Expression of Rab25 correlates with the invasion and metastasis of gastric cancer
2013-06-12ChuanwuCaoChenhuiLuJichongXuJiaxingZhangJunZhangMaoquanLi
Chuanwu Cao, Chenhui Lu, Jichong Xu, Jiaxing Zhang, Jun Zhang, Maoquan Li
1Department of Interventional Radiology, Shanghai 10thPeople’s Hospital, Tongji University, Shanghai 200072, China;2Department of Regenerative Medicine, Tongji University School of Medicine, Shanghai 200092, China
*Chuanwu Cao and Chenhui Lu contributed equally to this study.
Expression of Rab25 correlates with the invasion and metastasis of gastric cancer
Chuanwu Cao1*, Chenhui Lu1*, Jichong Xu1, Jiaxing Zhang1, Jun Zhang2, Maoquan Li1
1Department of Interventional Radiology, Shanghai 10thPeople’s Hospital, Tongji University, Shanghai 200072, China;2Department of Regenerative Medicine, Tongji University School of Medicine, Shanghai 200092, China
*Chuanwu Cao and Chenhui Lu contributed equally to this study.
Corresponding to:Maoquan Li, MD, PhD. Department of Interventional Radiology, Shanghai 10thPeople’s Hospital, Tongji University, No. 301, Yanchang Rd, Shanghai 200072, China. Email: drmaoquan.li@gmail.com.
The objective of this study was to determine the expression of the important vesicle traffickingregulating factor Rab25 in human gastric cancer tissues, to analyze the correlation between Rab25 protein expression with gastric cancer occurrence and development, and to discuss the correlation of Rab25 protein expression with gastric cancer cell metastasis. The overall aim was to provide experimental evidence that can be used to design future biological treatments of human gastric cancer. Human gastric cancer tissue and the adjacent normal gastric tissue were surgically removed, and immunohistochemistry and Western blotting were used to detect Rab25 protein expression. The correlation between Rab25 protein expression with the development and pathological characteristics of gastric cancer was analyzed. Using RNAi, Rab25 expression was reduced in the gastric cancer cell line MGC80-3, and the changes in MGC80-3 cell invasiveness were then monitored. Immunohistochemistry showed that the Rab25 protein expression rates were 78.21% and 23.08% in gastric carcinoma and the adjacent normal gastric tissue, respectively. Immunohistochemistry and Western blot results showed that Rab25 protein expression in gastric cancer was significantly higher than in adjacent normal gastric tissues (P<0.01). Less differentiated gastric cancer cells had higher expression of Rab25 protein (P<0.01). Gastric carcinomas from patients with a late pathological stage (III-IV) had significantly higher Rab25 protein expression than early stage (I-II) patients (P<0.01). Gastric carcinomas from patients with lymph node metastasis had significantly higher Rab25 protein expression than lymph node metastasisfree patients (P<0.01). Gastric carcinomas from patients with distant metastases had significantly higher Rab25 protein expression than the distant metastasis-negative patients (P<0.01). Rab25 protein expression in gastric cancer was not affected by the patients,sex, age, or tumor size (P>0.05). MGC80-3 cells transfected with Rab25 siRNA had significantly lower Rab25 protein expression (P<0.01) and a significantly lower number of cells that passed through a Transwell chamber compared with non-transfected controls and the transfected control group (P<0.01). Rab25 protein expression is associated with the development of gastric cancer. siRNA knockdown of Rab25 protein expression in MGC80-3 gastric cancer cells reduced MGC80-3 cell invasiveness and provided experimental evidence for potential future biological treatment strategies of human gastric cancer.
Rab25; human gastric cancer; immunohistochemistry; Western blot; siRNA; invasion
Scan to your mobile device or view this article at:http://www.thecjcr.org/article/view/1748/2480
Introduction
Gastric cancer is a malignant tumor that is derived from the epithelium. It is the most common gastrointestinal cancer and one of the most common tumors, with tumor morbidity and mortality rates ranking fourth and the second in the world, respectively (1). Every year there areapproximately 900,000 new gastric cancer patients, while approximately 700,000 people die of gastric cancer (2). Although the diagnosis and treatment of gastric cancer has been greatly improved, there has been no significant decline in morbidity and mortality (3-5). Gastric cancer remains a serious threat to human life and health with a five-year survival rate of less than 24% (6-9). The predominant characteristics of gastric cancer are invasion and a tendency to metastasize. Approximately 60% of gastric cancer patients had a metastasis when diagnosed, and approximately 70% of patients had recurrence and metastasis after being treated for gastric cancer. Metastasis is one of the major causes of death in patients with gastric cancer (10,11). Epidemiological data showed that because of the low cure rate and the fact that gastric tumor cells were prone to invasion, migration, and metastasis, most patients with gastric cancer ultimately died of multiple organ failure. As a complex biological process, gastric carcinoma migration is often accompanied by changes in certain cytokines and signaling pathways (12,13). The study of genes associated with the metastasis of gastric cancer could provide experimental evidence of metastasis and possible therapeutic targets.
