Effect of Impregnation Sequence on Propane Dehydrogenation Performance of PtSnNa/ZSM-5 Catalyst
2013-07-31LiuHuiZhouYumingZhangYiweiShengXiaoliZhangZewuZhouShijiang
Liu Hui; Zhou Yuming; Zhang Yiwei; Sheng Xiaoli; Zhang Zewu; Zhou Shijiang
(1. School of Chemistry and Chemical Engineering, Southeast Uniνersity, Nanjing 211189; 2. School of Chemistry and Chemical Engineering, Jiangsu Uniνersity, Zhenjiang 212013)
Effect of Impregnation Sequence on Propane Dehydrogenation Performance of PtSnNa/ZSM-5 Catalyst
Liu Hui1,2; Zhou Yuming1; Zhang Yiwei1; Sheng Xiaoli1; Zhang Zewu1; Zhou Shijiang1
(1. School of Chemistry and Chemical Engineering, Southeast Uniνersity, Nanjing 211189; 2. School of Chemistry and Chemical Engineering, Jiangsu Uniνersity, Zhenjiang 212013)
The effects of the sequence for impregnation of metal precursors on the performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation to propene were studied in this paper. Some methods such as XRD, TPDA, BET, H2-TPR, XPS, ICP, TEM and hydrogen chemisorption were used to characterize the catalysts. The structure of ZSM-5 zeolite was not destroyed by the introduction of metal components. Meanwhile the different impregnation sequence of metal precursors could affect the behavior of Sn4+species entering the ZSM-5 channel, and the interaction between platinum and tin species, as well as the degree for reduction of Pt and Sn components. As a result, the prepared catalysts exhibited different reaction activity and selectivity. Compared with the co-impregnation treated catalyst, the catalysts prepared by the sequential impregnation method showed better catalytic activity in propane dehydrogenation, especially the one prepared through impregnation with tin precursor at first. Finally, a model for the effect of impregnation sequence on the distribution of Pt and Sn species in PtSnNa/ZSM-5 catalyst was proposed.
impregnation sequence, propane dehydrogenation, PtSnNa/ZSM-5, propene
1 Introduction
With the fast growth in the demand for propene derivatives, various alternative technologies for producing propene, such as propane dehydrogenation, methanol-toolefin (MTO), and fluid catalytic cracking (FCC) technologies, have been studied[1-3]. Among these, propane dehydrogenation technology is of great interest to researchers because of its high propene selectivity.
PtSn/γ-Al2O3is a catalyst widely used in the area of propane dehydrogenation[4-5]. However, coke is usually formed and accumulated easily on the surface of γ-Al2O3at low pressure and high temperature, making PtSn/ γ-Al2O3catalyst inactivated quickly during the reaction. So new carriers, especially the zeolites, are garnering great interest of many researchers. The ZSM-5 zeolite contains the special three-dimensional channels, which can hinder the formation of large hydrocarbon molecules, thus preventing the so-called coke deposition and improving the catalytic stability. Moreover, the acidity of ZSM-5 zeolite can be changed randomly. At present, ZSM-5 zeolite is widely applied in such reactions as alkane dehydrogenation, aromatization, catalytic cracking, and isomerization[6-7].
For a supported multi-component catalyst prepared by impregnation, the method for preparation of the catalyst, especially the impregnation sequence of metal precursor, is known to be an important factor for the performance of catalysts. Pakornphant, Shu, and Deng studied the effect of impregnation sequence on the metal dispersion and reaction performance of the PtSn/Al2O3catalyst, the Pt-Ni catalyst, and the Cu-Co catalyst, respectively. The results indicated that the impregnation sequence could influence the dispersion of active components on the surface of carrier, the formation of metal-metal bonds and the interaction between various components and the carrier, thus affecting the performance of catalysts[8-10].
According to our previous researches, the PtSnNa/ZSM-5 catalyst showed optimum catalytic performance for propane dehydrogenation[11-13]. However, the co-impregnation method was used in all of our previous papers[14-16]when Pt and Sn species were loaded on ZSM-5 zeolite, whilethe effect of sequence for impregnation of zeolite with metal precursor on catalytic activity of PtSnNa/ZSM-5 catalyst has not been studied yet until now.
