荸荠皮酚性成分及其抗氧化活性研究
2013-09-12李行任罗杨合彭丽艳吴兴德杜如男赵勤实
李行任,罗杨合*,何 隽,彭丽艳,吴兴德,杜如男,赵勤实*
1贺州学院桂东特色资源研究与开发广西高校重点建设实验室,贺州 542899;2中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室,昆明 650204
Introduction
Eleocharis tuberosa(Cyperaceae)is one of the most popular hydrophytic vegetables in China and other Asia countries.The corms are consumed as fruit,grain or vegetable,and prepared of starch and canning.E.tuberosa has also been used as a traditional medicine to treat pharyngitis,laryngitis,enteritis,cough,hepatitis and hypertension[1].The peels of E.tuberosa contain puchiin,cytokinin,flavonoids and phenolics[1-3].However,little research was reported about its chemical constituents.In recent years,fresh-cut E.tuberosa corms are in high demand because of their unique taste,medicinal properties.A large number of peels were wasted.It was reported that the extracts of E.tuberosa peels showed strong bactericidal[2]and antioxidant activities[3].To comprehensive utilize it,the chemical constitutes of E.tuberosa peels were investigated.As a result,a new phenolic,named eleocharinol A(1)with 8 known phenolic compounds was isolated.The antioxidant properties of the isolated compounds,including reducing power and scavenging activities against DPPH radical,were evaluated.
Experimental
Instrumentation
Optical rotations(OR)were recorded on a JASCO-20C digital polarimeter.Ultraviolet absorption(UV)spectra were determined on a Shimadzu UV-2401A spectrophotometer(Shimadzus,Kyoto).Infrared spectroscopy(IR)spectra were measured on a Tensor 27 spectrometer with KBr pellets.Nuclear Magnetic Resonance(NMR)spectra were collected on Bruker AV-400,DRX-500 and AVANCE III-600 spectrometers with tetramethylsilane as an internal standard.Electrospray ionization-mass spectrometry(ESI-MS)and high-resolution(HR)EI-MS were recorded with a Bruker HCT/Esquire and Waters AutoSpec-P776 mass spectrometer,respectively.Semi-preparative HPLC was performed on an Agilent 1100 liquid chromatography with a Zorbax SB-C18 column(Agilent Technologies,USA).Column chromatography(CC)was performed using silica gel or polyamides(100-200 and 200-300 mesh,Qingdao Marine Chemical Co.Ltd.,Qingdao,China),MCI gel(75-150 μm;Mitsubishi Chemical Corporation,Japan),and Sephadex LH-20(Amersham Pharmacia Biotech,Sweden).
Plant materials
Peels offresh-cutE.tuberosa were collected in Hezhou,Guangxi province,People's Republic of China,in March 2012,and identified by Professor PAN Bai-ming of Hezhou University,Hezhou.A voucher specimen was kept in the laboratory for future reference.
Extraction and isolation
The air-dried and powdered peels of E.tuberosa(8.0 kg)were extracted three times with 70%aqueous acetone(32 L × 3),each for 24 h,at room temperature,and concentrated in vacuo to give a crude extract.The crude extract was suspended in water,followed by partitioning with EtOAc.The EtOAc fraction(120 g)was chromatographed on MPLC(MCI gel)eluted with a gradient of MeOH-H2O(5∶5,7∶3,9∶1 and 10∶0)to afford four fractions(A-D).Fraction A(21.5 g)was chromatographed on MCI CC and then on polyamides CC to give compound 9(13 mg)and compound 6(330 mg).Fraction B(9.8 g)was purified by a polyamides CC and eluted with gradient CHCl3-MeOH(from 5∶1 to 0∶1,v/v),followed by polyamides CC and silica CC with PE-acetone-H2O(49∶49∶2)to produce compounds 5(20 mg),TLC(CHCl3-MeOH-H2O=90∶9∶1)to afford 8(20 mg).Fraction C(31 g)was submitted to a polyamides CC and eluted with gradient CHCl3-MeOH(from 5∶1 to 0∶1,v/v),then separated by Sephadex LH-20 CC,followed by semi-preparative HPLC to give compound 2(120 mg),3(7 mg),and 4(15 mg),by MIC CC to produce 7(10 mg).Fraction D(28 g)was separated by CC on silica(PE-CHCl3=8∶2)to give compound 1(50 mg).
