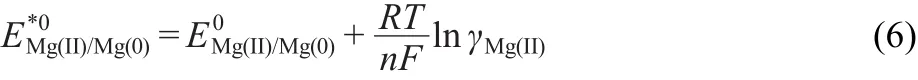
where γMg(II)=aMg(II)/XMg(II),is the activity coefficient of Mg(II),and XMg(II)is the molar fraction of Mg(II)in the salt.Combining Eq.(5)and Eq.(6),can be expressed as
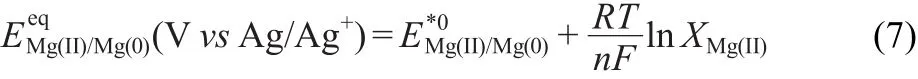
The potential data have been calculated versus the Cl2/Clreference electrode,according to the following equation:
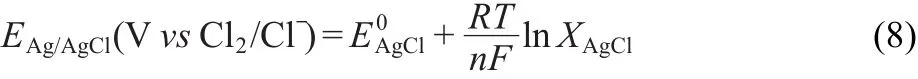
Based on extrapolation to infinite dilution of the data of Yang and Hudson32at low AgCl concentrations in the LiCl-KCl eutectic and other references,31,33the potential of the reference electrode used in this work(0.0039 mole fraction AgCl)versus Cl2/Cl-is given by the expression
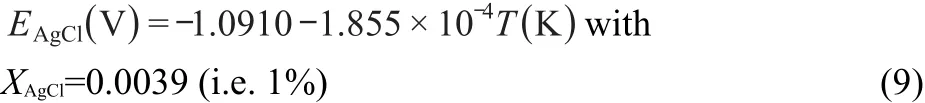
Table 2 summarizes the experimental data obtained from Mg deposited on a tungsten wire by open circuit potentiometry.Fig.6 shows the variation of standard potential with temperature computed from the experimental data of Table 2.The experimental data of the present study(shown by the line)can be described by
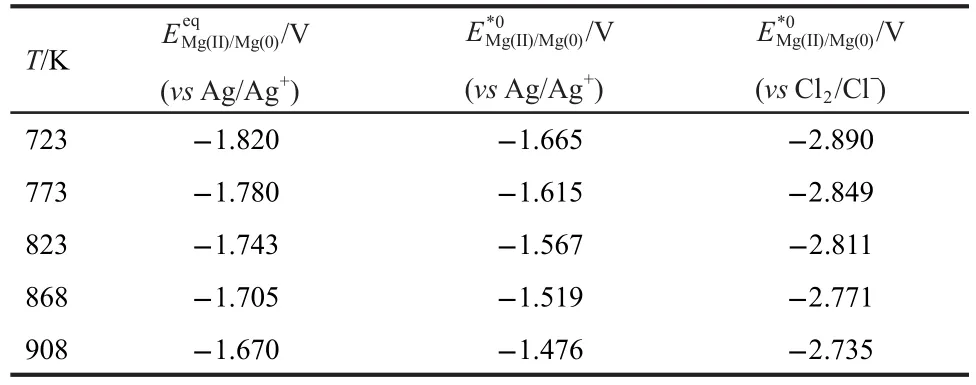
Table 2 Experimental data for apparent standard potential and standard potential of Mg(II)/Mg(0)in the LiCl-KCl eutectic melts
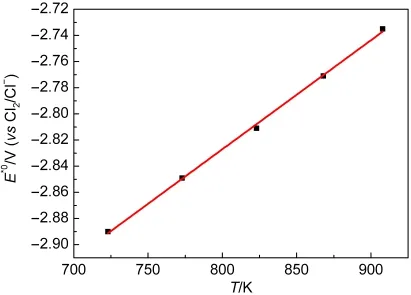
Fig.6 Variation of the standard potential of Mg(II)/Mg(0)with temperature in the LiCl-KCl eutectic melts

3.5 Gibbs free energy and activation coefficient
The standard potential relationship of Eq.(10)can be used to compute the Gibbs free energy of formation and subsequent dissolution of the reaction

according to the relationship

where ΔG*0MgCl2is the Gibbs free energy(kJ·mol-1)of the dissolved metal chloride,calculated from the experimentally-determined standard potential,E*0Mg(II)/Mg(0),Gibbs free energy is also a function of temperature,following the form

from which the enthalpy and entropy can be obtained.The values from the present study were plotted versus temperature,and the relationship was drawn to be

