超高效液相色谱测定肉制品中的罗丹明B、孔雀石绿和结晶紫及其代谢物残留
2013-07-02孙娜李挥孙汉文
孙娜,李挥,孙汉文
(1.河北大学化学与环境科学学院,河北省分析科学技术重点实验室,河北保定 071002;2.河北省食品质量监督检验研究院,河北石家庄 050091)
超高效液相色谱测定肉制品中的罗丹明B、孔雀石绿和结晶紫及其代谢物残留
孙娜1,李挥2,孙汉文1
(1.河北大学化学与环境科学学院,河北省分析科学技术重点实验室,河北保定 071002;2.河北省食品质量监督检验研究院,河北石家庄 050091)
提出了一种以超高效液相色谱同时检测肉制品中5种违禁合成色素的新方法.以甲醇-水(V(甲醇)∶V(水)=95∶5)作为提取剂,样品在80℃经微波辅助萃取5min,继以C18固相萃取柱净化,使用ACQUITY BEH C18分析柱,以乙腈-20mmol/L乙酸铵缓冲溶液(V(乙腈)∶V(乙酸铵)=80∶20,pH=5)作流动相,实现了5种色素的有效分离.在0.1~5.0μg/mL内,校准曲线呈良好的线性,相关系数r为0.994 5~0.999 5.该方法测定肉制品中罗丹明B、孔雀石绿、结晶紫、隐性孔雀石绿和隐性结晶紫的定量限分别为4.23,1.83,1.61,1.96,1.95μg/kg.对加标50μg/kg的牛肉香肠日内测定6次,5种分析物的精密度(以RSD表示)优于9.2%.在25μg/kg和75μg/kg添加水平,平均回收率为78.01%~109.2%,相对标准偏差小于10%.实验结果表明:该方法具有快速、灵敏和准确等优点,可用于肉制品中5种违禁色素的常规分析.
违禁色素;肉制品;微波辅助提取;固相萃取;超高效液相色谱
食用色素可以改善食品的色泽,增加人们的食欲,曾一度有100余种色素在食品生产加工领域使用.随着对色素安全性评估的深入,发现许多色素在生物体内转化为有害的衍生物,许多合成色素已被禁止作为食品色素[1].我国《食品添加剂卫生标准》规定:鱼、虾、蟹等水产制品中杜绝添加任何合成色素[2].因此,必须加强动物性食品中色素的监管.
目前,色谱法常用来检测食品中的色素[3].高效液相色谱荧光检测法已作为测定水产品中孔雀石绿和结晶紫残留量的标准方法(GB/T 20361-2006),其定量限为2μg/kg.最近,高效色谱法已用于养殖水产品中的罗丹明B和罗丹明6G[4-5]、孔雀石绿和隐形孔雀石绿[6],虾肉中孔雀石绿和结晶紫残留[7],水产品中的孔雀石绿、结晶紫及其代谢物[8],调味品中罗丹明B[9].高效色谱-质谱联用法已用于鱼中的孔雀石绿[10]、水产品中的孔雀石绿和隐形孔雀石绿[11-12]、鱼中孔雀石绿和结晶紫[13-14]和水产品中孔雀石绿、隐形孔雀石绿、结晶紫和隐形结晶紫[15-17].虽然高效色谱-质谱联用法具有高的灵敏度,但是仪器昂贵,运行成本高而难于在一般实验室普及.样品处理关系到方法的特效性和检测灵敏度.固相萃取[5,8-10]、浊点萃取[7]、加速溶剂萃取[16,1]已用于食品中色素的提取.本工作旨在将微波辅助提取和固相萃取净化相结合继以超快速高效液相色谱分离分析,首次实现同时检测肉制品中罗丹明B、孔雀石绿、隐性孔雀石绿、结晶紫和隐性结晶紫等5种违禁食品色素.
