13th Asia-Pacific Congress of Clinical Microbiology and lnfection Consensus Guidelines for diagnosis and treatment of liver failure
2013-07-03
13th Asia-Pacific Congress of Clinical Microbiology and lnfection Consensus Guidelines for diagnosis and treatment of liver failure
Organization Committee of 13th Asia-Pacific Congress of Clinical Microbiology and Infection
Introduction
Liver failure is defined as hepatocellular dysfunction manifested by severe synthetic and metabolic disturbance with extremely high mortality. Scientists and clinicians have been trying to explore its definition, classification, etiology, diagnosis, prognosis, and management. However, the documentation on liver failure is paucity. Polson and Lee, endorsed by the American Association for the Study of Liver Diseases (AASLD), published the management recommendations in 2005.[1]In October 2006, we published the first version ofDiagnostic and Treatment Guidelines for Liver Failure(Guidelinesin short) in China,[2]which included the definition, etiology, classification, diagnosis and management of a variety of liver failure. TheGuidelineswere in line with both international standards and actual clinical practice in China. The publications entitledAcute-on-Chronic Liver Failure: Consensus Recommendations of the Asian Pacific Association for the Study of the Liver(APASL,2009)[3]andAASLD Position Paper: the Management of Acute Liver Failure: Update 2011(AASLD,2011)[4]summarized the published literature pertaining to acute-on-chronic liver failure (ACLF) and acute liver failure (ALF) managements respectively. Some of the recommendations are adopted to these updated guidelines. The Consensus guidelines are recommendations forclinicians to better understand the disease and to make appropriate decisions on the diagnosis and treatment of liver failure. Since liver failure is complicated, it is impossible to include or solve all of the problems in clinical practice. An appropriate treatment plan should consider the status of illness, medical resources and patients' consents. This Consensus will update the advances in the diagnosis and treatment of liver failure based on clinical evidence.
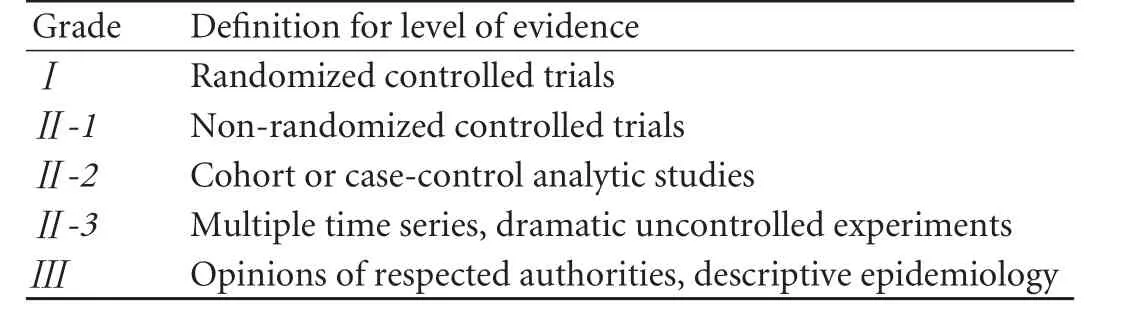
Table 1.Quality of evidence on which a recommendation is based
The Consensus follows the principles of evidencebased medicine (EBM) and the evidence used in this Consensus can be divided into 3 grades and 5 levels, which are presented by italic Roman numerals enclosed in parentheses (Table 1).
Definition and etiologies
Definition
Liver failure is the inability of the liver to perform its normal synthetic, metabolic, excretory and biotransformation functions. Liver failure is usually manifested as coagulopathy, jaundice, hepatic encephalopathy (HE) and ascites.
Etiologies
Hepatitis B is highly endemic in China. Hepatitisviruses (mainly hepatitis B virus) infections are the leading cause of liver failure in this country. Drugs and hepatotoxic substances (such as alcohol, chemicals, etc.) may also be the etiologies of liver failure. While in Western countries, drug use is the primary cause for acute and sub-acute liver dysfunction, and long-term alcohol consumption often leads to chronic liver disease. Liver failure in children is due to hereditary-related metabolic diseases (Table 2).