Therefore, three tri-component (Pt/Sn/Na) catalysts based on ZSM-5 zeolite were prepared in this work. The introduction of Na was the same for all of these catalysts and three different impregnation methods were utilized in the introduction of Pt species and Sn species, including coimpregnation and sequential impregnation (either preimpregnating Sn species firstly or pre-impregnating Pt species firstly). The first objective of this work was to evaluate how the impregnation sequence could affect the structure of catalysts and the interaction between Pt species, Sn species and the carrier. The second objective was to study the effects of impregnation sequence on the propane dehydrogenation performance, in order to select an optimum method for preparing the PtSnNa/ZSM-5 catalyst.
2 Experimental
2.1 Catalyst preparation
The first step for manufacture of three catalysts was to prepare the Na/ZSM-5 powder. The powdered HZSM-5 zeolite was impregnated with a solution of 0.427 mol/L of NaCl at 80 ℃ for 4 h, followed by drying at 80 ℃ for 3 h to obtain the required Na/ZSM-5 powder. Then three different metals deposition techniques were used in the following impregnation process.
1) Co-impregnation: Na/ZSM-5 powder was impregnated in a mixed solution composed of 0.033 mol/L of H2PtCl6and 0.153 mol/L of SnCl4at 80 ℃ for 4 h followed by drying at 80 ℃ for 3 h. Thus, the co-impregnation sample was prepared, which was defined as the PtSn catalyst;
2) Sequential impregnation was carried out in the following order: The zeolite was impregnated with H2PtCl6solution at first prior to drying, and then was impregnated with SnCl4solution, followed by drying. The impregnation conditions were the same as those adopted during the co-impregnation process. The obtained sample was defined as the Pt-Sn catalyst;
3) Sequential impregnation was conducted with the preparation process and conditions being the same as those used for impregnating the Pt-Sn catalyst, except for the sequence for impregnating the zeolite with the respective SnCl4solution and H2PtCl6solution. The sample was defined as the Sn-Pt catalyst. The nominal composition of each catalyst sample was equal to 0.5 m% of Pt, 1.0 m% of Sn, and 1.0 m% of Na.
All these samples were fully agglomerated with 5.0 m% of alumina during the process of agglomeration. After having been dried, the catalysts were calcined at 500 ℃for 5 h in an air flow and reduced in a flowing H2stream at 500 ℃ for 8 h.
2.2 Catalyst characterization
The XRD patterns of the catalyst samples were collected with a D/Max2200 X-ray powder diffractometer (Ricoh, Japan) having a Cu Kα radiation filter and an angular range 2θof between 5° and 40° using a scanning rate of 5(°)/min to determine the positions of the ZSM-5 peaks. The pore volume and specific surface area were measured from the adsorption and desorption data acquired on a Micromeritics ASAP 2020 adsorption and desorption apparatus. Each sample was pretreated at 350℃ under a vacuum of 5×10-3Pa for 15 h.
The temperature-programmed reduction (TPR) studies of samples were performed in a conventional TPD/TPR apparatus interfaced with a computer. A sample of 0.15 g was placed in a quartz tubular reactor. Prior to the TPR experiments, the catalysts were dried in a flowing N2at 400 ℃ for 1 h and then the TPR profiles were obtained by passing a 5% H2/N2flow (at a rate of 40 mL/min) through the sample. The temperature was increased at a rate of 10 ℃/min from room temperature to 650 ℃.
X-ray photoelectron spectroscopy (XPS) measurements were performed using a Kratos Amicus instrument with a non-monochromatic Mg Kα X-ray source (1 253.6 eV). Before the analyses, all samples were reduced in situ under a hydrogen flow at 500 ℃ for 1 h. Then the spectra were recorded at room temperature under a vacuum lower than 1×10-9mbar. All binding energy (B. E.) values were charge-corrected to the C1s excitation set at 284.8 eV.
The concentration of the acid sites was measured by temperature programmed desorption of ammonia (TPDA) and the platinum dispersion was obtained from hydrogen chemisorption experiment using the same apparatus used for the TPR experiments. The experimental details were in accordance with the reference [13].