In eukaryotic cells, cell growth, biosynthesis, secretion, and other important biological processes are all associated with protein transport between the organelles. Macromolecule transport and the dynamic balance of proteins between cell organelles depend on the two-way flow of tubular vesicles (14,15). One of the most important determinants of normal cellular activity is whether proteins can reach the appropriate cell organelles (16,17). The intracellular transport of proteins depends on vesicles (18). Rab is an important regulator of vesicle transport and is considered a “molecular switch” in vesicle transport. It has a role in vesicle formation, transport, adhesion, anchoring, and fusion, among other processes (19-21). Rab25 is a recently discovered Rab family member that belongs to the Ras oncogene family and is also known as CATX8 (22,23). The Rabs are closely related to tumorigenesis and metastasis. Studies have shown that Rab25 is related to ovarian cancer, colon cancer, breast cancer development, and metastasis (24-26). However, the correlation between Rab25 protein expression and the occurrence, development, and metastasis of gastric cancer has not yet been reported. In this study, immunohistochemistry and Western blotting showed differences in Rab25 protein expression between human gastric cancer tissue and normal adjacent gastric tissue. Using RNAi, Rab25 protein expression was reduced in the human gastric cancer cell line MGC80-3, and a change was observed in MGC80-3 cell invasiveness. The correlation between Rab25 protein expression and human gastric cancer development and metastasis will be discussed.
Materials and methods
Samples
Fresh specimens were collected from 78 gastric cancer patients (45 males and 33 females) that were treated in our hospital between June 2004 and June 2010. Half of each specimen was placed in 4% paraformaldehyde, and the other half was immediately frozen in liquid nitrogen. None of the patients had preoperative chemotherapy, radiotherapy, or other treatment history, and none had other inflammatory diseases. The gastric cancer tissue and adjacent normal gastric tissue (more than 5 cm from the cancer tissue) from the surgical resections of the 78 patients were all verified by pathologists in our hospital. The patients were aged 40-79 years with a mean age of 70.39 years. Twenty-six patients were less than 60 years old, and 52 patients were greater than or equal to 60 years old. Thirty-six patients had primary tumors smaller than 3 cm, and 42 cases had tumors larger than or equal to 3 cm. The differentiation statuses of the tumors were as follows: 49 cases of highly differentiated tumors and 29 cases of poorly differentiated tumors. Regarding the pathological stage, 47 cases were stages I-II, and 31 cases were stages III-IV. Thirty-five cases had lymph node metastasis, and 43 cases had no lymph node metastasis. Thirty-two cases had distant metastases, and 46 cases had no distant metastasis.
Reagents
The Rab25 antibody was a rabbit anti-human polyclonal antibody, and the β-actin antibody was a mouse antihuman monoclonal antibody. Both were purchased from Abcam (UK). The biotin-linked goat anti-rabbit IgG secondary antibody was purchased from Santa Cruz Biotechnology Inc. (USA), and the IRDye 800-conjugated affinity-purified goat anti-mouse IgG and IRDye 800-conjugated affinity-purified goat antirabbit IgG secondary antibodies were purchased from the LI-COR Corporation. The concentrated DAB chromogenic kit was purchased from Beijing Zhongshan Biotechnology Co., Ltd. The ready-to-use SABCimmunohistochemistry kit was purchased from Wuhan Boster Biological Engineering Co., Ltd. The Rab25 siRNA (5'-GGAGCUCUAUGACCAUGCU-3') and the negative control siRNA were purchased from Sigma Aldrich. MGC80-3 human gastric cancer cell lines were purchased from the Chinese Academy of Sciences, Shanghai Institute of Cells. RPMI 1640, trypsin, fetal bovine serum, and the Lipofectamine 2000 transfection reagent were purchased from Invitrogen. The cell culture plates and Transwell invasion chambers were purchased from the Corning Corporation.