1 实验部分
1.1 仪器与试剂
超高效液相色谱:ACQUITY超高效液相色谱仪(Waters公司);Explorer sp 48全自动微波合成仪(美国培安公司);SPE-30四通道全自动固相萃取仪(中国 天津博纳艾杰尔科技有限公司),C18固相萃取柱(500mg,6mL,美国Waters公司).标准品孔雀石绿(质量分数98.0%)、隐性孔雀石绿(质量分数97.0%)、结晶紫(质量分数80%)、隐性结晶紫(质量分数99%)、罗丹明B(质量分数99.0%)均购自Sigma公司.乙腈、甲醇、冰乙酸为色谱纯,实验用水均为超纯水,其他试剂为分析纯.
标准储备液:分别准确称取适量各色素的标准品,均用甲醇配成1.0mg/mL的标准储备溶液,置于冰箱中冷藏保存.用超纯水稀释标准储备溶液配制混合标准工作液.
1.2 样品前处理
购自当地超市的样品用粉碎机粉碎均匀后,置于冰箱(-20℃)中冷藏备用.准确称取约10g(精确至0.05mg)样品,加入15mL甲醇-水溶液(V(甲醇)∶V(水)=95∶5),混匀后,在压力0.63MPa、功率50W、温度80℃的条件下进行微波辅助萃取5min.冷却至室温,然后在15 000r/min和4℃条件下离心3min,上清液以预先经过6mL甲醇和6mL水活化的C18固相萃取柱净化.以4mL甲醇-乙酸(V(甲醇)∶V(水)=95∶5)洗脱,其流速为1mL/min.抽干柱中残留的液体,收集全部的流出液和洗脱液,在50℃下氮气吹干,用0.5mL的甲醇溶解残渣,经0.22μm滤膜过滤后,滤液上机测定.
1.3 色谱条件
色谱柱:ACQUITY BEH C18(2.1mm×50mm,1.7μm);流动相:乙腈-20mmol/L乙酸铵缓冲溶液(V(乙腈)∶V(乙酸铵)=80∶20,pH 5),流速0.3mL/min;柱温30℃;进样2μL.在254nm波长处检测罗丹明B,隐性孔雀石绿和隐性结晶紫,在600nm波长处检测孔雀石绿和结晶紫.
2 结果与讨论
2.1 微波辅助萃取条件的优化
2.1.1 溶剂的选择
溶剂的选择首先应考虑目标分析物的溶解度、溶剂与基质之间作用能力以及溶剂对微波的吸收特性.孔雀石绿、隐性孔雀石绿、结晶紫、隐性结晶紫和罗丹明B是弱极性和极性化合物,在甲醇中的溶解度高于乙腈,甲醇的极性高于乙腈,因此选择甲醇作微波萃取溶剂为宜.本实验进一步考察不同比例的甲醇-水溶液对萃取效率的影响,结果如图1所示.与单纯使用甲醇相比,以甲醇-水溶液(V(甲醇)∶V(水)=95∶5)为萃取溶剂,罗丹明B、孔雀石绿和隐性结晶紫的回收率有所提高,而隐性孔雀石绿和结晶紫的回收率有所降低.但是,随着水量的增加,5种分析物的回收率反而明显降低.本实验选用甲醇-水作为萃取溶剂,5种分析物可获得84%~105%的回收率.
2.1.2 提取温度与时间的选择
准确称取3份平行加标样品,每种分析物的加标质量分数为1.5mg/kg,分别在60,80,100℃下以甲醇-水(V(甲醇)∶V(水)=95∶5)萃取5min,结果表明:从60℃到80℃时,5种色素回收率可提高到85%以上.但是在100℃萃取,其回收率与80℃时的回收率基本一致.因此,萃取温度选为80℃.在萃取温度80℃的条件下,进一步考察了萃取时间对回收率的影响.图2表明:萃取时间从1min到5min,回收率逐渐提高,但是萃取7min和5min,回收率基本一致,为95%~107%,因此,萃取5min为宜.