Inductively coupled plasma (ICP) optical emission spectrometry was performed with a VISTA-MPX inductively coupled plasma quantometer (Varian, USA) to determine the metal content in each sample. Transmission electron microscopy (TEM) micrographs were performed with a JEOL-JEM-2010 (JEOL, Japan) operating at 200 kV.
2.3 Catalytic reaction
Propane dehydrogenation reaction was carried out in a conventional quartz tubular micro-reactor. The experimental setup was similar to the one that was described in a previous paper[17]. Reaction conditions were as follows: 1.5 g of catalyst was tested at a reaction temperature of 590 ℃, a reaction pressure of 0.1 MPa; a WHSV of 3.0 h-1and a H2/C3H8molar ratio of 0.25. The reaction gas products were analyzed with an online GC9890A gas chromatograph. Results from a reproduced experiment showed that the propane conversion and propene selectivity had an error of ±5%.
3 Results and Discussion
3.1 Characterization of catalysts
Figure 1 shows the XRD patterns of different samples. The representative peaks at 7°—8° and 23°—24° are shown in the XRD patterns of all samples, indicating that the characteristic structure of zeolite was not destroyed during the impregnation process.
It is worth noting that the diffraction peak intensity at 2θ=7°—8° in all the catalyst samples weakens slightly in comparison with that of pure HZSM-5 zeolite. In general, the decrease of the peak intensity in lower angle regions demonstrates that some amount of species has entered the channels of the support[18-19]. Moreover, it should be noted thatmight disperse on the surface of ZSM-5 zeolite, while Na+and Sn4+species could enter the channel during the impregnation process because of their difference in diameter. Among these catalysts, the lowest intensity of diffraction peak at 2θ=7°—8° is found in the Sn-Pt catalyst, which suggests that more metal ions may enter the channels of zeolite. Possibly, this behavior is hindered by chloroplatinic acid during the preparation of both of the PtSn catalyst and the Pt-Sn catalyst. For the PtSn catalyst, mutual competition coexists betweenspecies and Sn4+species. In the case of the Pt-Sn catalyst,species distributed on the surface and ostiole of zeolite preferentially may prevent the ingress of Sn4+species into the zeolite channels.

Figure 1 XRD patterns of different samples
The BET surface area, microporous volume and average pore size of three catalyst samples were measured and the results are presented in Table 1. It is obvious that the specific surface area, microporous volume and average pore size of catalyst samples changed with the difference in the sequence for impregnating the carrier with metal precursors. The related characterization values of the Sn-Pt catalyst decrease in comparison with other samples, which may imply once again that more Sn species in the Sn-Pt catalyst may enter the channels of ZSM-5.
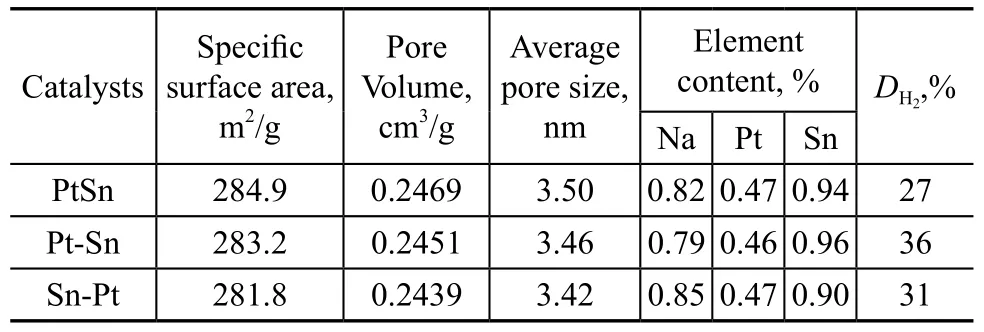
Table 1 The characterization data for the prepared catalysts
Table 1 also lists the element contents of different catalyst samples measured by the ICP method and the Pt metal dispersion obtained by the hydrogen chemisorption experiments. It is revealed that the element contents in the three catalysts are almost the same because of the similar preparation method using the same solution with the same concentration of reagents. It can be seen from the value ofthat the dispersity of Pt metal in the sequentially impregnated catalyst is enhanced in comparison with that in the co-impregnated catalyst. The test results indicate that the sequential impregnation method is favorable to the distribution of Pt metal, especially in the case of impregnating zeolite with Pt species prior to impregnating withSn species.