Immunohistochemistry
The 4% paraformaldehyde-fixed tumor tissue was paraffin embedded, cut into 5-µm serial sections, and blocked with normal goat serum at room temperature for 20 minutes. The tissue section was probed with the Rab25 antibody (at a working dilution of 1:200) at 4 ℃ overnight and then washed three times with PBS for 2 mins each. The section was subsequently probed with the biotinylated goat anti-rabbit IgG secondary antibody (at a working dilution of 1:150) at 4 ℃ overnight and washed three times with PBS for 2 min. The section was stained by SAB at 37 ℃ for 20 mins, was washed with PBS four times for 5 mins, and then was incubated in DAB at room temperature for 10 min. The section was stained by hematoxylin and observed using a Leica microscope.
Yellow and brown staining in the immunohistochemical results was considered positive. According to staining intensity and the percentage of cells staining positive, the immunohistochemistry results were divided into four levels (-, +, ++, +++) based on (i) the presence and depth of color (0 for no color, 1 for light yellow or yellow, 2 for pale brown, and 3 for brown) and (ii) the percentage of the area that stained positively (0 for <5% of the area positively stained, 1 for 5-25%, 2 for 25-50%, and 3 for >50%). The score for each specimen was calculated as (i+ii)/2, and the specimens were grouped as follows: 0 was considered negative (-); 0.5-1 was considered weakly positive (+); 1.5-2 was considered positive (++); and 2.5-3 was strongly positive (+++).
Western blot
A total of 50 mg of the specimen frozen in liquid nitrogen was ground, lysed in 1 mL of RIPA lysis buffer [150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris (pH 7.9), 10 mM NaF, PMSF, and 1× protease inhibitors (Complete cocktail tablets, Roche)], and homogenized. The homogenate was transferred to a 1.5-mL tube and centrifuged at 16,000 g for 30 minutes, and the protein content of the supernatant was measured using a bicinchoninic acid (BCA) assay (Shen Neng Bocai Biotechnology Co., Ltd.). The gastric carcinoma samples were made from a mixture of 10 µg of protein from each gastric carcinoma sample, and the adjacent normal tissue samples were made from a mixture of 10 µg of protein from each adjacent normal tissue sample. A 5% stacking gel and a 12% separating gel were made, and 50 µg protein per lane was separated by electrophoresis and transferred to a PVDF membrane (Bio-Rad, USA). The membrane was blocked with a TBST [10 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween-20] solution containing 5% skim milk at room temperature for 1 h and then probed with the rabbit anti-Rab25 polyclonal antibody (1:500 dilution) and the mouse anti-human β-actin monoclonal antibody (1:1,000 dilution) at 4 ℃ overnight. The membrane was then incubated with the appropriate IRDye 800-labeled secondary antibody (1:2,000 dilution in PBS) at 4 ℃ overnight. After the blot was washed with TBST, it was scanned using an Odyssey Infrared Imaging system (Rockland Company). The relative amount of Rab25 is shown as Rab25/β-actin and was quantified using Quantity One (Bio-Rad, USA).
Rab25 siRNA transfection and MGC80-3 cell invasiveness and metastasis ability
MGC80-3 cells were plated at 1×105cells/well in a 24-well cell culture plate. The next day, sense and antisense Rab25 siRNA were transfected into MGC80-3 cells using Lipofectamine 2000. A Western blot detecting the Rab25 protein expression levels was performed to determine the effect of RNA interference. A Transwell invasion assay was performed to examine the invasiveness of non-transfected MGC80-3 human gastric cancer cells, cells transfected with the control, and Rab25 siRNA-transfected cells. The cells that passed through the polycarbonate membrane were counted using a Leica microscope. Those passing through the polycarbonate membrane were counted as invading cells, and eight random fields were counted.
Statistical analysis
The Stata 7.0 statistical software was used for the statistical analysis of the experimental results. A Ridit analysis and a ttest were performed, and P<0.05 was considered statistically significant.