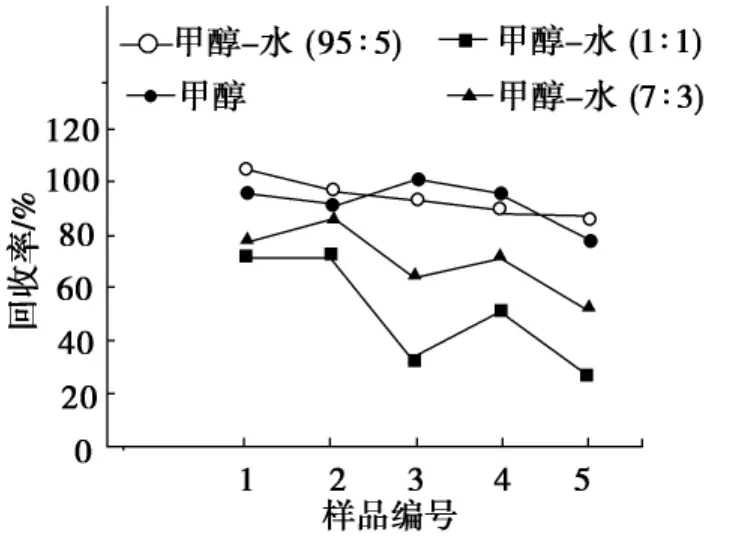
图2 萃取时间对5种色素回收率的影响Fig.2 Effect of extraction time on the recoveries of five colorants
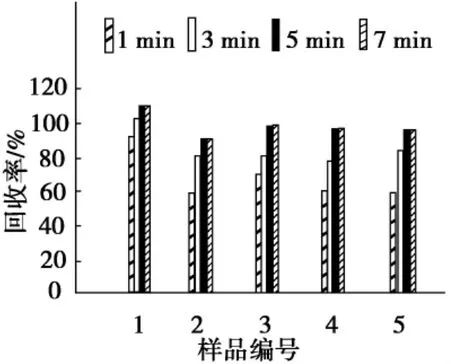
图1 不同溶剂对5种色素回收率的影响Fig.1 Effect of different solvents on recovery of five colorants
2.2 固相萃取净化条件的优化
比较了3种常用固相萃取柱净化色素萃取液的效果.以甲醇-乙酸作洗脱剂,其回收率列于表1中.MCX载体是阳离子交换反相吸附剂,HLB载体具有亲脂和亲水特性,其回收率分别为0~20.1%和19.8%~112%.C18固相萃取柱对色素具有高的回收率,因此选用C18固相萃取柱.进一步比较了以甲醇、甲醇-水(V(甲醇)∶V(水)=95∶5)、甲醇-乙酸(V(甲醇)∶V(乙酸)=95∶5)和甲醇-氨水(体积分数1%)对目标分析物的洗脱效果,结果如图3所示.选用甲醇-氨水(体积分数1%)作洗脱剂,5种分析物的回收率在92%~109%内.通过考察洗脱剂体积(2,3,4,5mL)对回收率的影响,结果表明其用量以4mL为佳.
