Development and Commercial Application of Ultra-Low Pressure Naphtha Reforming Technology with Continuous Catalyst Regeneration
2013-07-31MaAizengXuYouchunYangDongZhangXinkuanWangJieguang
Ma Aizeng; Xu Youchun; Yang Dong; Zhang Xinkuan; Wang Jieguang
(1. Research Institute of Petroleum Processing, SINOPEC, Beijing 100083; 2. SINOPEC Luoyang Petrochemical Engineering Company; 3. SINOPEC Guangzhou Company)
Development and Commercial Application of Ultra-Low Pressure Naphtha Reforming Technology with Continuous Catalyst Regeneration
Ma Aizeng1; Xu Youchun2; Yang Dong3; Zhang Xinkuan1; Wang Jieguang1
(1. Research Institute of Petroleum Processing, SINOPEC, Beijing 100083; 2. SINOPEC Luoyang Petrochemical Engineering Company; 3. SINOPEC Guangzhou Company)
The development history and major technological innovations of the ultra-low pressure naphtha reforming technology with continuous catalyst regeneration in China were introduced. This technology had been adopted by the 1.0 Mt/a CCR unit at the Guangzhou Company. The appropriate catalyst was selected to meet the demand of the unit capacity, the feedstock, and the product slate. The design parameters, including the reaction pressure, the octane number ofliquid product, the reaction temperature, the space velocity, the hydrogen/oil molar ratio, and the catalyst circulating rate, were chosen based on the study of process conditions and parameters. The commercial test results showed that the research octane number ofproduct reached 104 when the capacity of the CCR unit was 100% and 115% of the design value. The other technical targets attained or exceeded the expected value.
ultra-low pressure; continuous catalytic reforming; catalyst; regeneration; aromatics; gasoline; process; naphtha
1 Introduction
Naphtha catalytic reforming with continuous catalyst regeneration (CCR) is a process that can produce highoctane gasoline components, aromatics and hydrogen. High-octane gasoline components can be blended into gasoline pool to produce clean fuel. Aromatics are generally used as the raw material for manufacture of chemical fiber, plastics and rubber products. Hydrogen is a primary resource utilized by hydrogen-consuming units at refineries. Therefore, the CCR technology level can affect the technical progress and competitive edge of China’s petroleum and petrochemical industry. To exploit the selfdependent CCR technology, SINOPEC organized two research projects, the low pressure combined bed (LPCB) catalytic reforming technology and the continuous naphtha catalytic reforming technology, which had been commercialized at the refineries of Changling Company and Luoyang Company. After the successful application of the above-mentioned CCR technology, SINOPEC continued to organize the research and development of ultralow pressure naphtha catalytic reforming technology with continuous catalyst regeneration. This paper intends to outline its development history, major technological innovations and commercial applications in a 1.0 Mt/a CCR unit at SINOPEC Guangzhou Company refinery.
2 Development History and Major Technological Innovations
Since the 1990s, SINOPEC Research Institute of Petroleum Processing (RIPP) began to carry out the research and development of continuous catalytic reforming catalyst and related process technologies[1-15]. During this period, RIPP successfully developed the catalytic reforming reaction process model and the low-pressure combined bed catalytic reforming process, and filed many patents associated with the catalytic reforming reaction, the regeneration process and the regenerator. SINOPEC Luoyang Petrochemical Engineering Company (LPEC) also developed some patented technologies related to CCR regeneration process and apparatus and automatic controltechniques[16-20]. In the following years, LPEC and RIPP co-developed the low-pressure combined bed (LPCB) catalytic reforming process, which had been successfully commercialized at the SINOPEC Changling Company refinery[20].
After application of LPCB, LPEC and RIPP soon codeveloped the continuous catalytic reforming technology with independent intellectual property rights, which had been successfully applied in revamping the 700 kt/a CCR unit at the SINOPEC Luoyang Company refinery. However, the reaction pressure of the revamped reforming unit is 0.68 MPa due to the constraints of existing equipment. Higher pressure does not favor catalytic reforming reaction, so the yields of C5+ product, aromatics and H2delivered from the unit cannot reach the technical targets of ultra-low pressure continuous catalytic reforming process. Out of this concern, LPEC and RIPP decided to develop the ultra-low pressure naphtha catalytic reforming process with continuous catalyst regeneration and finally succeeded .