Antioxidant activity
Reducing power assay
A modification of the method described by Li Xia,et al[4]was used.A sample solution(1 mL,0.1 mg/mL in ethanol)was added into a phosphate buffer(2.5 mL,0.2 M,pH 6.6)and potassium ferricyanate(2.5 mL,10 mg/mL).The mixtures were incubated at 50oC for 20 min,and then 2.5 mL of trichloroacetic acid(100 mg/mL)was added and centrifuged at 2000 g for 10 min.The distilled water(2.5 mL)and ferric chloride(0.5 mL,1.0 mg/mL)was added to 2.5 mL of the supernatant.The absorbance was measured at 700 nm using a spectrophotometer.Rutin with the same concentration was used as the reference sample.The increase in absorbance represented the increase in reducing power.
DPPH radical scavenging assay
The 2,2-diphenyl-1-picrylhydrazyl(DPPH)assay was conducted according the reported method[5]with some modifications.The DPPH solution was prepared by dissolving 39.4 mg of DPPH with 500 mL of ethanol,and then was stored in a cool,dark area,until needed.0.1 mL of blank(ethanol)or sample solutions in ethanol at different concentrations(from 0.05 mg/mL to 16 mg/mL)was each added to 3.9 mL of a DPPH solution,which was diluted to 0.080 mM with ethanol.The solution in the testing tubes was shaken vigorously and incubated in the dark for 30 min at 37oC.Then,the absorbance of mixture was measured at 517 nm.The tests were carried out in triplicate.The antioxidant ac-tivity was expressed as the antioxidant activity index(AAI),calculated as follows as:
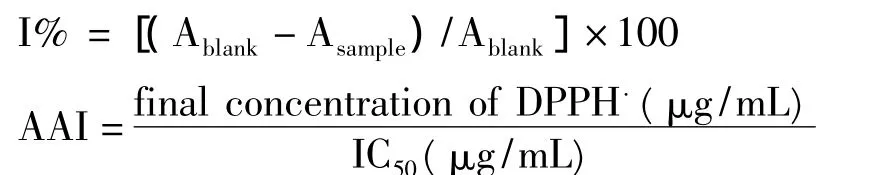
The IC50(concentration providing 50%inhibition)was calculated on a calibration curve by plotting the sample concentration and the corresponding scavenging effect.The sample would show poor,moderate,strong,or very strong antioxidant activity when AAI values were less than 0.5,between 0.5 and 1.0,between 1.0 and 2.0,or larger than 2.0,respectively.