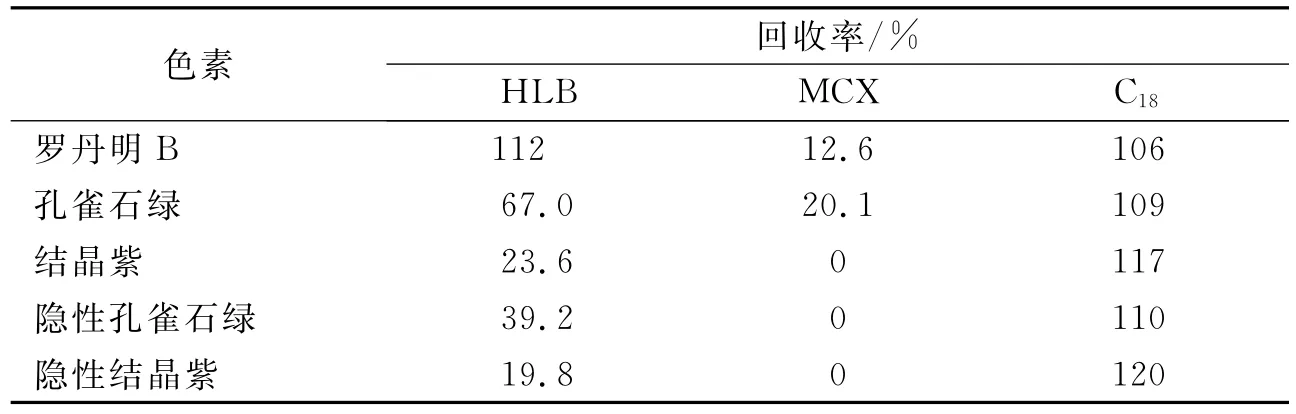
表1 使用不同固相萃取柱时5种色素的回收率Tab.1 Recoveries of 5colorants using different solid-phase extraction columns
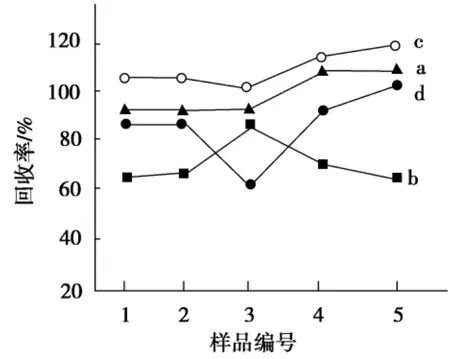
图3 不同淋洗剂对5种色素回收率的影响Fig.3 Recovery of five colorants using different eluents
2.3 色谱分离检测条件的选择
使用C18作固定相,流动相中需加入一种电解质以实现反相色谱分离离子化的色素.本工作以乙腈作流动相,考察了乙酸铵的浓度(0.2,2,20,30mmol/L)对分离效果的影响.结果表明:随着缓冲盐浓度的增大,峰形越尖锐,峰宽越窄.但是,如果盐的浓度太高则易导致堵塞色谱柱.以乙腈-20mmol/L乙酸铵(V(乙腈)∶V(乙酸氨)=80∶20)作流动相为宜.进一步考察了流动相pH(4,5,6,7)对色谱峰的影响,发现在pH=5时,峰形良好,无拖尾现象,可在12min内完成,实现了5种分析物的有效分离.
在200~800nm进行全波扫描,考察了5种色素的最大吸收波长.罗丹明、隐性孔雀石绿和隐性结晶紫在254nm波长处吸收较强,而孔雀石绿和结晶紫在600nm波长处吸收较强.色谱图如图4所示.
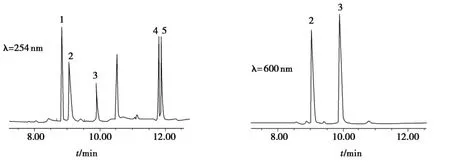
图4 萃取时间对5种色素的回收率的影响Fig.4 Effect of extraction time on the recoveries of five colorants
2.4 方法的线性、检出限和精密度
用水稀释,配制不同质量浓度的混合标准溶液,每种色素的质量浓度为0.1,0.5,1,5,10μg/mL.以混合标准溶液的质量浓度为横坐标、色谱峰面积为纵坐标,绘制标准曲线,其线性方程列于表2中.