Ultra-low pressure naphtha catalytic reforming process with continuous catalyst regeneration possesses independent intellectual property rights and its major technological innovations cover the following items, viz.: (1) Proposing a design idea of maximizing target aromatic yield for aromatic production unit and high octane-barrel for gasoline production unit through the investigation of catalytic reforming reaction laws; (2) Solving the problems related with the manufacture, transportation, installation and maintenance of large-scale reactors, and alleviating the difficulties in apparatus fabrication and installation by optimizing the configuration of reactors; (3) Avoiding the damage of regenerator internals caused by heat stress through improving the design of its internal grids, making the installation and replacement of internals easier; (4) Developing a reliable de-chlorination technology for regeneration recycle gas, avoiding the chlorine and caustic corrosion and pipeline scaling; (5) Optimizing the circuit of gas discharged from the drying and oxychlorination zone for realizing low coke burning, and preventing the electric heater for oxychlorination zone inlet gas from interlocking owing to low gas flowrate; (6) Developing a new type of automatic control system for lock hopper to make catalyst circulation and transfer stable, safe and accurate; and (7) Exploiting patented sulfidation and desulfidation technology, and preventing CCR units from coking without sulfur and poisoning by sulfur.
3 Investigation on Process Conditions of Guangzhou CCR Unit
3.1 Unit capacity
Before construction of the new CCR unit, the Guangzhou Company refinery has a CCR unit with a capacity of 400 kt/a. The refinery capacity was to be expanded to 13 Mt/a, the output of naphtha would additionally increase to 1.0 Mt/a after completion of the revamp, leading to a deluge of naphtha. Therefore, a new CCR unit with a capacity of 1.0 Mt/a was planned for construction, which not only can process the surplus naphtha, but also optimize the production ratio of gasoline components coupled with low-cost hydrogen.
3.2 Design feedstock and product target
According to the crude oil processing target envisaged in the 13 Mt/a refinery revamping plan, the reformer feedstock is designed to cope with two cases, including the lean feed case and the rich feed case. The lean feed consists of 120 kt/a of hydrocracking heavy naphtha and a mixture composed of 30 m% of heavy naphtha, 30 m% of medium naphtha and 40 m% of light naphtha derived from the Arabian crude. The rich feed is composed of 120 kt/a of hydrocracking heavy naphtha, 380 kt/a of Mangi heavy naphtha and a mixture consisting of 30 m% of heavy naphtha, 30 m% of medium naphtha and 40 m% of light naphtha derived from the Arabian crude. The compositions of design feedstock are given in Table 1.
In line with the overall process flow sheet of the refinery, the newly-built CCR unit at Guangzhou refinery not only can produce high-octane gasoline components, but also satisfy the hydrogen balance of the whole refinery. Furthermore, after consideration of the surplus aromatics in gasoline pool and the market demand for mixed xylenes,the product targets of the CCR unit are aimed at production of gasoline and aromatics. To meet the demand for hydrogen needed by the refinery, the hydrogen yield of the CCR unit should exceed 3.61 m%.
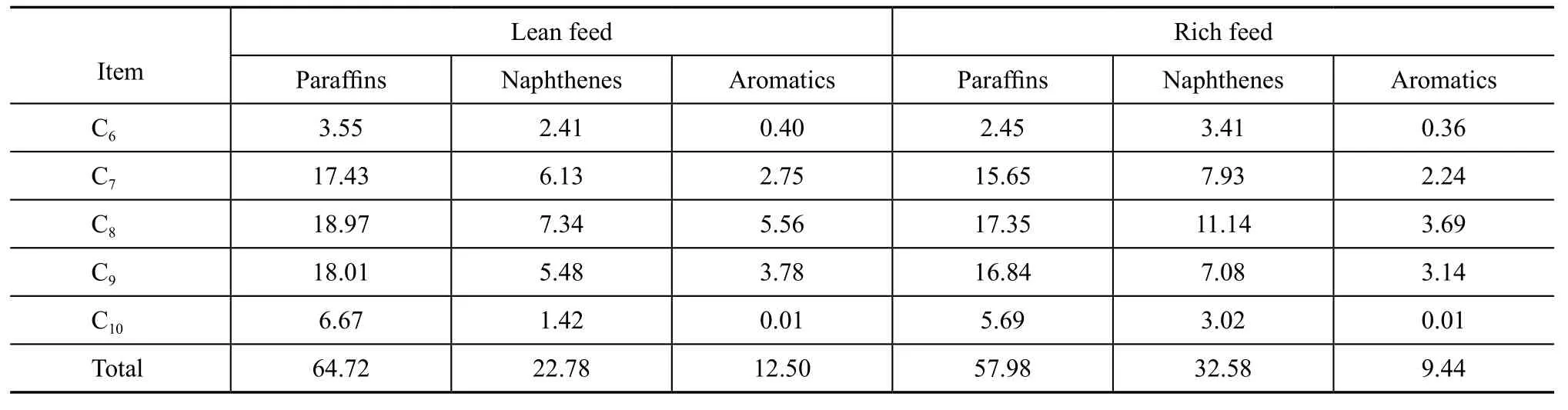
Table 1 Compositions of design feedstockw, %
3.3 Selection of catalyst
As we all know, the product yield of CCR reforming unit is dependent on the performance of catalyst. Therefore, the selected catalyst should meet the requirements for the octane barrel, the aromatics yield and the hydrogen yield provided by the newly-built CCR unit. It is necessary to compare the performance of catalysts developed by RIPP. The catalyst evaluation tests were performed in a lab-scale apparatus equipped with a recycle gas compressor. The properties of feedstock are listed in Table 2. The evaluation test conditions are shown in Table 3. The composition and physical properties of several catalysts are listed in Table 4, and the performance of catalyst is shown in Table 5.