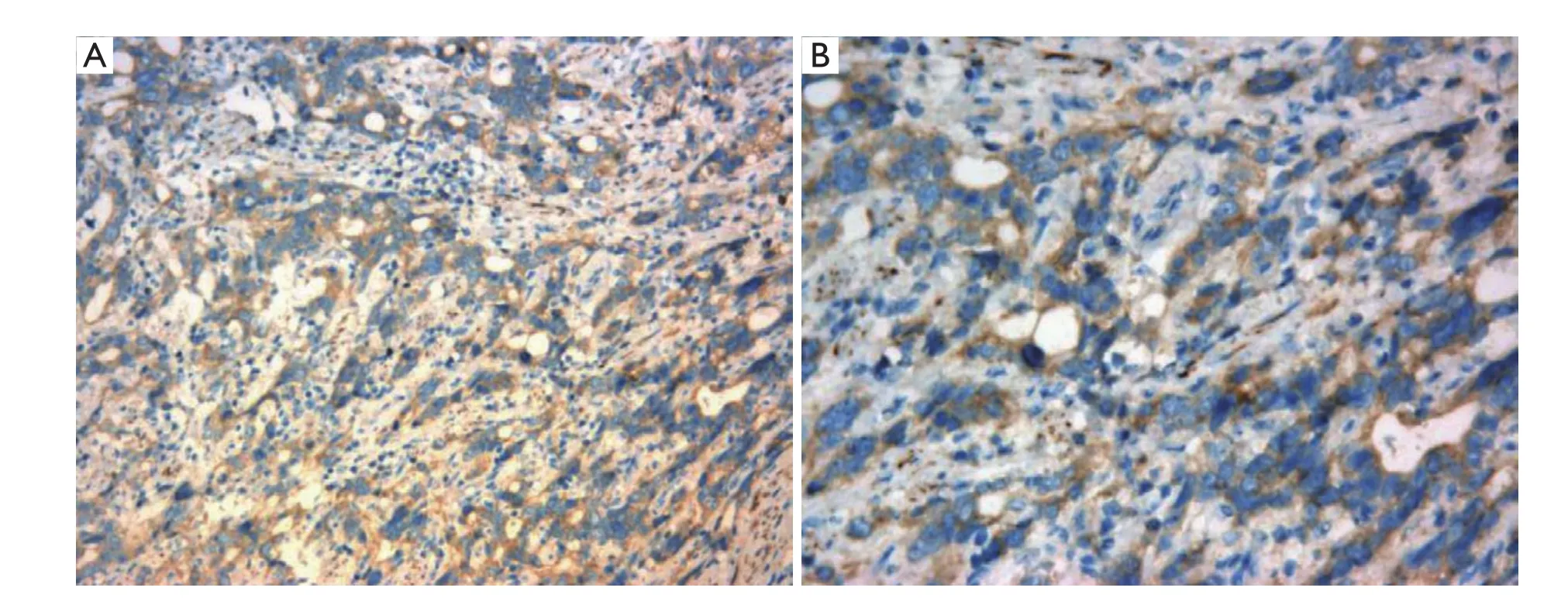
Figure 1 Rab25 expression in gastric cancer (A. ×200; B. ×400)
Results
Immunohistochemical detection of Rab25 protein in gastric carcinomas and its correlation with gastric cancer development and pathology
As shown inFigure 1, Rab25 staining was mainly localized in the cytoplasm, and no positive staining was detected in the nucleus. The expression rate of Rab25 protein in gastric carcinomas was 78.21%, and its expression intensity was primarily designated “++” to “+++.” The expression rate of Rab25 protein in adjacent relatively normal gastric tissue was 23.08%, and its expression intensity was mostly “+”. Rab25 protein expression in gastric carcinoma was significantly higher than in adjacent normal gastric tissues (P<0.01). The less differentiated the gastric cancer cells were, the higher the expression of Rab25 protein was (P<0.01). Rab25 protein expression in patients with late pathological stages of gastric carcinoma (III-IV) was significantly higher than that in patients with early stages (I-II, P<0.01). Rab25 protein expression in the gastric carcinomas of patients with lymph node metastasis was significantly higher than that of lymph node metastasisnegative patients (P<0.01). Rab25 protein expression in gastric carcinoma patients with distant metastases was significantly higher than that of the distant metastasisnegative patients (P<0.01). Rab25 protein expression in gastric cancer was not affected by the patients’ sex, age, or tumor size (P>0.05). These results are shown inTable 1.
Western blot detection of Rab25 protein expression in gastric cancer
The Western blotting results showed that Rab25 protein expression in gastric cancer tissues was significantly higher than that in adjacent normal gastric tissue (P<0.01). The less differentiated the gastric cancer cells were, the higher the Rab25 protein expression was (P<0.01). Rab25 protein expression in patients with late pathological stages of gastric carcinoma (III-IV) was significantly higher than that of patients with early stages (I-II, P<0.01). Rab25 protein expression in gastric carcinoma in patients with lymph node metastasis was significantly higher than that in lymph node metastasis-negative patients (P<0.01). Rab25 protein expression in the gastric carcinomas of patients with distant metastases was significantly higher than that in the distant metastasis-negative patients (P<0.01). These results are shown inFigure 2.