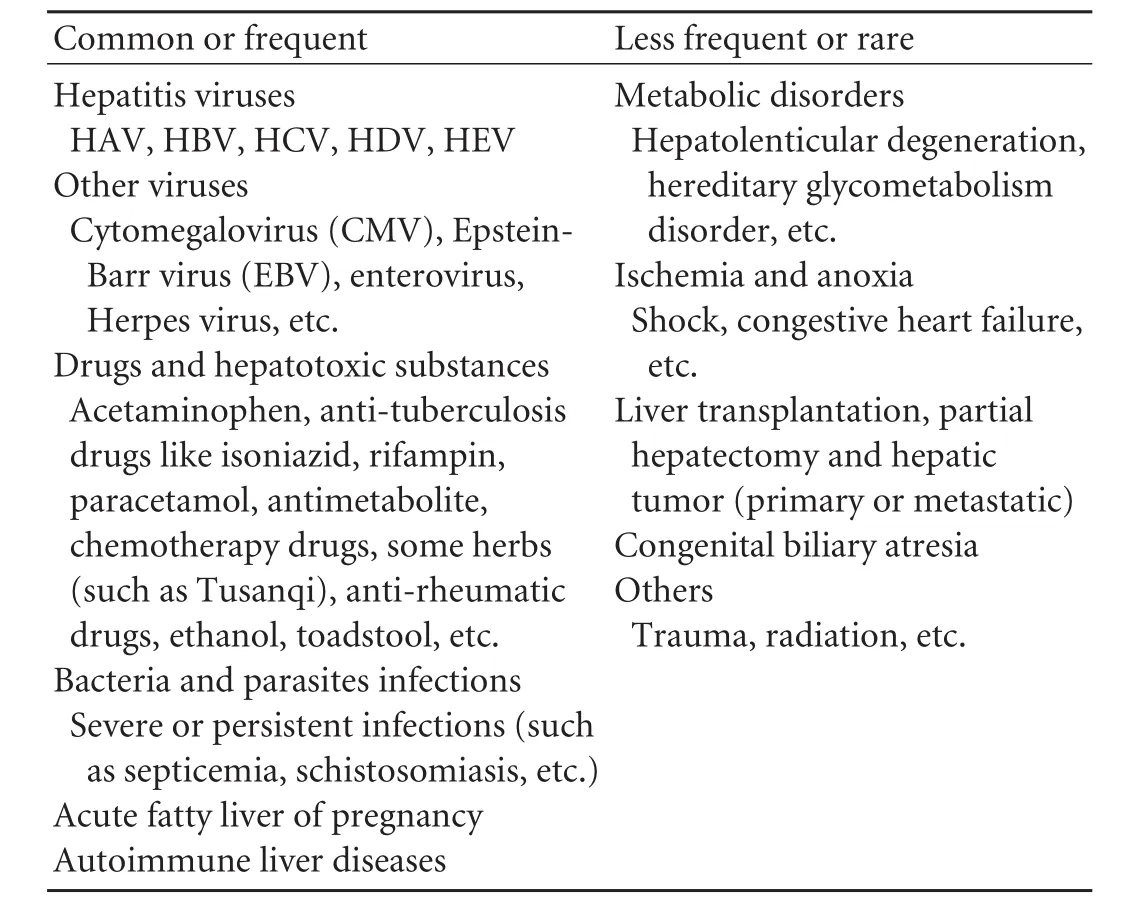
Table 2.Etiologies of liver failure
Pathogenesis
Host
a. Host genetic background plays a key role in hepatitis B. Although there are many studies on the role of hereditary factors in the infection and clearance of HBV and chronic HBV infection-related liver cirrhosis and liver cancer, genetic susceptibility for severe hepatitis B has rarely been reported.[5]The involved genes are tumor necrosis factor (TNF) including TNF-α and TNF-β, IL-10, interferon-inducible protein 10 (IP-10, CXCL-10), vitamin D receptor (VDR), and human leukocyte antigen (HLA).[5]
b. Host immune in pathogenesis of liver failure has been widely recognized. Cytotoxic T-lymphocyte (CTL), the core of the cellular immune, plays a key role in the clearance of intracellular virus, which is the main source of cell apoptosis or necrosis.
Virus
a. The components of virus damage the liver directly. Overexpression of HBsAg leads to hepatocyte damage and liver failure. HBV X protein also insults the liver directly. In the early stage of infection, X protein sensitizes the hepatocyte to inflammatory factors such as TNF-α and induces the necrosis, which may be related with severe hepatitis.
b. Gene mutation can also lead to necrosis and severe liver damage.[6]
Endotoxin
The function of Kupffer cells is seriously impaired in patients with severe liver diseases, resulting in a large amount of endotoxins from portal vein overflow into the circulation without detoxification. Endotoxin induces liver necrosis directly or through the activation of chemical mediators released by Kupffer cells, and endotoxin also aggravates liver necrosis induced by hepatotoxic substances such as galactosamine, CCl4and ethanol, etc.
Metabolism
Hepatic microcirculation disorder exists in all chronic liver diseases, which results in insufficient blood supply to hepatocytes. On the other hand, collateral circulation also depletes the blood flow from the liver and thus, a) The nutrients absorbed from the intestines cannot nourish the liver; b) The therapeutic effects of some drugs are decreased; and c) The metabolic wastes are accumulated and all of these further speed up the progress of liver diseases.
Epidemiology
Liver failure in China is mainly caused by HBV infection which ranks number eighth of the deaths in China. A majority of the patients are ACLF due to the following causes such as drugs or hepatotoxic substances (ethanol, chemical agents). Immune inhibitor is the major cause of HBV reactivation in China. Liver failure may develop in patients with positive HBV serum markers. Viral replication leads to the depletion of nutrients from hepatocytes and immune paralysis roots in the liver damage.
HBV-induced liver failure is usually severe and of high mortality. It has many complications and the treatment is difficult. HBV-induced liver failure is often found in males, and more frequently in young and middle-aged, which might because men are more likely to have severe hepatitis, and alcohol may also be related to the occurrence of liver failure. Most of patients are labors. Patient's environment, life style, healthcare status and level of education may also play roles. In China, amajority of the patients are Han ethnics.[7]With the advances of antiviral treatment, the incidence of acute and sub-acute liver failure is decreased, and most of the patients are with ACLF and chronic liver failure (CLF).