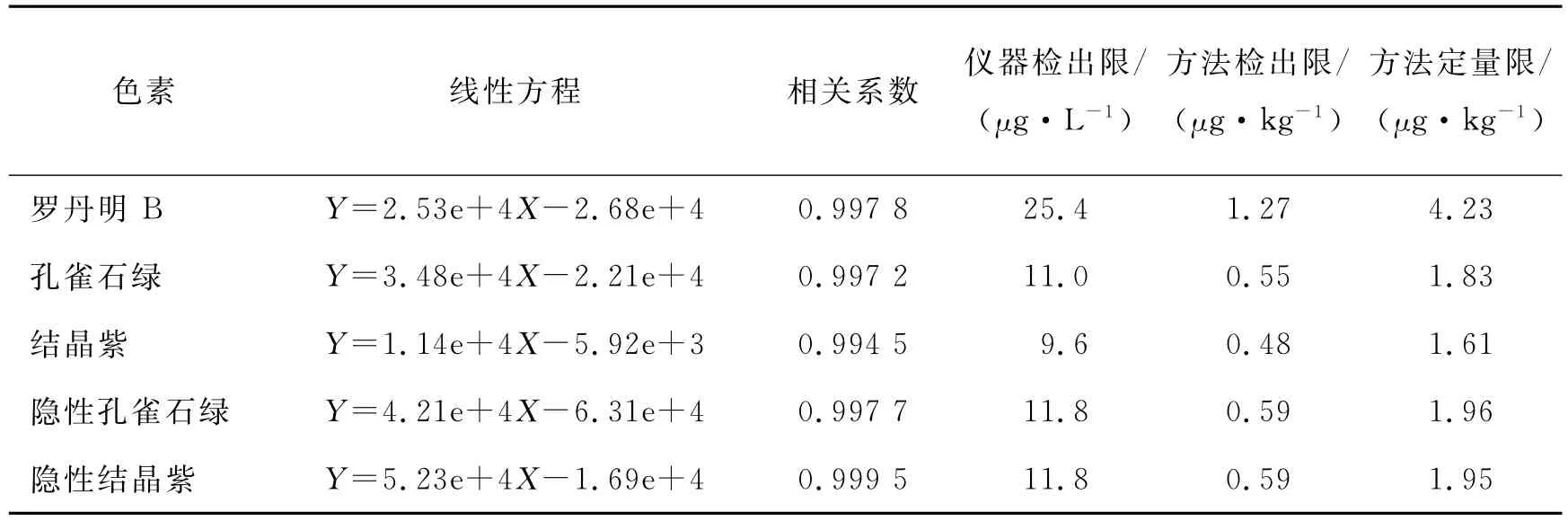
表2 线性方程、检出限(LOD)及定量限(LOQ)Tab.2 Linear equations,limits of detection(LOD)and limits of quantification(LOQ)
依据色谱峰信噪比S/N=3计算检出限,S/N=10计算定量限(见表2).5种分析物的仪器检出限为9.6~25.4μg/L.本方法取样量10g,最终试液0.5mL,则方法的检出限为0.48~1.27μg/kg,测定肉制品中罗丹明B、孔雀石绿、隐性孔雀石绿、结晶紫和隐性结晶紫的定量限分别为4.23,1.83,1.96,1.61,1.95μg/kg.本方法的检测灵敏度优于标准方法(GB/T 20361 2006)和文献报道的高效液相色谱法[4,7-9].
本方法的精密度以日内相对标准偏差来表示.对加标50μg/kg的牛肉香肠进行日内6次检测,相对标准偏差小于9.2%.
2.5 方法的应用
应用本方法对3份从当地超市购买的鱼肉肠和猪肉肠样品进行测定,均未检出5种目标分析物,其含量均低于方法的定量限.加标回收实验表明,加标浓度为25μg/kg和75μg/kg时,检测鱼肉肠样品中5种色素的回收率为78.01%~109.2%,相对标准偏差为2.04%~8.73%,检测牛肉肠样品中5种色素的回收率为78.24%~108.9%,相对标准偏差为2.13%~9.54%(见表3).