Table 2 Properties of feed used in lab-scale apparatus

Table 3 Conditions of catalyst evaluation test

Table 4 Composition and physical properties of some catalysts
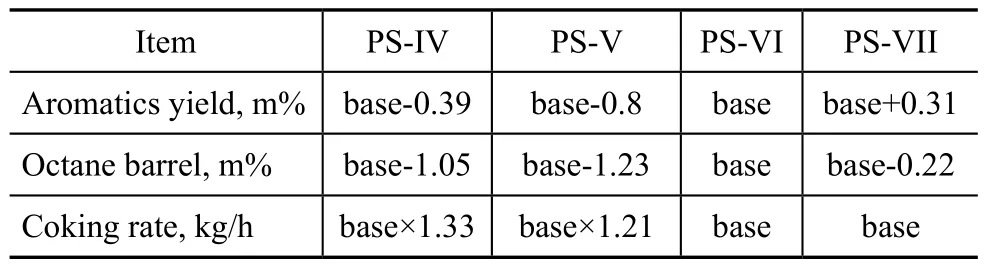
Table 5 Comparison of catalyst performance
It can be seen from Table 4 that the major metal elements of PS-IV and PS-V catalysts are Pt and Sn, and a new auxiliary agent M is additionally introduced into PS-VI and PS-VII catalysts; the Pt content of PS-VI and PS-VII catalysts is 0.35 m%, while that of PS-V and PS-VI catalysts is 0.28 m%. Table 5 indicates that the coking rate of PS-IV and PS-V catalysts is by 33 m% and 21 m% higher than that of PS-VI catalyst, while the aromatics yield and octane barrel value decrease. Therefore, PS-IV and PS-V catalysts are unsuitable for the newly-built CCR unit. Compared to the PS-VI catalyst, the aromatics yield of PS-VII catalyst increases by 0.31 percentage points, and the octane barrel value decreases by 0.22 percentage points at a same coking rate.
The relationship between catalyst activity andliquid yield is investigated in a lab-scale apparatus, using a feed given in Table 2. The evaluation test conditions cover a reaction pressure of 0.35 MPa, a LHSV of 1.2 h-1, and a hydrogen/hydrocarbon volume ratio of 1 300. The comparison on activity and liquid yield between PS-VI and PS-VII catalysts are shown in Figures 1 and 2. It can be seen from Figures 1 and 2 that the catalytic activity of PS-VII catalyst is slightly lower than that of PS-VI catalyst at lower reaction severity, and is slightly higher at higher reaction severity. During the initial stage of catalyst operation, theyield on PS-VII catalyst is slightly higher than that of PS-VI catalyst and then decreases gradually, but eventually reaches the similar level.