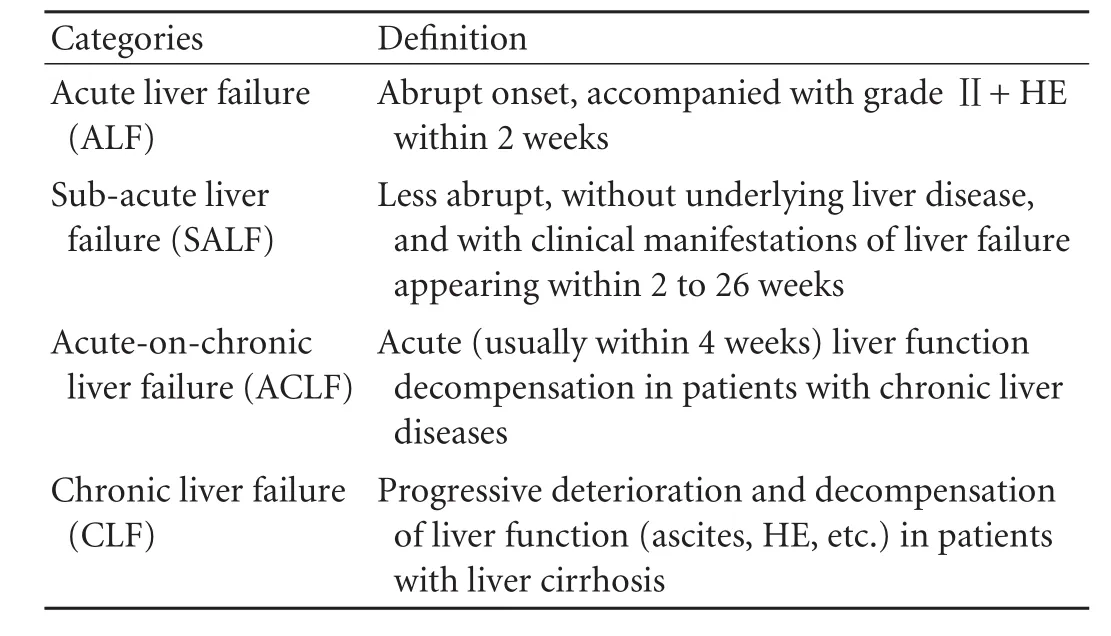
Table 3.Classification of liver failure
Classification and diagnosis
Classification
In terms of histopathological characteristics and the progression of disease, liver failure can be divided into 4 classes: ALF, sub-acute liver failure (SALF), ACLF, and CLF (Table 3).
Diagnosis
Clinical diagnosis
The clinical diagnosis of liver failure should be based on the comprehensive analysis of medical history, clinical manifestations and laboratory examination.
ALF: Abrupt onset, accompanied with a grade Ⅱor higher HE (according to the Ⅳ-grade classification) and the following symptoms are shown within 2 weeks: a) fatigue with severe gastrointestinal tract symptoms, such as anorexia, abdominal distension, nausea and vomiting; b) progressive aggravation of jaundice; c) hemorrhagic tendency with international normalized ratio (INR) ≥1.5 or prothrombin activity (PTA) ≤40% and other causes have been excluded; and d) progressive reduction in liver size.
SALF: Onset is slower than ALF, the following symptoms occur within 2-26 weeks: a) fatigue with gastrointestinal tract symptoms; b) rapidly deepening jaundice, with total bilirubin (TBil) 10 times greater than the upper limit of normality (ULN), or a daily increase ≥17.1 µmol/L; c) occurrence of HE; and d) hemorrhagic tendency with INR ≥1.5 or PTA ≤40% and ruling out other causes.
ACLF: Acute or sub-acute deterioration of liver function in patients with chronic liver diseases. It usually has the following symptoms: a) fatigue with gastrointestinal tract symptoms; b) rapidly deepening jaundice, with TBil 10 times greater than the ULN, or a daily increase ≥17.1 µmol/L; c) hemorrhagic tendency with INR ≥1.5 or PTA ≤40% and other causes have been excluded; d) progressive reduction in liver size; and e) occurrence of HE.
CLF: Progressive deterioration and decompensation of liver function in patients with liver cirrhosis. It usually has the following symptoms: a) a significant increase of TBil; b) a significant decrease of albumin; c) a hemorrhagic tendency with INR ≥1.5 or PTA ≤40% and other causes have been ruled out; d) ascites or other portal hypertension symptoms; and e) occurrence of HE.
Histopathology
Histopathological examination plays an important role in diagnosis, classification and prognosis of liver failure. However, liver biopsy is risky because patients with liver failure usually have coagulopathy. Extensive necrosis of hepatocytes can be observed in patients with liver failure except CLF. Necrosis can be focal and varies according to etiology and disease course. In terms of extent, necrosis can be divided into massive necrosis (involving an area over 2/3 of the liver), sub-massive necrosis (involving approximately 1/2-2/3 of liver), confluent necrosis (necrosis of neighboring hepatocytes), and bridging necrosis (extensive confluent necrosis and destruction of the liver structure). In liver tissue with different liver failure courses, single or multiple pathologic lesions representing old and new hepatocyte necrosis can be observed. Currently, experts have not come to an agreement on the correlation between the etiologies, classification, stages of liver failure and the changes of liver histology. Since most liver failures in China are caused by HBV infection, this Consensus takes HBV-induced liver failure as the example.[8]
ALF: Necrosis can be massive, sub-massive or bridging necrosis, accompanied by degeneration of surviving hepatocytes, and with no collapse or no complete collapse in hepatic sinus network scaffold.