Rab25 expression level affects in vitro invasiveness of MGC80-3 human gastric cancer cells
Rab25 protein expression was reduced using RNA interference, and the change in the invasiveness of MGC80-3 human gastric cancer cells was examined using a Transwell invasion chamber assay. MGC80-3 cells were divided into three groups: a non-transfected control group, a transfected control group, and a Rab25 siRNA transfection group. A Western blot analysis showed that MGC80-3 cells transfected with Rab25 siRNA had significantly lower Rab25 protein expression (P<0.01), and significantly fewer cells passed through the polycarbonate membrane ofthe Transwell invasion chamber (P<0.01). Rab25 protein expression and cell invasiveness were not significantly different between the untransfected and transfected control groups of MGC80-3 cells. The results shown inFigure 3demonstrate that reducing in Rab25 protein levels reduced the MGC80-3 human gastric cancer cell invasiveness. It was also found that the Rab25 protein expression level positively correlated with MGC80-3 cell invasiveness.
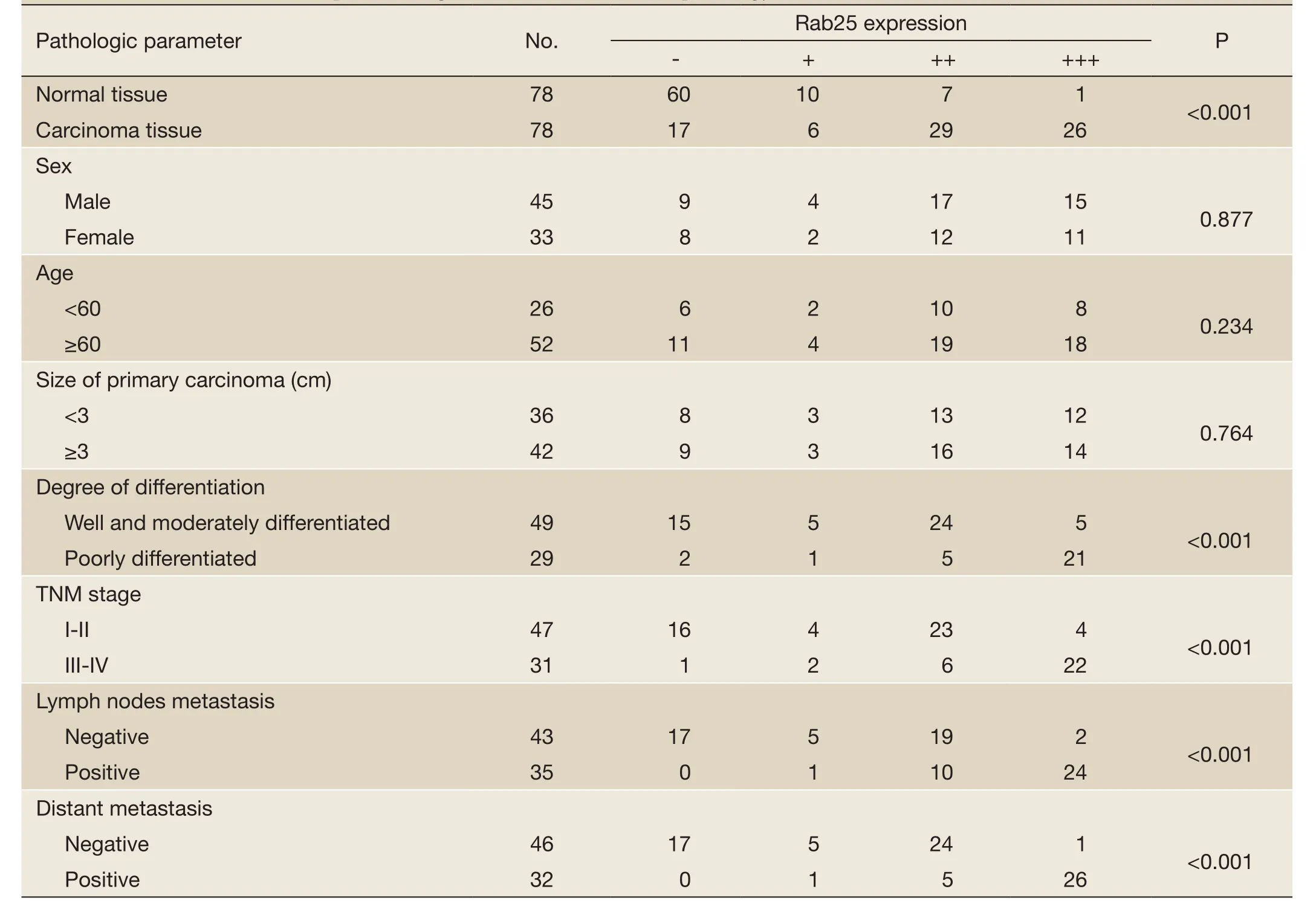
Table 1 Correlation of Rab25 expression in gastric cancer with clinical pathology
Discussion
Rab proteins are the largest subfamily of small molecular G proteins (the Ras superfamily). They are found in all eukaryotes and have been highly conserved during evolution. However, the quantity and function of these proteins also exhibit diversity among different species (27). The first studies on Rab proteins were performed by Novick and colleagues in the budding yeastS. cerevisiae. Many genes that are necessary for normal cell secretion were identified and named by the SEC nomenclature (28). Subsequently, Gallwitzet al.identified the YPT1 gene between the myosin gene and the tubulin gene (29). Touchot and colleagues cloned a SEC4/Yptl gene homolog with similar function from a rat (Rattus norvegicus) brain cDNA library and named it Rab (ras-like in rat brain) (30). In recent years, the Rab proteins that have been identified in yeast,C. elegans,Drosophila,Arabidopsis, human, and other organisms were shown to be distributed throughout various organelles and intracellular transport vesicles and to regulate a specific means of vesicle transport (31-33). The degree of conservation of the Rab proteins during evolution and the defects in vesicle trafficking caused by their mutation have been widely characterized (34).
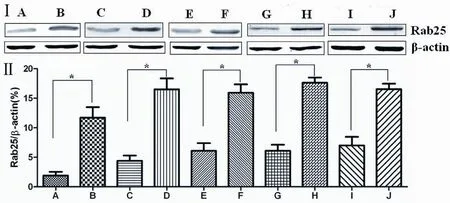
Figure 2 I. Correlation of Rab25 protein expression in gastric cancer and relatively normal adjacent gastric tissue with clinical pathological factors; II. Relative expression of Rab25 protein in gastric carcinoma and in adjacent normal gastric tissues (A. relatively normal adjacent gastric tissue; B. gastric cancer tissue; C. highly and moderately differentiated cancer tissue; D. poorly differentiated cancer tissue; E. patients with early pathological staging (I-II); F. patients with late pathological staging (III-IV); G. lymph node metastasis-negative patients; H. lymph node metastasis-positive patients, I. distant metastasis-negative patients; J. distant metastasis-positive patients. *indicates P<0.05)