Figure 2 shows the H2-TPR pro files of different catalysts. No obvious peak is found for HZSM-5 zeolite in the reduction process. Three peaks are identi fied from the profiles of all catalyst samples, which represent the reduction peak of Pt4+→ Pt0, Sn4+→ Sn2+, and Sn2+→ Sn0from low temperature to high temperature, respectively[11-12].

Figure 2 The H2-TPR profiles of different catalyst samples
Compared with the Sn-Pt catalyst, the Pt reduction peaks in Pt-Sn catalyst and PtSn catalyst shift to the higher temperature region and their peak area decreases, indicating that the reduction of Pt species in Pt-Sn catalyst and PtSn catalyst becomes more difficult, which is caused by the strong interaction of Pt and Sn[20]. Moreover, the reduction peaks at low temperature of Sn species in two catalyst samples, which represent the interaction between Pt species and Sn species, shift to the higher temperature region, attesting similarly to the enhancement of the interaction between Pt and Sn species.
The reduction peaks at high temperature of Sn species in PtSn catalyst and Pt-Sn catalyst, which represent the interaction between Sn species and the carrier, move to lower temperature region and the peak area increases in comparison with that in Sn-Pt catalyst. The result indicates that the interaction between Sn species and the carrier is weakened in PtSn catalyst and Pt-Sn catalyst, and the Sn species in the two catalysts exist mainly in the form of Sn0. The possible reason is that the position of strong interaction of metal with the carrier is occupied preferentially by Pt species, irrespective of whether the carrier is impregnated first with H2PtCl6solution or coimpregnated with H2PtCl6solution and SnCl4solution. Furthermore, as it can be seen from Figure 1 and Table 1, more amount of Sn4+has entered the channels of ZSM-5 zeolite in Sn-Pt catalyst, which can protect Sn species from being easily reduced to Sn0.
To further obtain information about the oxidation state of the metal phase (Sn) on the surface of ZSM-5 zeolite, the XPS spectra of different catalyst samples were obtained and the results of the Sn3d XPS region are shown in Figure 3. In addition, the measured binding energies of the Si2p, Al2p and Sn3d5/2levels and the atomic concentration of different elements on the surface of catalyst are summarized in Table 2.

Figure 3 Sn3d XPS spectra of the different catalysts
As shown in Figure 3, three peaks at about 484 eV, 486 eV and 487 eV are obtained in three catalysts, which correspond to different tin species. Generally, the component at low binding energy is associated with the zero valentSn, while the others are ascribed to the tin oxides[21]. It can be seen from the data in Table 2 that the percentage of Sn0in PtSn catalyst, Pt-Sn catalyst and Sn-Pt catalyst is 29.9%, 26.8% and 11.4%, respectively. The results show that more Sn species in Sn-Pt catalyst can exist in the form of tin oxides in comparison with those in other two catalysts.

Table 2 XPS binding energies and atomic concentration on the surface of carrier for different catalysts
Moreover, judging from the atomic concentration of different catalysts, it is found out that the percentage of Pt atoms on the surface of all the catalyst samples is similar, showing that Pt species can disperse on the surface of the sequential-impregnation catalyst and the co-impregnation catalyst. However, the percentage of Sn atoms on the surface in the Sn-Pt catalyst is far lower than that in other catalysts. The result indicates that more Sn species in Sn-Pt catalyst enter the channels of carrier, which is in accordance with the result of XRD analyses.