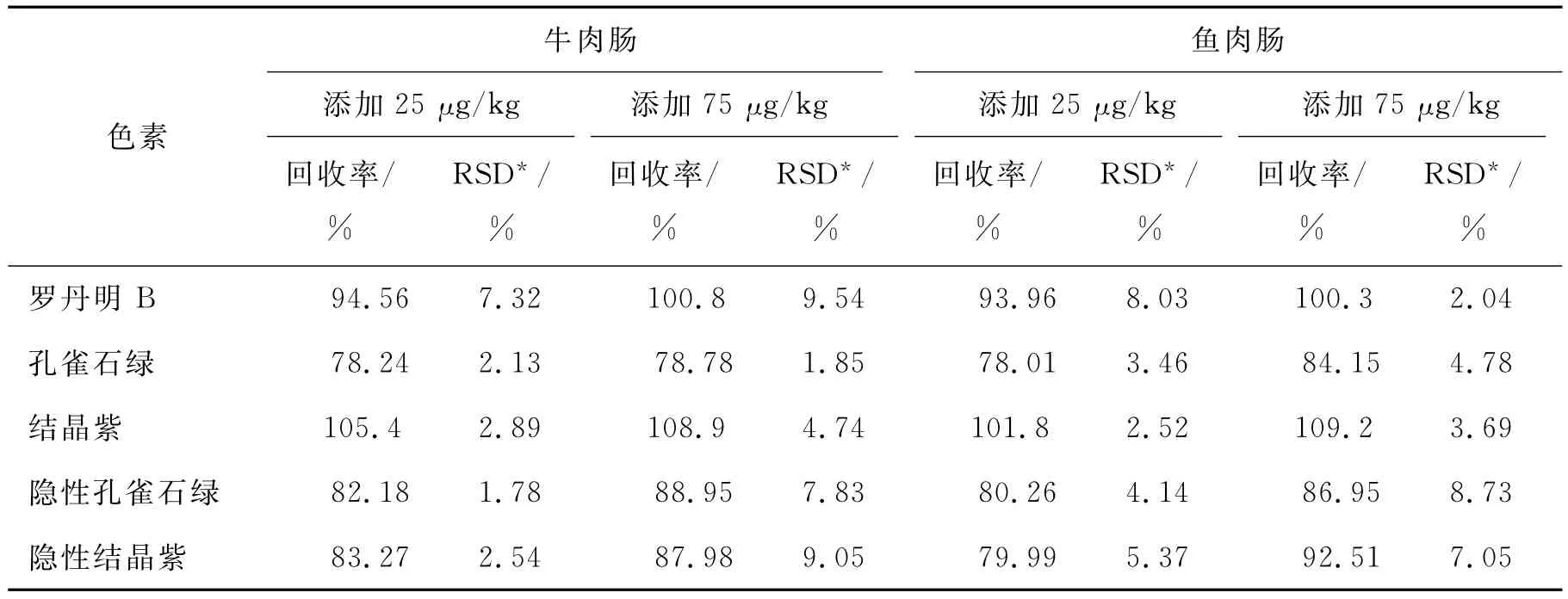
表3 鱼肉肠和牛肉肠中5种色素的测定Tab.3 Determination of five colorants in beef sausage and fish sausage
3 结论
将微波辅助提取和固相萃取净化相结合有效地减少了基体成分的干扰,提高了方法的检测灵敏度.以超快速高效液相色谱分离分析,在12min内5种目标分析物可完全达到基线分离,实现了同时、快速、准确定量检测肉制品中罗丹明B、孔雀石绿、隐性孔雀石绿、结晶紫和隐性结晶紫等5种违禁食品着色剂.该方法简单快速,结果准确可靠,并可节约大量溶剂,适用于对肉制品中5种合成色素残留的常规检测.
[1] 李娜,李晓丽,苗虹.食品中违禁色素检测方法的研究进展[J].中国食品卫生杂志,2012,24(2):185-189.
LI Na,LI Xiaoli,MIAO Hong.Progress on detection methods for banned dyes in food[J].Chinese Journal of Food Hygiene,2012,24(2):185-189.
[2] GB/T2760-2007,食品添加剂卫生标准[S].