Eukaryotic intracellular organelles are connected through vesicle transport, and the transport is generally divided into the formation of vesicles (budding), transport, adhesion, docking, and fusion (35,36). Rab proteins are ubiquitous components of vesicle transport and have both basic functions and fine regulatory features. Because Rab proteins have an important role in vesicle transport and intracellular signal transduction, the abnormal expression of Rab proteins may be associated with cell proliferation and malignancy. With further research, a number of Rab proteins have been found not only to regulate certain kinase activities but also directly or indirectly control the activation, metabolism, and signal transduction of many membrane receptors. Furthermore, Rab proteins affect cell growth, differentiation, and apoptosis. Therefore, abnormalRab protein expression may have an important role in tumor occurrence and development (37).
Cheng’s work and other studies have demonstrated that Rab25 protein levels were significantly higher in breast and ovarian cancer, and higher levels of the Rab25 protein correlated with a lower the survival rate of patients and poor prognosis (26,38). In this study, Rab25 protein mainly stained in the cytoplasm, and no staining was observed in the nucleus. The expression rate of Rab25 protein in gastric carcinoma was 78.21%, and its expression intensity was mostly categorized as “++” to “+++”. The expression rate of Rab25 protein in relatively normal adjacent gastric tissue was 23.08%, and its expression intensity was mostly categorized as “+”. Rab25 protein expression in gastric carcinomas was significantly higher than that in adjacent normal gastric tissues (P<0.01). The less differentiated the gastric cancer cells were, the higher the Rab25 protein expression was (P<0.01). The Rab25 protein expression in patients with late pathological stages of gastric carcinoma (III-IV) was significantly higher than that of patients with early stages (I-II, P<0.01). The Rab25 protein expression in the gastric carcinomas of patients with lymph node metastasis was significantly higher than that of lymph node metastasisnegative patients (P<0.01). The Rab25 protein expression in the gastric carcinomas of patients with distant metastasis was significantly higher than that of the distant metastasisnegative patients (P<0.01). Rab25 protein expression in gastric cancer was not affected by the patients’ sex, age, or tumor size (P>0.05). These results are consistent with those of previous studies.