SALF: Histology includes co-existence of previous and new sub-massive or bridging necrosis of liver tissues; collapse of reticular fiber in the older necrosis areas, or deposition of collagen fibers; regeneration of residual hepatocytes, and small bile duct hyperplasia and cholestasis.
ACLF: New hepatocyte necrosis develops upon pathologic damage caused by chronic liver diseases.
CLF: Primarily, the diffuse hepatic fibrosis andthe formation of pseudo lobules are accompanied by distributed hepatocyte necrosis.
Stages
Based on the severity of clinical manifestations, subacute and acute-on-chronic liver failure can be divided into early, middle, and end stages.
Early stage
a. Fatigue with obvious gastrointestinal tract symptoms such as anorexia, vomiting, and abdominal distention;
b. Progressively deepening jaundice (TBil ≥171 µmol/L or daily increase ≥17.1 µmol/L);
c. With hemorrhagic tendency, 30%<PTA ≤40% (or 1.5≤INR<1.9);
d. No HE or other complications.
Middle stage
The condition further develops upon the symptoms of early stage, and with one of the following symptoms:
a. Grade Ⅰ/Ⅱ HE, and (or) ascites;
b. Obvious hemorrhagic tendency (bleeding point or petechia), with 20%<PTA ≤30% (or 1.9 ≤INR<2.6).
End stage
The condition further aggravates with obvious hemorrhagic tendency (injections petechiae) with PTA≤20% (or INR ≥2.6), and with one of the following symptoms: hepatorenal syndrome, severe upper gastrointestinal bleeding, serious infection, and HE over grade Ⅱ.
The following symptoms should be noted in patients who have not developed liver failure:
a. Extremely fatigue with obvious gastrointestinal tract symptoms such as anorexia, vomiting and abdominal distention;
b. Severe jaundice (51 µmol/L ≤TBil ≤171 µmol/L) or daily increase ≥17.1 µmol/L;
c. With hemorrhagic tendency, 40%<PTA ≤50% (or 1.5<INR ≤1.6).
Therapeutic efficacy judgment
The primary index is survival rate (including 4-, 12-, 24- and 48-week survival rate). Secondary index includes improvement in symptoms like fatigue, anorexia, abdominal distension, oliguria, hemorrhagic tendency, HE, infection and ascites, decline in TBil, normalization of PTA (INR), and improvement in albumin.
The cure rate is used for acute and sub-acute liver failure, which includes: a) disappearance of clinical symptoms like fatigue, anorexia, abdominal distension, oliguria, hemorrhagic tendency, HE, infection, and ascites; b) subsidence of jaundice with normal liver size; c) normalization of liver function indices; and d) normal PTA (INR).
Improvement rate is used for acute-on-chronic and chronic liver failure, which includes: a) significant improvement in fatigue, anorexia, abdominal distension, hemorrhagic tendency, and disappearance of HE; b) significant relief of jaundice and ascites; c) significant improvement of liver function indices (TBil <5×ULN, PTA >40% or INR <1.6).
Assessment of prognosis
To the present, no sensitive and reliable assessment system is available for predicting the outcome of liver failure. Clinical analyses include multivariate prognostic evaluation models such as the King's College Hospital criteria (KCH), model for end-stage liver disease (MELD), sequential organ failure assessment (SOFA), Child-Turcotte-Pugh scores (CTP scores), and univariatefactor indicators including TBil, PTA, serum creatinine, bile alkali esterase, blood lipids, and serum sodium.
Treatment
Currently there are no proven therapeutic or medical measures for liver failure. Early diagnosis and treatment is preferred, and any comprehensive therapeutic program should be based on the etiology. Active prevention of various complications is also very important during the treatment. Early transfer to the intensive care unit (ICU) is highly recommended once diagnosed. If possible, artificial liver treatment should be performed and liver transplantation is contemplated according to the progress of the disease (Ⅲ).
Comprehensive therapy of internal medicine
General supportive therapy
a. Bed rest, reducing energy consumption, and alleviating the burden of the liver (Ⅲ);
b. Monitoring PTA/INR, blood ammonia and blood biochemical indices, analyzing arterial blood lactate, endotoxin, hepadnavirus markers, ceruloplasmin, antibodies of autoimmune liver diseases, and examining abdomen by B-ultrasound (liver, gallbladder, spleen and pancreas, ascites), taking chest X-ray and electrocardiography (ECG) (Ⅲ);[1,4]
c. Enteral nutrition is preferred, including highcarbohydrate, low-fat, moderate protein diet, providing 35-40 kcal/kg total calories; for patients with HE, proteinintake by intestinal should be limited; for patients with hypoalimentation, adequate liquid and vitamin supplementation should be delivered intravenously to guarantee a daily total energy intake (Ⅲ);
d. It is necessary to actively rectify low proteinemia, and supply albumin or fresh plasma and blood coagulation factors as appropriate (Ⅲ);
e. Monitoring arterial blood gas is done if required, correcting the disturbance of water and electrolytes as well as acid-base equilibrium, especially hyponatremia, hypochloraemia, hypopotassaemia and alkalosis (Ⅲ); Hyponatremia is a common complication of decompensated cirrhosis. Hyponatremia, refractory ascites and acute kidney injury (AKI) are commonly interrelated with each other. Water restriction, as appropriate, combined with diuretics, is effective for hyponatremia and thus, prevents the subsequent complications;
f. Antibiotic therapies include oral care and intestinal management so as to prevent nosocomial infection (Ⅲ).