Figure 4 shows the transmission electron micrographs of different catalysts. It can be seen from the low-magnif ication transmission electron micrographic images (Figure 4a, 4c and 4d) that the distribution of metal particles on the surface of Sn-Pt catalyst and the Pt-Sn catalyst is uniform which is in a narrow particle size range, while the agglomeration of particles is observed in the PtSn catalyst. It verifies that the sequential impregnation is beneficial to the distribution of metal particles, which is in agreement with the result ofDH2. The different metal phases on different catalysts are identified from the HRTEM images (Figure 4b, 4e and 4f) and thedvalues of these diffracted spots are calculated using the Gatan digital micrograph software. The PtSn (withd(110)=0.355 nm andd(101)=0.297 nm) and Pt3Sn (withd(110)=0.283 nm) alloy phases[5,22]are confirmed on the sequential-impregnation catalyst and theco-impregnation catalyst, respectively. Clearly, these phenomena indicate that the formation of Pt-Sn alloy is inevitable no matter which impregnation method is adopted. However, the probability of forming Pt-Sn alloy in the coimpregnation catalyst is more than that in the sequentialimpregnation catalyst as evidenced by Figure 4b, which is consistent with the conclusion of H2-TPR analysis.

Figure 4 Transmission electron micrographs of different catalysts
The TPDA profiles of different samples are presented in Figure 5. Two NH3desorption peaks at 241 ℃ and 445 ℃ are found in the profile of HZSM-5 zeolite, which represents weak acid sites and strong acid sites on the surface of zeolite, respectively. As regards all the catalyst samples, no high-temperature desorption peak is detected, showing that the strong acid centers on zeolite are preferentially neutralized. The low-temperature desorption peak shifts to the lower temperature region and the peak area decreases, indicating that the acidic intensity and amount of the weak acid sites are reduced. It is worth noting that the similar acidity for all of the catalyst samples is identified. It is believed that the addition of Na species plays an important role in determining the catalyst acidity. In this way, the influence of different impregnation methods for the introduction of Pt and Sn species on the acidity of catalysts is insignificant. In our experiments, the way for incorporating Na species is same for all the catalysts. Based on this point of view, the similar acidity of different catalysts is not surprising.

Figure 5 The TPDA profiles of different samples
3.2 Propane dehydrogenation over different catalysts
Figure 6 shows the catalytic performance versus the reaction time for different catalyst samples. As shown in Figure 6, the propane conversion of the stepwise impregnated catalysts (the Pt-Sn catalyst and Sn-Pt catalyst) is higher than that of the co-impregnation catalyst (the PtSn catalyst), which indicates that the stepwise introducing the metal components into the carrier is favorable to the dehydrogenation of propane. The highest initial propane conversion and initial propene selectivity are achieved by the Pt-Sn catalyst, but the fast deactivation of the catalyst is observed subsequently. After 9 h of reaction, the propane conversion decreases to 28.7% and the catalyst deactivation rate reaches 13.9%. However, the deactivation rate of Sn-Pt catalyst is only 5.0% in the same time and the final propane conversion is still close to 30%. Therefore, the different impregnation sequence of metal precursors has an obvious influence on the reaction performance of the catalyst. The stepwise impregnation method is the optimum impregnation method for preparation of PtSn-Na/ZSM-5 catalyst and the sequence of impregnating the support with Sn species prior to Pt species is an appropriate impregnation sequence.

Figure 6 Propane dehydrogenation performance vs reaction time of different catalysts
Yu, et al. reported that four reaction processes occurredduring the propane dehydrogenation reaction on the surface of ZSM-5 zeolite, including the dehydrogenation of propane to propene and hydrogen, the catalytic cracking of propane to methane and ethylene, the polymerization of propene and ethylene to aromatic hydrocarbons, and the hydrogenation of propene and ethylene to propane and ethane. Moreover, previous studies showed that the above processes were catalyzed by the carrier and the active components[23-24]. Thus, the dehydrogenation activity of PtSnNa/ZSM-5 catalyst is related with the acidity of ZSM-5 zeolite and the Pt metal particles dispersed on the zeolite. Judging from the TPDA profiles, the similar acidity is found in all the catalysts, therefore the dehydrogenation performance of the catalysts is determined by the Pt metal particles dispersed on the carrier.