Fanet al.found that reducing Rab25 protein expression by RNAi in A2780 ovarian cancer cells led to decreased invasiveness (39). Cheng’s work and other studies found that reduced Rab25 protein expression in A2780, DOV13, HEY, MCF-7, and other cells decreased the invasiveness of these cells (38). Our results showed that Rab25 protein expression levels were significantly lower in the Rab25 siRNA-transfected MGC80-3 cells (P<0.01) and that the number of cells that passed through the polycarbonate membrane in a Transwell chamber invasion assay was significantly less (P<0.01). This result demonstrates that Rab25 protein expression and MGC80-3 cell invasiveness are positively correlated. The results of this study are consistent with those of previous studies.
Metastasis and recurrence are the leading cause of death in patients with gastric cancer. Metastasis is the interaction between the tumor and host and is the result of many factors and a complex mechanism. The identification and study of the genes related to gastric carcinoma metastasis and recurrence will lay a foundation for exploring the nature of gastric cancer metastasis and recurrence while providing a scientific basis for the treatment of gastric cancer. Our results showed that Rab25 protein expression positively associated with the development of gastric cancer and that Rab25 is involved in the invasion and metastasis of gastric cancer cells. However, the exact mechanism must be further studied. The Rab25 protein may regulate intracellular signal transduction in tumor development and thereby have an important role in this process. Although it is not a cancer gene product, it can alter cancer cell proliferation, survival, and invasiveness and could be a potential future therapeutic target for gastric cancer.
Acknowledgements
Disclosure:The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
1. Yang D, Hendifar A, Lenz C, et al. Survival of metastatic gastric cancer: Significance of age, sex and race/ethnicity. J Gastrointest Oncol 2011;2:77-84.
2. Chen WQ, Zheng RS, Zhang SW, et al. Report of incidence and mortality in china cancer registries, 2008. Chin J Cancer Res 2012;24:171-80.
3. Jimenez P, Pathak A, Phan AT. The role of taxanes in the management of gastroesphageal cancer. J Gastrointest Oncol 2011;2:240-9.
4. Ling TC, Kang JI, Slater JD, et al. Proton therapy for gastrointestinal cancers. Transl Cancer Res 2012;1:150-8.
5. Yang X, Chen J, Xia L, et al. Laparoscopic radical gastrectomy for gastric cancer. Transl Gastrointest Cancer 2012;1:189-201.
6. Ge SH, Wu XJ, Wang XH, et al. Over-expression of Metastasis-associated in Colon Cancer-1 (MACC1) Associates with Better Prognosis of Gastric Cancer Patients. Chin J Cancer Res 2011;23:153-9.
7. Tan KK, Quek TJ, Wong N, et al. Emergency surgery for perforated gastric malignancy: An institution,s experience and review of the literature. J Gastrointest Oncol 2011;2:13-8.
8. Zhang XD, Shu YQ, Liang J, et al. Combination chemotherapy with paclitaxel, cisplatin and fluorouracilfor patients with advanced and metastatic gastric or esophagogastric junction adenocarcinoma: a multicenter prospective study. Chin J Cancer Res 2012;24:291-8.
9. Saglam S, Aykan NF, Sakar B, et al. A pilot study evaluating the safety and toxicity of epirubicin, cisplatin, and UFT (ECU regimen) in advanced gastric carcinoma. J Gastrointest Oncol 2011;2:19-26.
10. Graziosi L, Evoli LP, Cavazzoni E, et al. The role of 18FDG-PET in gastric cancer. Transl Gastrointest Cancer 2012;1:186-8.
11. Hopkins S, Yang GY. FDG PET imaging in the staging and management of gastric cancer. J Gastrointest Oncol 2011;2:39-44.
12. Achyut BR, Yang L. Transforming growth factor- in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology 2011;141:1167-78.