Figure 1 Comparison of activity between PS-VI and PS-VII Catalysts
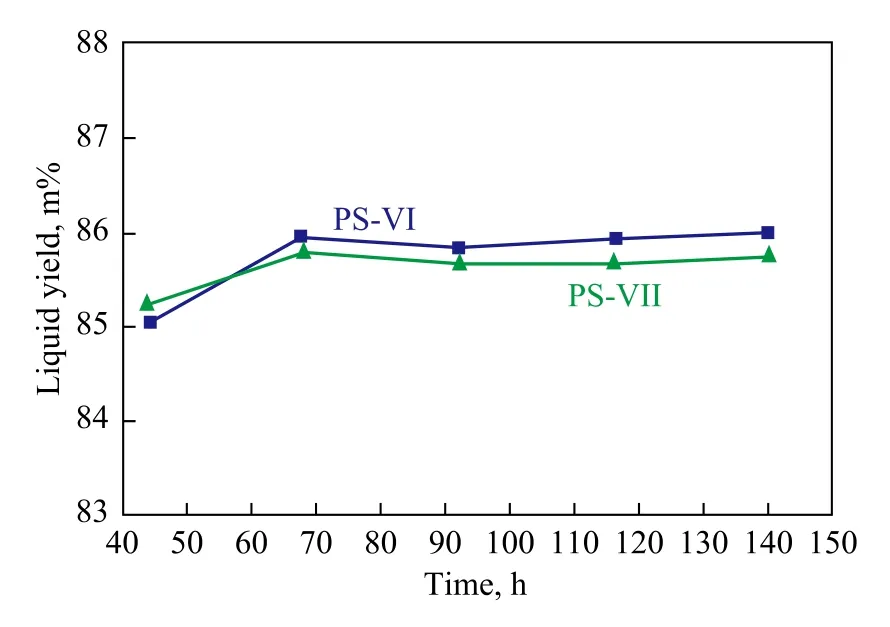
Figure 2 Comparison of liquid yield between PS-VI and PS-VII catalysts
In summary, PS-VI and PS-VII catalysts can both meet the requirements of the CCR unit. However, the Pt content of PS-VII catalyst is 25 m% higher than that of PSVI catalyst. Therefore, PS-VI catalyst is selected in an attempt to reduce the initial investment cost.
3.4 Main reaction conditions
The effects of main process parameters on product distribution are investigated through using the catalytic reforming reaction model developed by RIPP and the operating data of commercial CCR units in China.
3.4.1 Reaction pressure
Lower pressure can favor catalytic reforming reaction towards aromatics formation[12]. Therefore, catalytic reforming will proceed along the direction of lower reaction pressure. Up to now, the reaction pressure of CCR unit has decreased to 0.35 MPa. If the reaction pressure further decreases, the energy consumption and equipment investment of catalytic reforming unit would increase sharply. After full consideration of positive and negative factors related with lower operating pressure, the average reaction pressure of the newly-built CCR unit is speci fied at 0.35 MPa.
3.4.2 Reaction severity (RONC ofproduct)
Figures 3—6 depict the effects of RONC ofliquid product on hydrogen yield, aromatics yield, coking rate and octane barrel obtained during processing of lean feed and rich feed in the presence of PS-VI catalyst at a reaction pressure of 0.35 MPa, a LHSV of 1.2 h-1, and a H2/hydrocarbon ratio of 2.5. Figure 3 indicates that the hydrogen yield of rich feed is higher than that of lean feed at the same RONC ofproduct. The hydrogen yield of lean feed and rich feed both increases with the increase in RONC ofproduct. For lean feed, the hydrogen yield can reach 3.61 m% when RONC ofproduct attains or exceeds 102. For this reason, the bottom value of RONC ofproduct is designed as 102.

Figure 3 Effect of RONC ofproduct on H2yield
Figures 4 and 5 show that the aromatics yield of rich feed is higher than that of lean feed at the same RONC ofproduct, and increases with the increase in RONC ofproduct. The coking rate of rich feed is lower than that of lean feed, and increases sharply with the increase in RONC ofproduct. The coking rate of rich feed and lean feed increases by 86 m% and 59 m%, respectively, as the RONC ofproduct increases to 105 from 102. The coking rate increases by 15 m%—28 m% on oneunit of RONC increase.

Figure 4 Relationship between aromatics yield and RONC ofproduct

Figure 5 Effect of RONC ofliquid product on coking rate
It can be seen from Figure 6 that the octane barrel of rich feed is higher than that of lean feed, and reaches a peak value when RONC ofproduct is 104. So, the upper value of RONC of C5+ product is set at 104.