Results and Discussion
Structural elucidation
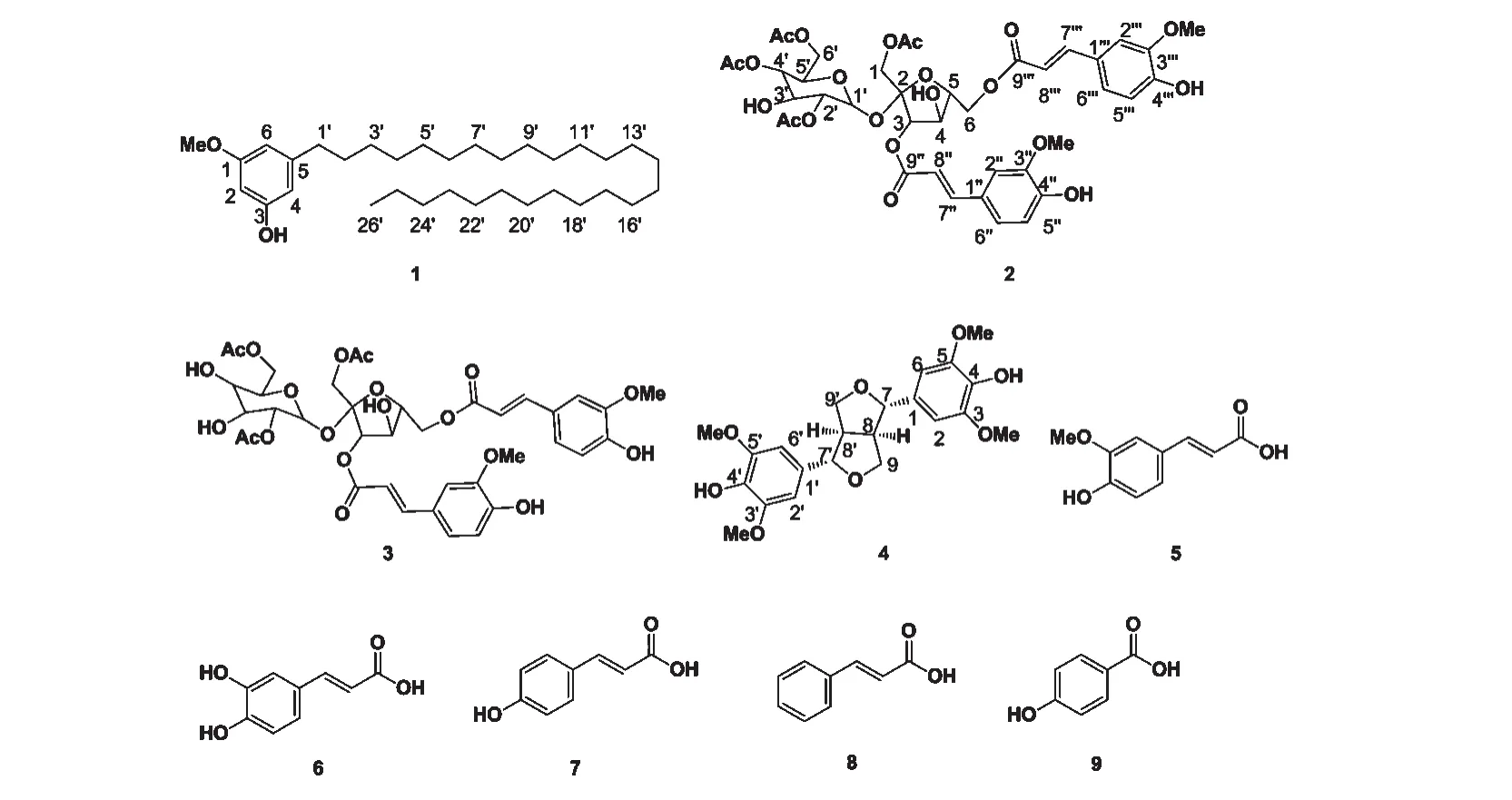
Fig.1 Compounds isolated from E.tuberosa
Compound 1 Compound 1 had the molecular formulaas deduced from HR-EI-MS at m/z 488.4608[M]+(calcd 488.4593),in accordance to four degrees of unsaturation.Its UV spectrum showed the typical absorptions of a phenolic group at 203 and 275 nm and its IR spectrum revealed band for saturated longchain groups(2918,2850,1467,720 cm-1).The1H NMR spectrum showed a hydroxyl group[4.97(1H,s,3-OH)],three aromatic singlets[δH6.33(1H,s,H-6),6.26(1H,s,H-4),and 6.24(1H,d,J=1.7 Hz,H-2)],and a extraordinary saturated multiplet[1.34-1.25(46H,overlapped,H-3'to 25')].The13C NMR spectra revealed the presence of 16 overlapped carbons.All of the saturated carbons were secondary carbons,except for two of methyl groups[55.2(q,1-OCH3)and 14.1(q,C-26')],and suggested it contained a long-chain alkyl.In1H-1H COSY spectrum,the multiplet at δH1.57(2H,m,H-2')was coupled with H-1'[2.51(2H,t,J=7.7 Hz)]and H-3'[1.31(overlapped)],and the triplets at 0.88 [(3H,t,J=6.6 Hz,H-26')] showed cross-peaks with H-25'[1.28(overlapped)].In the HMBC spectrum,the singlet at δH6.24(H-2)was coupled with C-1(δC160.7),C-3(δC156.4),and C-4(δC107.8),and the protons at δH6.26(H-4)and δH6.33(H-6)also showed obvious cross-peaks with their Cαand Cβ,respectively.The signals at δC31.9 and δC22.7 were assigned to C-24'and C-25',respectively,on the basis of HMBC correlations.As a result,all proton and carbon signals were fully assigned based on HMQC,HMBC and H-H COSY spectral analysis,and the positions of the substitution and saturated long-chain skeleton were determined.Consequently,the structure of compound 1 was determined as 3-hexacosyl-5-methoxyphenol,and named as Eleocharinol A.