Etiological therapy
Any pathogen identified needs further investigation, which is important for treatment as it can influence the prognosis of liver failure.
For HBV DNA (+) patients with liver failure, antiviral therapy is relatively effective in the early and middle stages; but for patients with advanced stage of liver failure, due to the severely damage of residual hepatocytes and regenerative capacity of the liver, antiviral treatment is unlikely to improve outcome. Antiviral drugs should select potent nucleoside class of drugs. Studies[9-11]demonstrated that lamivudine, entecavir, and telbivudine can reduce HBV DNA levels and patient's mortality. Therefore, when appropriate, individual or combined use of drugs such as entecavir, lamivudine, telbivudine, adefovir dipivoxil, and tenofovir can be applied as soon as possible (Ⅱ). Physicians also need to be aware of virus mutation and exacerbation after drug withdrawal. Presently the effects of these drugs on ALF caused by hepatitis A and E virus have not yet been proven (Ⅲ). For confirmed or suspected herpes simplex virus or varicella-zoster virus in patients with ALF, acyclovir (5 to 10 mg/kg every 8 hours intravenous) should be given, and liver transplantation may be considered (Ⅲ).
For drug-induced liver injury (DILI), any presumed or possible offending agent should be stopped immediately (Ⅲ), documenting all agents taken over the past six months including prescription drugs, non-prescription drugs, herbs, dietary supplements (recording the quantity ingested and the time period) (Ⅲ), and trying to determine the non-prescription drug ingredients (Ⅲ). For patients affected by paracetamol, N-acetylcysteine (NAC) therapy is preferred (Ⅰ).[12,13]For confirmed or suspected patients with ALF caused by acetaminophen (APAP) within 4 hours, optimal therapy is to take activated charcoal orally before the administration of NAC (Ⅰ).[14]NAC is given promptly to the patients with a large quantity of APAP ingestion, since the increase of serum drug level or aminotransferases indicates impending or evolving liver injury (Ⅱ-2). Administration of NAC is recommended in any case of ALF when APAP overdose is suspected (Ⅲ). If necessary, artificial liver absorption treatment should be performed. NAC may also improve the outcome for patients with ALF caused by non-APAP. For toadstool poisoning, silymarin or penicillin G[15-18]can be used (Ⅲ).
For ALF induced by acute fatty liver of pregnancy/ HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome, the immediate termination of pregnancy is recommended. If the disease continues to progress, artificial liver support and liver transplantation are recommended (Ⅲ).
Other treatments
Glucocorticoid: Experts have not come to an agreement on the application of glucocorticoid in the treatment of liver failure. It is optimally indicated for non-viral infectious liver failure such as autoimmune liver diseases, etc, and glucocorticoid (prednisone, 40 to 60 mg/d) may be considered. For early stage liver failure caused by other etiologies, if the disease develops rapidly and without complications such as serious infections and hemorrhages, glucocorticoid is also recommended (Ⅲ).
Hepatocyte regeneration therapy: To relieve the necrosis and promote the regeneration of hepatocytes, drugs including hepatocyte growth-promoting factors and prostaglandin E1 (PGE1) liposome (Ⅲ) can be used, but their efficacies need further observation.
Microecological therapy: Patients with liver failure exhibit gut microflora imbalance with reduced normal flora and increased pathogenic intestinal bacteria.[19]The application of intestinal probiotics may improve the prognosis. Microecological modulators such as lactulose or lactitol can be used to reduce the enteric bacteria translocation, endotoxemia, or HE (Ⅲ).
Prevention of complications
Cerebral edema: a) For patients with intracranial hypertension, mannitol (0.5-1.0 g/kg) can be used (Ⅱ-2)[20]; b) Loop diuretics, usually furosemide, can be used with osmotic dehydrant alternatively (Ⅲ); c) Artificial liver support therapy is used (Ⅲ); d)Glucocorticoid is not recommended for intracranial hypertension (Ⅰ);[21]e) Hypothermia therapy is effective in preventing cerebral edema, and decreasing intracranial pressure (Ⅲ).[22,23]
HE: a) eliminating the inciting factors such as serious infections, hemorrhage, electrolyte disturbance, etc. (Ⅲ); b) protein-restricted diet (Ⅲ); c) oral lactulose or lactitol, or transferred by high enema, which can acidify the intestinal tract, accelerate the excretion of ammonia, modulate intestinal microecology, and reduce the absorption of enterotoxin (Ⅲ); d) using antiammonia drugs optionally, like arginine, ornithineaspartic acid according to the electrolyte and acid-base equlibrium (Ⅲ); e) for CLF and ACLF patients, using branched chain amino acid or mixed preparation of branched chain amino and with arginine to rectify the inbalance of amino acid (Ⅲ); f) tracheal intubation for patients with grade Ⅲ+HE (Ⅲ); g) patients with hyperspasmia who may be given short halflife of phenytoin or benzodiazepines but preventive medication is not recommended (Ⅲ); h) artificial liver support therapy (Ⅲ).
Bacterial or fungal infections: a) Routine examination of blood and body fluids is performed (Ⅲ); b) Prophylactic use of antibacterial agents is not recommended except for patients with CLF who may orally take quinolones to prevent intestinal infections (Ⅲ); c) Once infected, broad spectrum antimicrobial agents or combined use of antibiotics are preferred, and the therapy is adjusted according to the results of the sensitivity test (Ⅱ-2). Superinfection of fungi is prevented during potent antibiotics treatment or antibiotics plus adrenal cortical hormone treatment (Ⅲ).