The activity coefficient of MgCl2in LiCl-KCl eutectic melts was determined from the difference between the Gibbs energy of formation derived from the electrochemical measurements and the Gibbs energy of formation for pure compounds in the supercooled state,according to the equation:
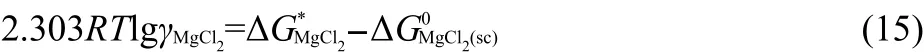
Where ΔGM0gCl2(sc)is the Gibbs energy of formation from reaction of the pure compounds in the supercooled(sc)state.The Gibbs energy of formation of MgCl2in the supercooled state was calculated according to Eqs.(16,17),in which the thermochemical data of Mg,Cl2,and MgCl2in 298 K were obtained from literature.34The thermodynamic data are summarized in Table 3.
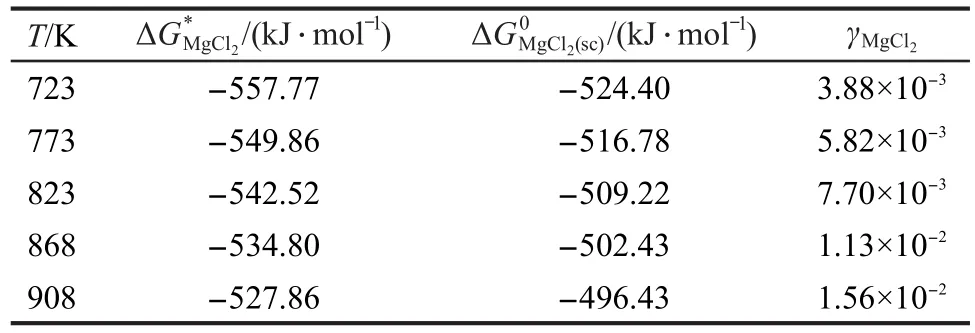
Table 3 Activity coefficient for MgCl2(XMg(II)=7.002×10−3)in the LiCl-KCl eutectic melts
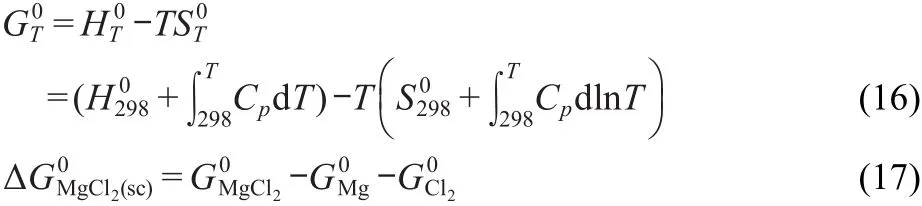
4 Conclusions
Electrochemical properties of MgCl2were studied using cyclic voltammetry and square wave voltammetry in the eutectic LiCl-KCl mixture in the temperature range of 723-908 K on a W electrode.The reduction of Mg(II)to Mg metal is a quasireversible system and occurs with two electrons exchange.The diffusion coefficients of the Mg(II)ions were calculated at several temperatures.At 903 K(a suitable temperature to co-reduction of Mg-RE alloys),the diffusion coefficient is 3.58×10-5cm2·s-1,and the data reveal that a temperature dependence complies with Arrhenius law.Moreover,by using EMF measurements the standard apparent potential of Mg(II)/Mg(0)redox systems is found to beE*0Mg(II)/Mg(0)=-2.742V(vs Cl2/Cl-).According to the standard apparent potentials at different temperatures,the valuesofenthalpy and entropy changes are-674.3 and 0.1608 kJ·mol-1,respectively.Furthermore,we have established the method to calculate the activity coefficient of MgCl2in the LiCl-KCl eutectic melts at different temperatures.
(1)Alam,M.E.;Han,S.;Nguyen,Q.B.;Hamouda,A.M.S.;Gupta,M.J.Alloy.Compd.2011,509,8522.doi:10.1016/j.jallcom.2011.06.020
(2) Hassan,S.F.;Gupta,M.J.Alloy.Compd.2006,419,84.doi:
10.1016 /j.jallcom.2005.10.005
(3) Kojima,Y.Mater.Trans.2001,42,1154.doi:10.2320/matertrans.42.1154
(4) Nguyen,Q.B.;Gupta,M.J.Alloy.Compd.2008,459,244.doi:10.1016/j.jallcom.2007.05.038
(5)Lü,Y.;Wang,Q.;Zeng,X.;Ding,W.;Zhai,C.;Zhu,Y.Mater.Sci.Eng.2000,A278,66.
(6) Sanschagrin,A.;Tremblay,R.;Angers,R.Mater.Sci.Eng.1996,A220,69.
(7)Wu,R.Z.;Qu,Z.K.;Zhang,M.L.Rev.Adv.Mater.Sci.2010,24,35.
(8) Iida,T.;Nohira,T.;Ito,Y.Electrochim.Acta 2003,48,2517.doi:10.1016/S0013-4686(03)00293-7
(9)Li,J.X.;Lai,H.;Zhang,Z.C.;Zhuang,B.;Huang,Z.G.Acta Phys.-Chim.Sin.,2007,23(8),1301.[李加新,赖 恒,张志城,庄 彬,黄志高.物理化学学报,2007,23(8),1301.]doi:10.3866/PKU.WHXB20070832
(10) Li,G.R.;Tong,Y.X.;Liu,G.K.Acta Phys.-Chim.Sin.2003,19(7),630.[李高仁,童叶翔,刘冠昆.物理化学学报,2003,19(7),630.]doi:10.3866/PKU.WHXB20030713