3.4.3 Hydrogen to hydrocarbon molar ratio
The in fluence of H2to hydrocarbon molar ratio on reaction performance of lean feed is given in Table 6. Table 6 shows that the yield ofliquid, the octane barrel, the hydrogen and aromatics yields slightly decrease with an increase in hydrogen to hydrocarbon molar ratio, and the coking rate also drops abruptly. The impact of H2to hydrocarbon molar ratio on reaction performance of rich feed follows the same rules.

Figure 6 Effect of RONC ofliquid product on octane barrel
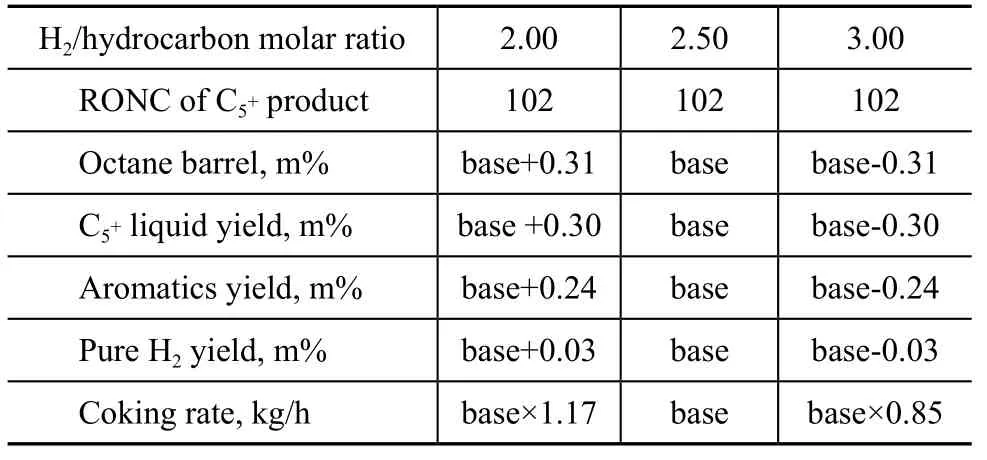
Table 6 Effect of H2/HC molar ratio on catalyst performance
With an increasing H2to hydrocarbon ratio, the coking rate declines so that the regeneration capacity can be reduced, resulting in a decreased equipment investment and energy consumption. However, the equipment cost and energy consumption of recycle gas compressor increase with the increase in the H2to hydrocarbon molar ratio[21-22]. Based on the above-mentioned facts, the H2to hydrocarbon molar ratio is set at 2.50.
3.4.4 Catalyst regeneration capacity
The CCR catalysts should be regenerated continuously in order to restore their activity and selectivity. The regeneration capacity is generally denoted by catalyst circulation rate. The higher the catalyst circulation rate is, the greater the regeneration capacity would be. Catalyst regeneration rate depends on the coking rate of catalyst and the coke-burning process. The suitable coke content on spent catalyst is generally 3 m%—5 m% based on the characteristics of coke-burning process involved in the ultra-low pressure naphtha catalytic reforming technology with continuous catalyst regeneration.
Coke deposition on catalyst is associated with the composition and properties of feed, the reaction pressure, the H2/hydrocarbon molar ratio, and RONC ofproduct. When the reaction pressure is 0.35 MPa, the H2/hydrocarbon molar ratio is 2.5, and the upper value of RONC ofproduct is 104, the coking rate is calculated by means of the reforming reaction model for rich feed and lean feed. The results show that the coking rate of rich feedand lean feed is 44.7 kg/h and 56.75 kg/h, respectively. Upon designing the CCR regenerator, the maximum coke content on spent catalyst is generally set at 5 m%. Based on the value of coking rate and coke content, the calculated circulation rate for the two cases is 885.4 kg/h and 1 135 kg/h, respectively. Therefore, the maximum catalyst circulation rate is set at 1 135 kg/h.
3.4.5 Reaction temperature and space velocity
Nowadays the olefin content of reformate from CCR units in China continues to increase, resulting in shorter service life of clay used for removing the olefins. Thus, the clay is replaced more frequently to shoot up the operating cost and affect the safe and smooth operation of CCR units. Much research shows that it is the ever increasing reaction temperature that leads to the elevation of olefin content in reformate. Upon using the feedstock listed in Table 2, the relationship between the olefin content in reformate and the reaction temperature is investigated at a lab-scale apparatus, which operates at a reaction pressure of 0.35 MPa, a LHSV of 1.2 h-1, and a H2/hydrocarbon volume ratio of 1 300. The experimental results are shown in Figure 7. It can be seen from Figure 7 that the olefin content in reformate increases sharply with the increase in reaction temperature. This indicates that it is necessary to decrease the reforming reaction temperature as far as possible in order to reduce the olefin content in reformate. After full consideration of the investment cost of equipment and catalyst, the upper value of the designed WAIT is specified at 530 ℃.