Hyponatremia and intractable ascites: Hyponatremia is a common complication of decompensated cirrhosis. Hyponatremia, intractable ascites and AKI are commonly correlated. Etiological treatment for hyponatremia is the key to prevent subsequent complications. Dilution hyponatremia induced by water and sodium retention is common. The existing diuretics can lead to the loss of blood sodium, and traditional complement of sodium is not efficient, but can lead to pontine myelinolysis. Tolvaptan is an arginine vasopressin V2 receptor blocker, which can promote free water drainage through selectively blocking collecting duct principal cells V2 receptors, and has become a new method for the treatment of hyponatremia and intractable ascites.
AKI and hepatorenal syndrome: a) Effective circulating blood volume is maintained, and intravenous infusion of normal saline is recommended for patients with hypotension (Ⅲ); b) Systemic vasoactive drugs such as terlipressin, norepinephrine, albumin can be used for patients with intractable hypovolemic hypotension, but not for severe encephalopathy patients with intracranial hypertension who have the risk of exacerbation of cerebral edema with the increase of cerebral blood flow (Ⅱ-2);[24]c) Mean arterial pressure of ≥75 mmHg was maintained (Ⅱ);[24]d) Fluid intake is restricted, and the total intake should not exceed the urine volume plus 500-700 mL within 24 hours (Ⅲ); e) Artificial liver support therapy is recommended (Ⅲ).
Hemorrhage: a) Prophylactic use of histamine-2 (H2) receptor blocking agents or proton pump inhibitors is recommended (Ⅰ);[25]b) For patients with portal hypertension hemorrhage, somatostatin analogues are preferred, and vasopression (or combined use of nitrate drugs) can also be used (Ⅲ); Sengstaken-Blakemore tube compression hemostasis, endoscopic sclerotherapy or band ligation, and interventional therapy such as transjugular intrahepatic portosystemic shunt (TIPS) can be used for patients with esophageal-gastric fundal variceal bleeding (Ⅲ); c) For patients with coagulation disorders, fresh plasma, prothrombin composite and fibrinogen can be given to supplement blood coagulation factors. For patients with dramatically reduced platelets, platelet-infusion can be effective (Ⅲ). For patients with disseminated intravascular coagulation (DIC), low-dose low molecular weight heparin (LMWH) or unfractionated heparin can be given. For patients with evidence of hyperfibrinolysis, anti-fibrinolysis drugs like tranexamic acid or hemostatic aryl acid can be used (Ⅲ); d) Since patients with liver failure are often associated with vitamin K deficiency, the routine use of vitamin K (5 to 10 mg) is recommended (Ⅲ).[26]
Hepatopulmonary syndrome: oxygen therapy is indicated when PaO2<80 mmHg; low-flow oxygen (2 to 4 L/min) is administrated via a nasal cannula or mask; and for those who require more oxygen, pressurized mask ventilation or endotracheal intubation is necessary (Ⅲ).
Artificial liver support therapy
Mechanism and methods
Artificial liver support therapy is effective for liver failure. Artificial liver support therapy originates from natural process of liver cell regeneration. It can eliminate noxious substances and temporarily take the place of the failed liver to create an environment for the regeneration of hepatocytes or liver transplantation. Artificial liver support system can be classified as 3 types: non-biological, biological and combined systems.
Non-biological artificial liver has been widely used in clinical practice and its effect has been proved (Ⅱ-2).[27]According to the principle of blood purification, plasmaexchange (PE), hemoperfusion (HP), plasma perfusion (PP), hemofiltration (HF), plasma bilirubin absorption (PBA) and continuous hemofiltration (CHDF), experts in China have created a non-biological artificial liver support system as Li non-bioartificial liver (LiNBAL). Following the principles of individualized treatment, we should choose appropriate methods according to the specific circumstances of each patient: PE combined with plasma filtration (PF) or PE combined with plasma dialysis (PD) or PE combined with PP for drugs and poison-induced liver failure; PE combined with PF for serious infection-induced liver failure; PE for early stage liver failure induced by viral hepatitis; PE combined with PF for middle stage liver failure induced by viral hepatitis; PE combined with PF or PE combined with PD for patients associated with cerebral edema or renal failure; PE combined with PF or PE combined with PD for patients associated with water and electrolyte disturbance; and PBA for patients with significant cholestatic symptoms. LiNBAL can also be applied to liver failure induced by other causes. Standard operations should be followed during the treatment.
Biological and combined biological artificial livers not only have detoxification functions, but also incorporate synthesis and biotransformation functions. They are the direction of future development. They have partly completed the Phase Ⅱ/Ⅲ clinical trials and proved the effectiveness on liver failure.[28,29]Current research focuses on the safety and how to improve its efficacy. Additionally, stem cells are a potential direction for the treatment of liver failure, but its biologic mechanism is still unknown. Although stem cell therapy in animal experiments has achieved satisfactory results,[30]it is not mature enough for clinical application.[31,32]
Indications[33](Ⅲ)
Early and middle stage liver failure is induced by various causes, with INR between 1.5 and 2.6, and platelet>50×109/L. Non-biological artificial liver can also be used for patients with end stage liver failure though there are more complications and the risk is higher. For those without liver failure, it can also be used for early intervention.