13. St Hill CA. Interactions between endothelial selectins and cancer cells regulate metastasis. Front Biosci 2011;16:3233-51.
14. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009;10:513-25.
15. Svetina S. Vesicle budding and the origin of cellular life. Chemphyschem 2009;10:2769-76.
16. Barth J, Volknandt W. Proteomic investigations of the synaptic vesicle interactome. Expert Rev Proteomics 2011;8:211-20.
17. Kelly BT, Owen DJ. Endocytic sorting of transmembrane protein cargo. Curr Opin Cell Biol 2011;23:404-12.
18. Neto H, Collins LL, Gould GW. Vesicle trafficking and membrane remodelling in cytokinesis. Biochem J 2011;437:13-24.
19. Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011;91:119-49.
20. Wang T, Ming Z, Xiaochun W, et al. Rab7: role of its protein interaction cascades in endo-lysosomal traffic. Cell Signal 2011;23:516-21.
21. Angers CG, Merz AJ. New links between vesicle coats and Rab-mediated vesicle targeting. Semin Cell Dev Biol 2011;22:18-26.
22. Agarwal R, Jurisica I, Mills GB, et al. The emerging role of the RAB25 small GTPase in cancer. Traffic 2009;10:1561-8.
23. Chia WJ, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta 2009;1795:110-6.
24. Goldenring JR, Nam KT. Rab25 as a tumour suppressor in colon carcinogenesis. Br J Cancer 2011;104:33-6.
25. Cheng JM, Volk L, Janaki DK, et al. Tumor suppressor function of Rab25 in triple-negative breast cancer. Int J Cancer 2010;126:2799-812.
26. Cheng KW, Lu Y, Mills GB. Assay of Rab25 function in ovarian and breast cancers. Methods Enzymol 2005;403:202-15.
27. Horgan CP, McCaffrey MW. Rab GTPases and microtubule motors. Biochem Soc Trans 2011;39:1202-6.
28. Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A 2006;103:11821-7.
29. De Antoni A, Schmitzová J, Trepte HH, et al. Significance of GTP hydrolysis in Ypt1p-regulated endoplasmic reticulum to Golgi transport revealed by the analysis of two novel Ypt1-GAPs. J Biol Chem 2002;277:41023-31.
30. Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell 2011;103:209-21.
31. Li Y, Hu J. Small GTPases and cilia. Protein Cell 2011;2:13-25.
32. Hoffman NJ, Elmendorf JS. Signaling, cytoskeletal and membrane mechanisms regulating GLUT4 exocytosis. Trends Endocrinol Metab 2011;22:110-6.
33. Itzen A, Goody RS. GTPases involved in vesicular trafficking: structures and mechanisms. Semin Cell Dev Biol 2011;22:48-56.
34. Mitra S, Cheng KW, Mills GB. Rab GTPases implicated in inherited and acquired disorders. Semin Cell Dev Biol 2011;22:57-68.
35. Pinot M, Goud B, Manneville JB. Physical aspects of COPI vesicle formation. Mol Membr Biol 2010;27:428-42.
36. Budnik A, Stephens DJ. ER exit sites--localization and control of COPII vesicle formation. FEBS Lett 2009;583:3796-803.
37. Agola J, Jim P, Ward H, et al. Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities. Clin Genet 2011;80:305-18.
38. Cheng KW, Lahad JP, Kuo WL, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med 2004;10:1251-6.
39. Fan Y, Xin XY, Chen BL, et al. Knockdown of RAB25 expression by RNAi inhibits growth of human epithelial ovarian cancer cells in vitro and in vivo. Pathology 2006;38:561-7.
Cite this article as:Cao C, Lu C, Xu J, Zhang J, Zhang J, Li M. Expression of Rab25 correlates with the invasion and metastasis of gastric cancer. Chin J Cancer Res 2013;25(2):192-199. doi: 10.3978/j.issn.1000-9604.2013.03.01

10.3978/j.issn.1000-9604.2013.03.01
Submitted Feb 14, 2013. Accepted for publication Accepted for publication Mar 19, 2013.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Efficacy and safety of albumin-bound paclitaxel in treating recurrent advanced non-small-cell lung cancer
- Doublecortin-like kinase 1 exhibits cancer stem cell-like characteristics in a human colon cancer cell line
- Primary gastrointestinal mantle lymphoma with massive bleeding: a case report and literature review
- microRNAs and ceRNAs: RNA networks in pathogenesis of cancer
- Antitumor effects of electrochemical treatment
- Autophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cells