(11) Gibilaro,M.;Massot,L.;Chamelot,P.;Taxil,P.Electrochim.Acta 2009,54,5300.doi:10.1016/j.electacta.2009.01.074
(12) Gibilaro,M.;Massot,L.;Chamelot,P.;Taxil,P.J.Nucl.Mater.2008,382,39.doi:10.1016/j.jnucmat.2008.09.004
(13) Gibilaro,M.;Massot,L.;Chamelot,P.;Cassayre,L.;Taxil,P.Electrochim.Acta 2009,55,281.doi:10.1016/j.electacta.2009.08.052
(14)Yan,Y.D.;Tang,H.;Zhang,M.L.;Xue,Y.;Han,W.;Cao,D.X.;Zhang,Z.J.Electrochim.Acta 2012,59,531.doi:10.1016/j.electacta.2011.11.007
(15)Yan,Y.D.;Xu,Y.L.;Zhang,M.L.;Xue,Y.;Han,W.;Huang,Y.;Chen,Q.;Zhang,Z.J.J.Nucl.Mater.2013,433,152.doi:10.1016/j.jnucmat.2012.09.008
(16)Tang,H.;Yan,Y.D.;Zhang,M.L.;Li,X.;Huang,Y.;Xu,Y.L.;Xue,Y.;Han,W.;Zhang,Z.J.Electrochim.Acta 2013,88,457.doi:10.1016/j.electacta.2012.10.045
(17)Cao,P.;Zhang,M.L.;Han,W.;Yan,Y.D.;Wei,S.Q.;Zheng,T.J.Rare Earths 2011,29,763.doi:10.1016/S1002-0721(10)60538-8
(18)Han,W.;Tian,Y.;Zhang,M.L.;Ye,K.;Yan,Y.D.;Zhao,Q.Y.;Wei,S.Q.J.Rare Earths 2010,28,227.doi:10.1016/S1002-0721(09)60085-5
(19)Xue,Y.;Yan,Y.D.;Zhang,M.L.;Han,W.;Zhang,Z.J.J.Rare Earths 2012,30,1048.doi:10.1016/S1002-0721(12)60177-X
(20) Castrillejo,Y.;Hernández,P.;Rodriguez,J.A.;Vega,M.;Barrado,E.Electrochim.Acta 2012,71,166.doi:10.1016/j.electacta.2012.03.124
(21) Martínez,A.M.;Børresen,B.;Haarberg,G.M.;Castrillejo,Y.;Tunold,R.J.Appl.Electrochem.2004,34,1271.doi:10.1007/s10800-004-1761-6
(22) Støre,T.;Haarberg,G.M.;Tunold,R.J.Electroanal.Chem.2000,30,1351.
(23)Hamer,W.J.;Malmberg,M.S.;Rubin,B.J.Electrochem.Soc.1956,103,8.doi:10.1149/1.2430236
(24) Laitinen,H.A.;Liu,C.H.J.Am.Chem.Soc.1958,80,1015.doi:10.1021/ja01538a001
(25) Pletcher,D.;Greef,R.;Peat,R.;Peter,L.;Robinson,J.Instrumental Methods in Electrochemistry;Southampton Electrochemistry Group,University of Southampton,Horwood:London,2001.
(26) Bard,A.J.;Faulkner,L.R.Electrochemical Methods,Fundamental and Applications;Wiley:New York,2001.
(27) Nicholson,M.M.J.Am.Soc.1954,76,2539.doi:10.1021/ja01638a072
(28) Osteryoung,J.;Osteryoung,R.A.Anal.Chem.1985,57,101.doi:10.1021/ac00279a004
(29)Ramaley,L.;Krasue,M.S.Anal.Chem.1969,41,1362.doi:10.1021/ac60280a005
(30) Berzins,T.;Delahay,P.J.Am.Chem.Soc.1953,75,555.doi:10.1021/ja01099a013
(31) Cassayre,L.;Serp,J.;Soucek,P.;Malmbeck,R.;Rebizant,J.;Glatz,J.P.Electrochim.Acta 2007,52,7432.doi:10.1016/j.electacta.2007.06.022
(32) Yang,L.;Hudson,R.G.J.Electrochem.Soc.1959,106,986.doi:10.1149/1.2427195
(33) Fusselman,S.P.;Roy,J.J.;Grimmett,D.L.;Grantham,L.F.;Krueger,C.L.;Nabelek,C.R.;Storvick,T.S.;Inoue,T.;Hijikata,T.;Kinoshita,K.;Sakamura,Y.;Uozumi,K.;Kawai,T.;Takahashi,N.J.Electrochem.Soc.1999,146,2573.doi:10.1149/1.1391974
(34) Barin,I.;Knacke,O.Thermochemical Properties of Inorganic Substances;Springer:Berlin,1973,Supplement,1997.