Non-biological artificial liver is also suitable for patients with end stage liver failure waiting for liver transplantation, those with rejection after liver transplantation, and those in non-function period after liver transplantation.
Relative contraindications
a. Patients with serious active hemorrhage or disseminated intravascular coagulation;
b. Patients who are highly allergic to the blood preparations or drugs like heparin and protamine used during the treatment;
c. Patients with circulatory function failure;
d. Patients with cardiocerebral infarction in an unstable period;
e. Third trimester of pregnancy (Ⅲ).
Complications
The complications of artificial liver support therapy are hemorrhage, coagulation, hypotension, secondary infection, allergic reactions, hypocalcemia and disequilibrium syndrome. Full assessments before the treatment, and close monitoring of the potential complications throughout the treatment are required. With the development of artificial liver technology, the occurrence of complications will decrease (Ⅲ).
Liver transplantation
Liver transplantation is the most effective therapy for advanced liver failure (Ⅱ-3).[34,35]Prognostic scoring systems such as MELD is valuable for end stage liver diseases, but not for ALF. Hence the determination of candidates for liver transplantation cannot completely depend on these scoring systems (Ⅲ).
Indications[34,36]
a. Middle and end stage liver failure induced by various causes, who have not acquired desired efficacy with internal medicine and artificial liver therapy;
b. Terminal cirrhosis.
Contraindications
Absolute contraindications: a) pulmonary infection, sepsis, abdominal infection, intracranial infection, and active tuberculosis which are out of control; b) incurable extra-hepatic malignant tumor; c) combined serious organic pathologic lesion in vital organs like the heart, brain, lung and kidney, including severe heart failure, intracranial hemorrhage, brain death, renal insufficiency with renal replacement therapy longer than one month; d) human immunodeficiency virus (HIV) infection; e) severe alcoholism or drug abuser; f) psychological diseases which are difficult to control.
Relative contraindications: a) patients over 65 years old; b) combined dysfunctional lesions in vital organs like the heart, brain, lung and kidney; c) hepatic malignant tumor with portal vein tumor thrombosis; d) extensive portal vein thrombosis and cavernous transformation of the portal vein which obstruct theportal vein inflow.
Prevention and treatment of hepatitis virus re-infection after liver transplantation
HBV re-infection: to prevent HBV re-infection, prophylactic use of nucleot(s)ide analogues before the operation and long-term application of high titer anti-HBV immunoglobulin and nucleot(s)ide analogues (lamivudine, adefovir dipivoxil, entecavir, telbivudine, and tenofovir disoproxil) during and after the operation are recommended.[37,38]In recent years, nucleot(s)ide analogues treatment alone can successfully prevent postoperative HBV recurrence.
HCV re-infection: to effectively prevent the recurrence of hepatitis C after liver transplantation, interferon α plus ribavirin treatment is recommended before the operation, which can reduce the postoperative infection, but the occurrence of drug-related adverse effects will increase. Whether postoperative antiviral prophylaxis is needed is inconclusive (Ⅲ).[39,40]Small molecular substances, such as protease inhibitors (which have so far been confined to European countries) provide a new option, but need further confirmation.
Funding:This work is supported by the Chinese High Tech Research & Development (863) program (2011AA020104) and National Science and Technology Major Project (2012ZX10002004).Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Polson J, Lee WM. American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005;41:1179-1197.
2 Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Diagnostic and treatment guidelines for liver failure. Zhonghua Gan Zang Bing Za Zhi 2006;14:643-646.
3 Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009;3:269-282.
4 Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology 2012;55:965-967.
5 Deng G, Zhou G, Zhang R, Zhai Y, Zhao W, Yan Z, et al. Regulatory polymorphisms in the promoter of CXCL10 gene and disease progression in male hepatitis B virus carriers. Gastroenterology 2008;134:716-726.
6 Sato S, Suzuki K, Akahane Y, Akamatsu K, Akiyama K, Yunomura K, et al. Hepatitis B virus strains with mutations in the core promoter in patients with fulminant hepatitis. Ann Intern Med 1995;122:241-248.
7 Liu C, Wang YM, Fan K. Epidemiological and clinical features of hepatitis B virus related liver failure in China. World J Gastroenterol 2011;17:3054-3059.
8 Sun YL, Zhao JM, Zhou GD, Wang SS, Li WS, Meng EH, et al. Cut-off period of subclassification and pathological features of severe hepatitis based on clinical and pathological analyses. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2003;17:270-273.
9 Zoulim F, Perrillo R. Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol 2008;48:S2-19.
10 Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, et al. A revisit of prophylactic lamivudine for chemotherapyassociated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology 2008;47:844-853.
11 Liao CA, Lee CM, Wu HC, Wang MC, Lu SN, Eng HL. Lamivudine for the treatment of hepatitis B virus reactivation following chemotherapy for non-Hodgkin's lymphoma. Br J Haematol 2002;116:166-169.
12 Keays R, Harrison PM, Wendon JA, Forbes A, Gove C, Alexander GJ, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ 1991;303:1026-1029.
13 Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988;319:1557-1562.
14 Sato RL, Wong JJ, Sumida SM, Marn RY, Enoki NR, Yamamoto LG. Efficacy of superactivated charcoal administered late (3 hours) after acetaminophen overdose. Am J Emerg Med 2003; 21:189-191.
15 Moroni F, Fantozzi R, Masini E, Mannaioni PF. A trend in the therapy of Amanita phalloides poisoning. Arch Toxicol 1976;36:111-115.