It was known in previous studies that platinum metal was not only located directly on the carrier surface to form multiple Pt centers (M1) but was also located on the SnOxmodified carrier surface to form new active sites with“sandwich structure” (M2centers) in platinum-tin bicomponent catalyst. The M1center had great effect on the hydrogenolysis and coking reactions, and the M2center was favorable to the dehydrogenation reaction. Moreover, the PtSn alloy was easily formed when Sn species existed in the form of Sn0. The formation of PtSn alloy would change the nature of platinum metal on the catalyst surface and affect the absorption of C3H7group by the platinum metal, hindering the propane dehydrogenation reaction and causing the deactivation of catalysts[25-26]. Therefore the retention of Sn species in the oxidation state is of utmost importance for propane dehydrogenation. It can be seen from the results of TPR profiles and XPS spectra that more Sn species in the Sn-Pt catalyst exist in the form of the oxidation state. And introducing Sn species firstly during the experiment can make Pt metal exist more easily as M2centers. So the Sn-Pt catalyst shows a best catalytic stability. With respect to the Pt-Sn catalyst, the Pt species can highly disperse on the surface of ZSM-5 zeolite, so the highest initial propane conversion is observed. However, the interaction between Sn species and the carrier is weakened; therefore most Pt species probably form the M1centers or the Pt3Sn alloy, resulting in a quick deactivation of the Pt-Sn catalyst.
3.3 Model for the effect of impregnation sequence on catalytic performance of PtSn-Na/ZSM-5 catalyst
Based on the results described above, a model describing the effect of impregnation sequence on the distribution of Pt and Sn species in PtSnNa/ZSM-5 catalyst is proposed, which is shown in Figure 7. For a co-impregnation catalyst (Figure 7a), a part of Sn species and Pt species can be dispersed on the surface of ZSM-5 zeolite, and a part of them are located on the metal species dispersed on the surface of ZSM-5 zeolite, and a few Sn species can enter the channels of ZSM-5 zeolite because of the impediment of Pt species. In this way, the interaction between Sn species and Pt species is strengthened, which would result in the difficulty for reduction of Pt species and Sn species. Meanwhile the interaction between Sn species and the carrier is weakened. It means that more Sn species are easily reduced to Sn0. Therefore, the PtSn catalyst showed the worst catalytic activity.

Figure 7 Model describing the effect of impregnation sequence on the distribution of Sn species and Pt species in the catalysts
Figure 7b depicts the model of the catalyst made by stepwise impregnation with pre-impregnated Sn species. When Na/ZSM-5 powder mixes with the Sn precursor firstly, Sn species are highly dispersed on the surface of ZSM-5 zeolite and are dominantly located on the sites of strong interaction with the carrier. And a plenty of Sn4+ions enter the channels of ZSM-5 zeolite without interference. Thus the interaction between the carrier and Sn species strengthens in this case and the reduction of Sn species to Sn0becomes quite difficult. Subsequently, the Pt species are easily located on the surface of Sn species during the introduction of Pt species and would readily form M2centers after the reduction of catalyst, which can improve the catalytic performance of catalyst. So the Sn-Pt catalyst exihibts the best stability and catalytic activity. The model of the catalyst prepared by successive impregnation through pre-impregnating the carrier with Pt species is shown in Figure 7c. After impregnating the zeolite with H2PtCl6solution firstly, Pt species distribute dominantly on the surface and pore mouths of ZSM-5 zeolite, and would result in a strong interaction with the carrier firstly. And then, a part of Sn species can be located on the surface of Pt species; and a part of them are located on the sites of weak interaction with ZSM-5 zeolite; and few Sn species can enter the channels of ZSM-5 zeolite because of the blockage imposed by Pt species. Thus, the interaction between Sn species and the carrier can be weakened. It means that more Sn species are easily reduced to Sn0with Pt3Sn alloy being easily formed in the catalyst, which is disadvantageous to the propane dehydrogenation reaction. Moreover, with respect to this catalyst, the dispersity of Pt species is higher than that in other catalysts. So the Pt-Sn catalyst possesses a highest intial propane conversion but can be deactivated quickly in the reaction.