At a reaction pressure of 0.35 MPa, a H2to hydrocarbon molar ratio of 2.5, and a RONC of C5+ product of 104, the relationship between LHSV and reaction temperature is plotted in Figure 8. Figure 8 depicts that the reaction temperature for lean feed and rich feed both increases with an increasing LHSV, but the reaction temperature for lean feed is higher than the case of rich feed at certain space velocities. When the WAIT is 530 ℃, the LHSV for lean feed and rich feed is 1.2 h-1and 1.4 h-1, respectively. Hence, the design value of LHSV should be less than 1.2 h-1in order to ensure that WAIT can be maintained at below 530 ℃.

Figure 7 Effect of reaction temperature on olefin yield

Figure 8 Relationship between LHSV and WAIT
4 Commercial Test of the CCR Unit at Guangzhou Company
The construction of the CCR unit was started on October 26, 2007 and was completed on January 15, 2009. This unit was put into operation on April 12, 2009. The performance evaluation of the CCR unit was carried out when the capacity reached 100% and 115% of the design target in September, 2009 and May, 2010, respectively. The feedstock for the performance evaluation is listed in Table 7. The main reaction conditions and results are given in Table 8.
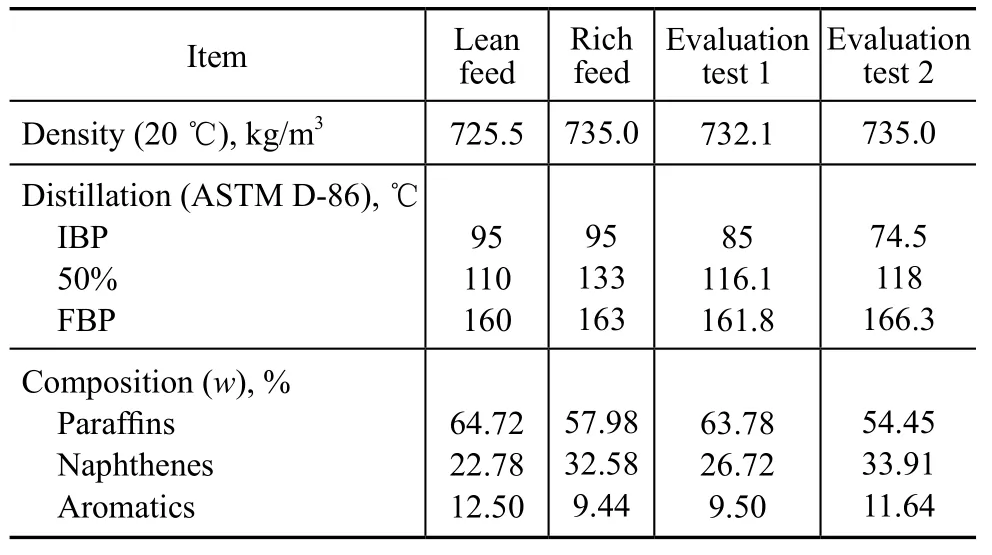
Table 7 Properties and composition of CCR unit feedstock

Table 8 Main reaction parameters and results on evaluation of 1 Mt/a CCR unit
It can be seen from Tables 7 and 8 that the distillation range of evaluation feed 1 is in the range of from 85 ℃to 161.8 ℃ and the mass fraction of naphthenes and aromatics is 36.22 m%, which is similar to the design value of lean feed. The RONC ofliquid product reaches 104 when the WAIT is 522.4 ℃, which is 7.6 ℃ lower than the expected temperature. The correspondingliquid yield is 87.58 m%, which is 0.52 percentage points higher than the expected value. The aromatics yield is 72.75 m%, which is 0.29 percentage points higher than the design value. The pure hydrogen yield is 3.90 m%, which is 0.29 percentage points higher than the target value. The octane barrel is 91.08 m%, which is 0.54 percentage points higher than the design value. The coke content on spent catalyst is 3.83 m%, which can satisfy the needs of coke burning process. The consumption of catalyst is 1.5 kg/h, which is 6.7 kg/d lower than the expected value. Compared to the design case of rich feed, the distillation range of the evaluation feed 2 is in the range of from 74.5 ℃ to 166.3 ℃ and the mass fraction of naphthenes and aromatics is 45.55 m%, which is 3.53 percentage points higher than the design value of rich feed, and the density is commensurate to the design value. The RONC ofliquid product reaches 104.1 at a WAIT value of 524 ℃. The correspondingliquid yield is 89.47 m%, which is 1.72 percentage points higher than the expected value. The aromatics yield is 73.71 m%, which is 1.21 percentage points higher than the design value. The pure hydrogen yield is 3.96 m%, which is 0.27 percentage points higher than the target value. The octane barrel is 93.14 m%, which is 1.88 percentage points higher than the design value. The coke content on spent catalyst is 3.51 m%, which can satisfy the needs of coke burning process. The consumption of catalyst is 4.8 kg/d, which is 3.4 kg/d less than the expected value. In summary, the commercial tests of the 1.0 Mt/a CCR unit at Guangzhou Company, which utilizes the selfdeveloped ultra-low pressure naphtha reforming technology with continuous catalyst regeneration, have shown that the research octane number ofproduct reached 104 when the naphtha reforming capacity was 100% and 115% of the design value. The other technical targets have attained or exceeded the expected value.