[3] KUCHASKA M,GRABKA J.A review of chromatographic methods for determination of synthetic food dyes[J].Talanta,2010,80:1045-1051.
[4] 孙磊龙,杨志华.高效液相色谱测定食品中的罗丹明B[J].中国卫生检验杂志,2011,21(7):1648-1649.
SUN Leilong,YANG Zhihua.HPLC determination of Rhodamine B in foods[J].Chinese Journal of Health Laboratory Technology,2011,21(7):1648-1649.
[5] CHIANG T L,WANG Y C,DING W H.Trace determination of rhodamine B and rhodamine 6Gdyes in aqueous samples by solid-phase extraction and high-performance liquid chromatography coupled with fluorescence detection[J].Journal of Chinese Chemical Society(Taibei),2011,59:1-5.
[6] LONG C,MAI Z,ZHU B,et al.New oxidant used for the post-column derivatization determination of malachite green and leucomalachite green residues in cultured aquatic products by high-performance liquid chromatography[J].Journal of Chromatography A,2008,1203:21-26.
[7] 陈建伟,姚志云,毛健伟,等.浊点萃取-高效液相色谱法测定虾肉中孔雀石绿和结晶紫残留的研究[J].南京农业大学学报,2010,33(1):94-98.
CHEN Jianwei,YAO Zhiyun,MAO Jianwei,et al.Determination of residues of malachite green and crystal violet in shrimp samples by cloud point extraction-high performance liquid chromatography[J].Journalof Nanjing Agricultural U-niversity,2010,33(1);,94-98.
[8] 葛宝坤,王云凤,常春艳,等.固相萃取-液相色谱法快速测定水产品中的孔雀石绿、结晶紫及其代谢物[J].中国卫生检验杂志,2006,16(1):45-47.
GE Baokun,WANG Yunfeng,CHANG Chunyan,et al.Rapid determination of malachite green,crystal violet and their metabolites in aquatic products by solid phase extraction-liquid chromatography[J].Chinese Journal of Health Laboratory Technology,2006,16(1):45-47 2.
[9] 刘敏,李小林,别玮,等.固相萃取-高效液相色谱法同时测定调味品中15种工业合成染料[J].色谱,2011,29(2):162-167.
LIU Min,LI Xiaolin,BIE Wei,et al.Simultaneous determination of 15industrial synthetic dyes in condiment by solid phase extraction-high performance liquid chromatography[J].Chinese Journal of Chromatography,2011,29(2):162-167.
[10] MARTÍNEZ BUENO M J,HERRERA S,UCLÉS A,et al.Determination of malachite green residues in fish using molecularly imprinted solid-phase extraction followed by liquid chromatography-linear ion trap mass spectrometry[J].Analytica Chimica Acta,2010,665:47-54.
[11] HALL Z,HOPLEY C,ÓCONNOR G.High accuracy determination of malachite green and leucomalachite green in salmon tissue by exact matching isotope dilution mass spectrometry[J].Journal of Chromatography B,2008,874:95-100.
[12] ARROYO D,ORTIZ M C,SARABIA L A,et al.Determination and identification,according to European union decision 2002/657/EC,of malachite green and its metabolite in fish by liquid chromatographyetandem mass spectrometry using an optimized extraction procedure and three-way calibration[J].Journal of Chromatography A,2009,1216:5472-5482.
[13] ANDERSEN W C,TURNIPSEED S B,KARBIWNYK,et al.Multiresidue method for the triphenylmethane dyes in fish:Malachite green,crystal(gentian)violet,and brilliant green[J].Analytica Chimica Acta,2009,637:279-289.
[14] 张志刚,施冰,陈鹭平,等.液相色谱法同时测定水产品中孔雀石绿和结晶紫残留[J].分析化学,2006,34,663-667.
ZHANG Zhigang,SHI Bing,CHEN Luping,et al.Simultaneous determination and confirmation of malachite dreen,crystal violet and their leuco metabolites residues in aquaticproduct by liquid chromatography-visible detection and tandem mass spectrometric detection[J].Chinese Journal of Analytical Chemistry,2006,34:663-667.