4 Conclusions
The effect of impregnation sequence on the dehydrogenation activity and selectivity of supported Na/Pt/Sn tricomponent catalysts based on HZSM-5 zeolite for the dehydrogenation of propane was studied. Judging from the TPDA profiles, the acid amount of catalysts prepared with different impregnation sequences is almost the same, but the dispersion of metal precursors, the degree of activation and reduction of metal precursors and the interaction between Pt and Sn are affected greatly by the impregnation sequence of metal precursors. The behavior of Sn4+entering the channel of ZSM-5 is hindered by [PtCl6]2-formed during the pre-impregnation of the support with H2PtCl6solution and the co-impregnation of the support with H2PtCl6solution and SnCl4solution. Moreover there is a relatively strong interaction between Pt species and Sn species in these two catalysts, resulting in more difficulty for reduction of Pt species and more easiness for formation of more Sn0species. The results of propane dehydrogenation have revealed that the high activity of catalysts prepared by the sequential impregnation is verified, especially in the case of catalyst, in which the carrier is impregnated firstly with the Sn solution.
Acknowledgement:The authors are grateful to the financial supports of the National Natural Science Foundation of China (Grant No.21376051, 21106017, 21306023, and 51077013), the Natural Science Foundation of Jiangsu (Grant No. BK20131288), the Fund Project for Transformation of Scienti fic and Technological Achievements of Jiangsu Province of China (Grant No. BA2011086), the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20100092120047), the Key Program for the Scienti fic Research Guiding Fund of Basic Scienti fic Research Operation Expenditure of Southeast University (Grant No. 3207043101), and the Instrumental Analysis Fund of Southeast University.
[1] Bulanek R, Kaluzova A, Setnicka M, et al. Study of vanadium based mesoporous silicas for oxidative dehydrogenation of propane andn-butane[J]. Catal Today, 2012, 179(1): 149-158
[2] Xu X L, Ran X L, Cui Q K, et al. ZSM-5-and MgAl2O4-based bifunctional additives for enhancing the production of propene and removal of SO2in the fluid catalytic cracking (FCC) process[J]. Energ Fuel, 2010, 24: 3754-3759
[3] Lesthaeghe D, Van der M J, Vandichel M, et al. Full theoretical cycle for both ethane and propene formation during methanol-to- olefin conversion in H-ZSM-5[J]. Chem Cat Chem, 2011, 3(1): 208-212
[4] Li Q, Sui Z J, Zhou X G, et al. Kinetics of propane dehydrogenation over Pt-Sn/Al2O3catalyst[J]. Appl Catal A: General, 2011, 398(1/2): 18-26
[5] Vu B K, Song M B, Ahn I Y, et al. Pt-Sn alloy phases andcoke mobility over Pt-Sn/Al2O3and Pt-Sn/ZnAl2O4catalysts for propane[J]. Appl Catal A: General, 2011, 400(1/2): 25-33
[6] Llias S, Bhan A. Tuning the selectivity of methanol-to-hydrocarbons conversion on H-ZSM-5 by co-processing olefin or aromatic compounds[J]. J Catal, 2012, 290: 186-192
[7] Toch K, Thybaut J W, Vandegehuchte B D, et al. A singleevent micro kinetic model for “ethylbenzene dealkylation/ xylene isomerization” on Pt/H-ZSM-5 zeolite catalyst[J]. Appl Catal A: General, 2012, 425: 130-144
[8] Chantaravitoon P, Chavadej S, Schwank J. Pt-Sn/Al2O3catalysts: Effect of catalyst preparation and chemisorption methods on H2and O2uptake[J]. Chem Eng J, 2004, 98(1/2): 99-104
[9] Shu Y Y, Murillo L E, Bosco J P, et al. The effect of impregnation sequence on the hydrogenation activity and selectivity of supported Pt/Ni bimetallic catalysts[J]. Appl Catal A: General, 2008, 339(2): 169-179
[10] Deng S Y, Chu W, Xu H Y, et al. Effects of impregnation sequence on the microstructure and performances of Cu-Co based catalysts for the synthesis of higher alcohols[J]. J Nat Gas Chem, 2008, 17(4): 369-37