Data of compound 1:White amorphous solid(CHCl3);:-12.48(c 1.01,MeOH);UV(MeOH)λmax(log ε)275.20(3.05),203.40(4.33)nm;IR(KBr)νmax3426,2918,2850,1624,1467,1148,1057,720 cm-1;1H NMR(400 MHz,CDCl3)and13C NMR(100 MHz,CDCl3)data,see Table 1;ESI-MS(positive-ion mode)m/z 489[M+H]+;HR-EI-MS m/z 488.4608 [M]+(calcd forC33H60O2,488.4593).

Table 1 1H and13C NMR spectroscopic data for compound 1a(δ in ppm)
Compound 2 Colorless amorphous solid(MeOH);1H NMR(400 MHz,MeOD-d4)δ:4.22(3H,m,H-1,5'),5.38(1H,d,J=7.7 Hz,H-3),4.45(3H,m,H-4,4',5),4.10(3H,m,H-6,3'),5.67(1H,d,J=3.4 Hz,H-1'),4.80(1H,m,H-2'),4.66(2H,m,H-6'),7.25(1H,s,H-2''),6.80(2H,d,J=8.1 Hz,H-5'',5'''),7.09(2H,d,J=9.8 Hz,H-6'',6'''),7.69(1H,d,J=15.9 Hz,H-7''),6.44(1H,d,J=15.2 Hz,H-8''),7.18(1H,s,H-2'''),7.64(1H,d,J=16.0 Hz,H-7'''),6.40(1H,d,J=15.5 Hz,H-8'''),1.85(3H,s,1-OCOCH3),2.01(3H,s,2'-OCOCH3),2.08(3H,s,4'-OCOCH3),2.10(3H,s,6'-OCOCH3),3.89(6H,s,3'',3'''-OCH3);13C NMR(100 MHz,MeOD-d4)δ:66.6(t,C-1),103.9(s,C-2),79.5(d,C-3),73.9(d,C-4),81.5(d,C-5),64.1(t,C-6),90.5(d,C-1'),73.8(d,C-2'),70.0(d,C-3'),72.2(d,C-4'),69.8(d,C-5'),64.7(t,C-6'),127.7(s,C-1''),111.7(d,C-2''),149.5(s,C-3''),151.1(s,C-4''),116.6(d,C-5''),124.7(d,C-6''),148.3(d,C-7''),115.1(d,C-8''),168.8(s,C-9''),127.5(s,C-1'''),111.6(d,C-2'''),149.4(s,C-3'''),150.7(s,C-4'''),116.5(d,C-5'''),124.4(d,C-6'''),147.3(d,C-7'''),114.3(d,C-8'''),168.0(s,C-9'''),171.8(s,1-OCOCH3),20.7(q,1-OCOCH3),172.1(s,2'-OCOCH3),20.8(q,2'-OCOCH3),172.2(s,4'-OCOCH3),20.9(q,4'-OCOCH3),172.7(s,6'-OCOCH3),21.0(q,6'-OCOCH3),56.5(q,3'',3'''-OCH3);ESI-MS(negative-ion mode)m/z 861[MH]-.The NMR data were in accordance with the reported data[6,7].Therefore,compound 2 was characterized as 1,2',4',6'-tetraacetyl-3,6-diferuloyl-sucrose.