[15] CHEN G,MIAO S.HPLC determination and MS confirmation of malachite green,gentian violet,and their leuco metabolite residues in channel catfish muscle[J].Journal of Agricultural and Food Chemistry,2010,58:7109-7114.
[16] TAO Yanfei,CHEN Dongmei,CHAO Xiaoqin,et al.Simultaneous determination of malachite green,gentian violet and their leuco-metabolites in shrimp and salmon by liquid chromatographyetandem mass spectrometry with accelerated solvent extraction and auto solid-phase clean-up[J].Food Control,2011,22:1246-1252.
[17] 罗瑞峰,罗小玲,马小宁.水产品中孔雀石绿、结晶紫及其代谢产物检测方法的探讨[J].化学分析计量,2011,20(3):40-42.
LUO Ruifeng,LUO Xiaoling,MA Xiaoning.Determination method of malachite green,crystal violet and itscorresponding leuco compounds in aquatic products[J].Chemical Analysis and Meterage,2011,20(3):40-42.
[18] 郝昀,李挥,孙汉文.加速溶剂萃取在动物源食品农兽药残留分析中的应用进展[J].河北大学学报:自然科学版,2012,32:434-448.
HAO Yun,LI Hui,SUN Hanwen.Application advancement of multi-residure analysis of veterinary drugs and pesticides in animal origin foods by Accelerated solvent extraction[J].Journal of Hebei University:Natural Science Edition,2012,32:434-448.
(责任编辑:梁俊红)
Simultaneous development of rhodamine B,malachite green,crystal violet and their metabolites residues in meat by UPLC
SUN Na1,Ll Hui2,SUN Hanwen1
(1.College of Chemistry and Environmental Science,Key Laboratory of Analytical
Science and Technology of Hebei Province,Hebei University,Baoding 071002,China;2.Hebei Institute of Food Quality Supervision and Research,Shijiazhuang 050091,China)
A new ultra-high performance liquid chromatographic method was developed for analysis of five banned rhodamine B,malachite green,crystal violet and their metabolites residues in meat.The meat samples were extracted by microwave-assisted extraction with methanol-water(95∶5,V/V),followed by clean up with C18solid phase extraction column.The effective separation of five colorants in meat matrixes was achieved using ACQUITY BEH C18analytical column with acetonitrile-20mmol/L acetic ammonium buffer(80∶20,V/V,pH=5)and no interfering peaks could be detected at the retention time of the analytes.The calibration curves in the range of 0.1- 5.0μg/mL for each analyte showed good linearitywithcorrelation coefficients of 0.994 5 0.999 5.The method limits of quantification for rhodamine B,malachite green,crystal violet,leucomalachite green,and leucocrystal violet in meat samples were 4.23,1.83,1.61,1.96,1.95μg/kg.For beef sausage spiked with 50μg/kg for each analyte,the intra-day precision(as RSD)for five analytes was less than 9.2%for six determinations within a day.The average recovery of the five analytes from meat samples spiked with 25and 75μg/kg was 78.01%-109.2%with RSD<10%.This method has the advantages of being rapid,sensitivity,and accuracy,and can be applied for multiresidue analysis of five banned and Rhodamine B food colorant in meat samples.
banned colorants;meat;microwave-assisted extraction;solid phase extraction;ultra-high performance liquid chromatography
O 657.7
A
1000-1565(2013)02-0154-07
10.3969/j.issn.1000-1565.2013.02.009
2012-12-15
河北省自然科学基金资助项目(B2008000583);河北省应用基础研究计划重点基础研究项目(10967126D)
孙娜(1989-),女,河北邢台人,河北大学在读硕士研究生
孙汉文(1945-),男,河北魏县人,河北大学教授,主要从事农兽药残留分析研究.E-mail:Hanwen@hbu.cn