5 Conclusions
(1) The ultra-low pressure naphtha reforming technology with continuous catalyst regeneration provided with independent intellectual property rights had been successfully developed after many years’ research efforts. The design ideas for maximizing the target aromatic yield in line with the aromatics production mode or for boosting the octane barrel in line with the gasoline production mode were put forward. Many technological innovations were realized, including the con figuration of reactors, the structure of regenerator’s internal grids, the technology for dechlorination of regenerator recycle flue gas, the process for burning low coke content of catalyst, the automatic control system of lock hopper, and the startup procedures.
(2) To meet the requirements of Guangzhou re finery, the capacity of CCR unit was set at 1.0 Mt/a, with the target products consisting of gasoline and aromatics. The PS-VI catalyst was selected for use in the CCR unit.
(3) Based on the investigations on process conditions and parameters, the design value of reaction pressure, the RONC ofliquid product, the maximum reactor inlet temperature, the LHSV, the H2to hydrocarbon molar ratio, and the catalystcirculation rate for the CCR unit is set at 0.35 MPa, 102—104, 530 ℃, 1.2 h-1, 2.5, and 1 135 kg/h, respectively.
(4) The commercial test results showed that the research octane number ofproduct reached 104 when the reformer capacity was 100% and 115% of the design value. The other technical targets had attained or exceeded the expected value.
Acknowledgement:Financial support form the SINOPEC Research Program(No.107025) is gratefully acknowledged.
[1] Ma Aizeng. Catalytic reforming catalysts and technology in China [J]. China Petroleum Processing & Petrochemical Technology, 2003(4): 15-24
[2] Pan Jincheng, Ma Aizeng, Yang Sennian. Research and evaluation of PS-VI CCR catalyst[J]. Petroleum Refinery Engineering, 2002, 32(7): 53-55 (in Chinese)
[3] Pan Maohua, Ma Aizeng. Industrial application experiments of PS-VI CCR catalyst [J]. Petroleum Processing and Petrochemicals, 2003, 34(7): 5-8 (in Chinese)
[4] Ye Xiaodong, Xu Wuqing, Ma Aizeng. Industrial application of PS-VI catalyst in IFP first generation CCR unit[J]. Petroleum Processing and Petrochemicals, 2003, 34(5): 1-4 (in Chinese)
[5] Zhang Baozhong, He Zhimin, Ma Aizeng. Industrial application experiments of PS-VI catalyst[J]. Chemical Reaction Engineering and Technology, 2007, 23(3): 273-278 (in Chinese)
[6] Wang Ying, Ma Aizeng, Pan Jincheng, et al. Effect of Eu on activity of Pt-Sn/γ-Al2O3reforming catalyst [J]. Journal of Molecular Catalysis, 2003, 17(2): 151-155 (in Chinese)
[7] Liang Weijun, Ma Aizeng, Pan Jincheng. Effect of Fe on catalytic activity of Pt-Sn reforming catalyst[J]. Petroleum Processing and Petrochemicals, 2004, 35(11): 15-19 (in Chinese)
[8] Ma Aizeng, Pan Jincheng, Yang Sennian. Research and evaluation of PS-VII CCR catalyst with high Pt content and low coke deposition rate [J]. Petroleum Refinery Engineering, 2004, 34(12): 45-47 (in Chinese)
[9] Ma Aizeng, Pan Jincheng, Yang Sennian. Development and commercial application of high-platinum CCR catalyst with low coke deposition rate[J]. China Petroleum Processing and Petrochemical Technology, 2007(4): 13-20