Compound 3 Colorless amorphous solid;1H NMR(500 MHz,MeOD-d4)δ:4.17-4.10(6H,m,H-1,3',5',6'),5.44(1H,d,J=8.4 Hz,H-3),4.52-4.40(4H,m,H-4,5,6),5.63(1H,d,J=3.6 Hz,H-1'),4.60(1H,m,H-2'),3.33(1H,overlapped in water peak,H-4'),7.27(1H,s,H-2''),6.81(2H,d,J=8.1 Hz,H-5'',5'''),7.12(1H,d,J=9.4 Hz,H-6''),7.71(1H,d,J=15.9 Hz,H-7''),6.47(1H,d,J=15.9 Hz,H-8''),7.22(1H,s,H-2'''),7.11(1H,d,J=8.4 Hz,H-6'''),7.66(1H,d,J=15.9 Hz,H-7'''),6.42(1H,d,J=16.0 Hz,H-8'''),2.07(3H,s,1-OCOCH3),2.09(3H,s,2'-
OCOCH3),2.12(3H,s,6'-OCOCH3),3.90(6H,s,3'',3'''-OCH3);13C NMR(125 MHz,MeOD-d4)δ:66.3(t,C-1),103.6(s,C-2),79.1(d,C-3),74.3(d,C-4),81.3(d,C-5),65.1(t,C-6),90.6(d,C-1'),72.2(d,C-2'),73.8(d,C-3'),71.9(s,C-4'),72.1(d,C-5'),65.2(t,C-6'),127.8(s,C-1''),115.2(d,C-2''),147.2(s,C-3'',3'''),151.1(s,C-4''),116.6(d,C-5'',5'''),124.5(d,C-6''),149.5(d,C-7''),112.1(d,C-8''),168.8(s,C-9''),127.5(s,C-1'''),114.4(d,C-2'''),150.9(s,C-4'''),124.3(d,C-6'''),148.3(d,C-7'''),111.9(d,C-8'''),168.3(s,C-9'''),172.1(s,1-OCOCH3),20.7(q,1-OCOCH3),172.4(s,2'-OCOCH3),20.9(q,2'-OCOCH3),172.9(s,6'-OCOCH3),21.0(q,6'-OCOCH3),56.6(q,3'',3'''-OCH3);ESI-MS(positive-ion mode)m/z 843[M+Na]+.The spectral data were matched with literature[6,7],hence it was identified as 1,2',6'-triacetyl-3,6-diferuloylsucrose.
Compound 4 Colorless needle(MeOH);1H NMR(500 MHz,MeOD-d4)δ:6.65(4H,s,H-2,2',6,6'),4.70(2H,d,J=3.9 Hz,H-7,7'),3.13(2H,m,H-8,8'),4.25(2H,dd,J=8.8,6.6Hz,H-9a,9'a),3.88(2H d,J=3.0 Hz,H-9b,9'b),3.83(12H,s,-OCH3×4);13C NMR(125 MHz,MeOD-d4)δ:133.2(s,C-1,1'),104.4(d,C-2,2',6,6'),149.3(s,C-3,3',5,5'),136.0(s,C-4,4'),87.6(d,C-7,7'),55.5(d,C-8,8'),72.8(t,C-9,9'),56.8(q,-OCH3× 4);ESI-MS(positive-ion mode)m/z 441,[M+Na]+.Compound 4 was identified as(+)-syringaresinol by a comparison of its spectral data with the reported[8].
Compound 5 White amorphous powders(MeOH);1H NMR(400 MHz,MeOD-d4)δ:7.16(1H,s,H-2),6.78(1H,d,J=8.1 Hz,H-5),7.04(1H,d,J=8.1 Hz,H-6),7.56(1H,d,J=15.9 Hz,H-7),6.29(1H,d,J=15.9 Hz,H-8),3.87(3H,s,3-OMe);13C NMR(100 MHz,MeOD-d4)δ:127.8(s,C-1),111.6(d,C-2),150.4(s,C-3),149.3(s,C-4),116.4(d,C-5),124.0(d,C-6),146.8(d,C-7),116.1(d,C-8),171.1(s,C-9),56.4(q,3-OCH3);ESI-MS(negative-ion mode)m/z 193[MH]-.The NMR data were in accordance with those reported in literature[6],and compound 5 was identified as trans-ferulic acid.
Compound 6 White amorphous powders(MeOH);1H NMR(400 MHz,MeOD-d4)δ:7.03(1H,d,J=2.0 Hz,H-2),6.77(1H,d,J=8.2 Hz,H-5),6.93(1H,dd,J=8.2,1.9 Hz,H-6),7.53(1H,d,J=15.9 Hz,H-7),6.22(1H,d,J=15.9 Hz,H-8);13C NMR(100 MHz,MeOD-d4)δ:127.8(s,C-1),115.0(d,C-2),146.8(s,C-3),149.5(s,C-4),116.5(d,C-5),122.9(d,C-6),147.0(d,C-7),115.5(d,C-8),171.1(s,C-9);ESI-MS(negative-ion mode)m/z 179[M-H]-.Comparing the NMR data with references[9],compound 6 was determined to be caffeic acid.