[11] Duan Y Z, Zhou Y M, Sheng X L, et al. Influence of alumina binder content on catalytic properties of PtSnNa/AlSBA-15 catalysts[J]. Micropor Mesopor Mat, 2012, 161: 33-39
[12] Zhang Peixin, Zhou Yuming, Duan Yongzheng, et al. Influence of alumina content on catalytic performance of PtSn/Al2O3/SBA-15 catalysts for propane dehydrogenation[J]. China Petroleum Processing and Petrochemical Technology, 2012, 14(4): 9-16
[13] Xu M W, Zhou Y M, Zhang Y W, et al. Effect of cerium addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. J Nat Gas Chem, 2012, 21(3): 324-331
[14] Xue Mengwei, Zhou Yuming, Huang Li, et al.Effect of mischmetal addition on catalytic performance of PtSnNa/ ZSM-5 for propane dehydrogenation[J]. China Petoleum Processing and Peteochemical Technology, 2011, 13(3): 47-52
[15] Liu Xuan, Zhou Yuming, Zhang Yiwei, et al. Effect of Ga addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. China Petroleum Processing and Petrochemical Technology, 2011, 13(4): 45-52
[16] Huang Li, Zhou Shijian, Zhou Yuming, et al. Effect of strontium addition to platinum catalyst for propane dehydrogenation[J]. China Petroleum Processing and Petrochemical Technology, 2012, 14(3): 75-82
[17] Zhang Y W, Zhou Y M, Qiu A D, et al. Effect of alumina binder on catalytic performance of PtSnNa/ZSM-5 catalyst for propance dehydrogenation[J]. Ind Eng Chem Res, 2006, 45(7): 2213-2219
[18] Li B, Li S J, Li N, et al. Structure and acidity of Mo/ZSM-5 synthesized by solid state reaction for methane dehydrogenation and aromatization[J]. Micropor Mesopor Mat, 2006, 88(1/3): 244-253
[19] Wang J W, Jin G Q, Zhang Z X, et al. Aromatization of propane over La/HZSM-5 catalyst[J]. J Chin Rare Earth Soc, 2001, 19(2): 103-106 (in Chinese)
[20] Rodriguez D, Sanchez J, Arteaga G. Effect of tin and potassium addition on the nature of platinum supported on silica[J]. J Mol Catal A: Chem, 2005, 228(1/2): 309-317
[21] Lewera A, Barczuk P J, Skorupska K, et al. Influence of polyoxometallate on oxidation state of tin in Pt/Sn nanoparticles and its importance during electrocatalytic oxidation of ethanol combined electrochemical and XPS study[J]. J Electroanal Chem, 2011, 662(1): 93-99
[22] Shashikala V, Jung H, Shin C H, et al.n-Butane dehydrogenation on PtSn/carbon modified MgO catalysts[J]. Catal Lett, 2013, 143(7): 651-656
[23] Yu S Y, Yu G J, Li W, et al. Kinetics and reaction pathways for propane dehydrogenation and aromatization on Co/HZSM-5 and H-ZSM-5[J]. J Phys Chem B, 2002, 106(18): 4714-4720
[24] Farjoo A, Khorasheh F, Niknaddaf S, et al. Kinetic modeling of side reactions in propane dehydrogenation over Pt-Sn/γ-Al2O3catalyst[J]. Sci Iran C, 2011, 18(3): 458-464
[25] Zhang Y W, Zhou Y M, Qiu A D, et al. Propane dehydrogenation on PtSn/ZSM-5 catalyst: Effect of tin as a promoter [J]. Catal Commun, 2006, 7(11): 860-866
[26] Yang W S, Wu R A, Lin L W. Investigation on dehydrogenation of propane over supported bi-component catalysts. V. The process of dehydrogenation of propane on PtSn/Al2O3catalyst[J]. Chin J Catal, 1992, 13(3): 161-166 (in Chinese)
Recieved date: 2013-05-24; Accepted date: 2013-11-09.
Zhou Yuming, Tel: +86-25-52090617; Fax: +86-25-52090618; E-mail: ymzhou@seu.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Effects of Ultrasonic Treatment on Residue Properties
- Sources of Particular Pollutants in Ambient Air at a Petrochemical Enterprise
- Methane Adsorption Study Using Activated Carbon Fiber and Coal Based Activated Carbon
- Development and Application of CDOS Series Catalysts for Bottoms Cracking
- Study on Preparation of Waterproofing Agent for Mineral Wool Board from Modified C9Petroleum Resin
- Effect of Ultraviolet Aging on Asphalt Rheological Properties