16 Hruby K, Csomos G, Fuhrmann M, Thaler H. Chemotherapy of Amanita phalloides poisoning with intravenous silibinin. Hum Toxicol 1983;2:183-195.
17 Broussard CN, Aggarwal A, Lacey SR, Post AB, Gramlich T, Henderson JM, et al. Mushroom poisoning--from diarrhea to liver transplantation. Am J Gastroenterol 2001;96:3195-3198.
18 Enjalbert F, Rapior S, Nouguier-Soulé J, Guillon S, Amouroux N, Cabot C. Treatment of amatoxin poisoning: 20-year retrospective analysis. J Toxicol Clin Toxicol 2002;40:715-757.
19 Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011;54:562-572.
20 Canalese J, Gimson AE, Davis C, Mellon PJ, Davis M, Williams R. Controlled trial of dexamethasone and mannitol for the cerebral oedema of fulminant hepatic failure. Gut 1982;23:625-629.
21 Rakela J, Mosley JW, Edwards VM, Govindarajan S, Alpert E. A double-blinded, randomized trial of hydrocortisone in acute hepatic failure. The Acute Hepatic Failure Study Group. Dig Dis Sci 1991;36:1223-1228.
22 Muñoz SJ, Moritz MJ, Bell R, Northrup B, Martin P, Radomski J. Factors associated with severe intracranial hypertension in candidates for emergency liver transplantation. Transplantation 1993;55:1071-1074.
23 Diringer MN, Reaven NL, Funk SE, Uman GC. Elevated body temperature independently contributes to increased length ofstay in neurologic intensive care unit patients. Crit Care Med 2004;32:1489-1495.
24 Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol 2009;6:542-553.
25 Selby NM, Kubba AK, Hawkey CJ. Acid suppression in peptic ulcer haemorrhage: a 'meta-analysis'. Aliment Pharmacol Ther 2000;14:1119-1126.
26 Pereira SP, Rowbotham D, Fitt S, Shearer MJ, Wendon J, Williams R. Pharmacokinetics and efficacy of oral versus intravenous mixed-micellar phylloquinone (vitamin K1) in severe acute liver disease. J Hepatol 2005;42:365-370.
27 Stange J, Hassanein TI, Mehta R, Mitzner SR, Bartlett RH. The molecular adsorbents recycling system as a liver support system based on albumin dialysis: a summary of preclinical investigations, prospective, randomized, controlled clinical trial, and clinical experience from 19 centers. Artif Organs 2002;26:103-110.
28 Demetriou AA, Brown RS Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg 2004;239:660-670.
29 Millis JM, Cronin DC, Johnson R, Conjeevaram H, Conlin C, Trevino S, et al. Initial experience with the modified extracorporeal liver-assist device for patients with fulminant hepatic failure: system modifications and clinical impact. Transplantation 2002;74:1735-1746.
30 Li J, Zhang L, Xin J, Jiang L, Li J, Zhang T, et al. Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology 2012;56:1044-1052.
31 Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and longterm outcomes. Hepatology 2011;54:820-828.
32 Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, et al. Safety and efficacy of autologous bone marrow stem cell transplantation through hepatic artery for the treatment of chronic liver failure: a preliminary study. Transplant Proc 2008;40:1140-1144.
33 Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline for non-bioartificial liver support system in the treatment of liver failure. Chin J Clin Infect Dis 2009;2:321-325.
34 Mochida S. Indication criteria for liver transplantation for acute liver failure in Japan. Hepatol Res 2008;38:S52-55.
35 Steadman RH, Van Rensburg A, Kramer DJ. Transplantation for acute liver failure: perioperative management. Curr Opin Organ Transplant 2010;15:368-373.
36 Rao HY, Guo F, Wei L. Introduction and comments on AASLD practice guidelines: diagnosis and treatment of acute liver failure and assessment of liver transplant patients. Zhonghua Gan Zang Bing Za Zhi 2006;14:154-156.
37 Cholongitas E, Vasiliadis T, Antoniadis N, Goulis I, Papanikolaou V, Akriviadis E. Hepatitis B prophylaxis post liver transplantation with newer nucleos(t)ide analogues after hepatitis B immunoglobulin discontinuation. Transpl Infect Dis 2012;14:479-487.
38 McCaughan GW. Prevention of post liver transplant HBV recurrence. Hepatol Int 2011;5:876-881.
39 García-Pajares F, Almohalla C, Lorenzo Pelayo S, Ruiz Zorrilla R, Pinto P, Ramos C, et al. Early and extended therapy for recurrent hepatitis C after liver transplantation. Transplant Proc 2012;44:1571-1573.
40 Everson GT, Terrault NA, Lok AS, Rodrigo del R, Brown RS Jr, Saab S, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology 2013;57:1752-1762.
February 22, 2013
Accepted after revision June 5, 2013
Lan-Juan Li, MD, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Email: ljli@zju.edu.cn)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60055-7
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Biliary-colonic fistula caused by cholecystectomy bile duct injury
- Hepatic abscess associated with Salmonella serotype B in a chronic alcoholic patient
- A new veno-venous bypass type for ex-vivo liver resection in dogs
- Effect of L-cysteine on remote organ injury in rats with severe acute pancreatitis induced by bile-pancreatic duct obstruction
- Endobiliary radiofrequency ablation for malignant biliary obstruction
- The diagnostic value of high-frequency ultrasonography in biliary atresia