Compound 7 White amorphous powder(MeOH);1H NMR(400 MHz,MeOD-d4)δ:7.44(2H,d,J=8.4 Hz,H-2,6),6.80(2H,d,J=8.5 Hz,H-3,5),7.58(1H,d,J=15.8 Hz,H-7),6.29(1H,d,J=15.9 Hz,H-8);13C NMR(100 MHz,MeOD-d4)δ:127.3(s,C-1),131.0(d,C-2,6),116.8(d,C-3,5),161.0(s,C-4),146.3(d,C-7),116.1(d,C-8),171.5(s,C-9);ESI-MS(negative-ion mode)m/z 163[M-H]-.Compound 7 was identified as p-coumaric acid by comparison of the spectral data[6].
Compound 8 White amorphous powder(MeOH);1H NMR(400 MHz,MeOD-d4)δ:7.50(2H,d,J=7.1 Hz,H-2,6),7.40(1H,d,J=16.0 Hz,H-7),7.37-7.26(3H,m,H-3,4,5),6.50(1H,d,J=15.9 Hz,H-8);13C NMR(100 MHz,MeOD-d4)δ:137.2(s,C-1),128.5(d,C-2,6),129.8(d,C-3,5),130.1(d,C-4),141.1(d,C-7),126.6(d,C-8),175.6(s,C-9);ESI-MS(negative-ion mode)m/z 147 [MH]-.The spectral data were agreed with the literature values of cinnamic acid[10].
Compound 9 White amorphous powder(MeOH);1H NMR(500 MHz,Pyridine-d5)δ:8.45(2H,d,J=8.5 Hz,H-2,6),7.24(2H,d,J=8.4 Hz,H-3,5);13C NMR(100 MHz,MeOD-d4)δ:118.3(s,C-1),132.8(d,C-2,6),116.1(d,C-3,5),163.2(s,C-4),169.4(s,CO);ESI-MS(negative-ion mode)m/z 137[M-H]-.The NMR data were in accordance with 4-hydroxylbenzoic acid[11].
Antioxidant activity
All the isolated compounds were evaluated for antioxidant activities by reducing power assay and DPPH radical scavenging assay.The results were showed in Table 2.In terms of reducing power,compounds 5 and 6 showed very strong reducing power.The reducing power of compound 9 was moderate,and those of the others were poor.In the DPPH radical scavenging assay,compounds 4,5,6,and 9 showed very strong DPPH radical scavenging activities.The DPPH radical scavenging activities of compounds 2 and 3 were moderate,and those of the others were poor.

Table 2 Reducing Power and DPPH Radical Scavenging Activity of Isolated Compounds
1 State Administration of Traditional Chinese Medicine.Chinese Materia Medica.Shanghai:Shanghai Scientific and Technical Publishers,1999.Vol 24,566-568.
2 Liu X,Hao SX,Zhao LC,et al.Study on tracking bacteriostatic components in puchiin.Food Sci,2005,26:78-81.
3 You Y,Duan X,Wei X,et al.Identification of major phenolic compounds of Chinese water chestnut and their antioxidant activity.Molecules,2007,12:842-852.
4 Li X,Zhang JY,Gao WY,et al.Chemical composition and anti-inflammatory and antioxidant activities of eight pear cultivars.J Agric Food Chem,2012,60:8738-8744.
5 Scherer R,Godoy HT.Antioxidant activity index(AAI)by the 2,2-diphenyl-1-picrylhydrazylmethod.Food Chem,2009,112:654-658.
6 Lee SY,Choi SU,Lee JH,et al.A new phenylpronpane glycoside from the rhizome of Sparganium stoloniferum.Arch Pharm Res,2010,33:515-521.
7 Sun X,Zimmermann ML,Campagne JM,et al.New sucrose phenylpropanoid esters from Polygonum perfoliatum.J Nat Prod,2000,63:1094-1097.
8 Park KH,Yeon SW,Cho JG,et al.Lignans from Silkworm droppings and their promotional activities on Heme Oxygenase-1(HO-1).J Korean Soc Appl Biol Chem,2010,53:734-739.
9 Zhang WD,Chen WS,Kong DY,et al.Studies on the chemical constituents of Erigeron breviscapus.Chin Pharm J ,2000,35:514-516.
10 Yang LJ,Yang XD,Li L.Study on chemical constituents of Lagotis yunnanensis.Chin Med Mat,2005,28:767-768.
11 Zhou LY,Zhang XH,Chen CX.Chemical study on Rhodiola from Lijiang.Nat Prod Res Dev(天然产物研究与开发),2004,16:410-414.