[10] Zhou Mingqiu, Chen Guoping, Ma Aizeng. Industrial application of PS-VII CCR catalyst[J]. Petroleum Processing and Petrochemicals, 2008, 39(4): 26-30 (in Chinese)
[11] Zhang Lanxin, Zhao Rendian, Meng Xianping, et al. Research and development of low pressure combined bed reforming technology[J]. Petroleum Processing and Petrochemicals, 2002, 33(10): 1-5 (in Chinese)
[12] Zhao Zhihai. Development of new regeneration process of CCR catalyst[J]. Petroleum Refinery Engineering, 2008, 38(2): 46-50 (in Chinese)
[13] Zhao Zhihai, Shi Feng, Zhao Rendian. Investigation on moisture content in regeneration recycle flue gas during the regeneration of CCR catalyst[J]. Petroleum Refinery Engineering, 2002, 32(10): 36-39 (in Chinese)
[14] Zhao Zhihai. Comparison and analysis of IFP and UOP CCR regeneration coke burning process[J]. Petroleum Refinery Engineering, 2002, 32(1): 14-17 (in Chinese)
[15] Ma Aizeng. Technology selection of CCR with the target products of aromatics and gasoline[J]. Petroleum Processing and Petrochemicals, 2007, 38(1): 1-6 (in Chinese)
[16] Ma Aizeng, Shi Feng, Li Bin, et al. Research on revamping process and catalyst selection of CCR unit at Luoyang refinery[J]. Petroleum Processing and Petrochemicals, 2008, 39(3): 1-5 (in Chinese)
[17] Fu Jinhui, Liu Zhenhua, Jin Xin, et al. Startup of a catalytic reformer with naphtha[J]. Petroleum Processing and Petrochemicals, 1998, 29(5): 4-7 (in Chinese)
[18] Zhao Zhihai. Reduce ole fins in FCC naphtha by mild catalytic reforming[J]. Petroleum Re finery Engineering, 2008, 38(3): 9-12 (in Chinese)
[19] Xu Youchun, Han Yucai. Technical economy exploration of low pressure combined bed reforming unit[J]. Petroleum Re finery Engineering, 2002, 32(12): 38-41 (in Chinese)
[20] Liu Hongyun, Zhu Xuedong, Yang Baogui, et al. Development of new-type centrifugal radial reactor and application[J]. Petroleum Refinery Engineering, 2009, 39(1): 29-31 (in Chinese)
[21] Liu Dehui. Development of a new lock hopper[J]. Petroleum Re finery Engineering, 2002, 32(4): 38-40 (in Chinese)
[22] Liu Dehui, Peng Shihao, Liu Taiji, et al. Dynamic mathematical models for regeneration system of catalytic reforming unit with continuous catalyst regeneration[J]. Petroleum Re finery Engineering, 1998, 28(1): 37-41 (in Chinese)
[23] Xu Youcun, Yan Guanliang. Design and running of reforming unit with low pressure combined bed[J]. Petroleum Re finery Engineering, 2002, 32(1): 8-13 (in Chinese)
[24] Liu Dehui, Xu Youchun, Yang Baogui, et al. Base energy consumption of catalytic reforming[J]. Catalytic Reforming Newsletter, 2003(4): 38-42 (in Chinese)
[25] Bao Wei. Progress of CCR as well as its design parameters selection[J]. Contemporary Chemical Industry, 2006, 35(3): 183-186 (in Chinese)
Recieved date: 2013-06-09; Accepted date: 2013-06-27.
Dr. Ma Aizeng, E-mail: maaizeng. ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Methane Adsorption Study Using Activated Carbon Fiber and Coal Based Activated Carbon
- Deep Hydrodesulfurization of Diesel Fuel over Diatomite-dispersed NiMoW Sulfide Catalyst
- Effect of Ultraviolet Aging on Asphalt Rheological Properties
- Effects of Ultrasonic Treatment on Residue Properties
- Study on Preparation of Waterproofing Agent for Mineral Wool Board from Modified C9Petroleum Resin
- Sources of Particular Pollutants in Ambient Air at a Petrochemical Enterprise
