Laparoscopic versus open resection of gastric gastrointestinal stromal tumors
2013-06-12
Department of Gastrointestinal Surgery, China-Japan Union Hospital, Jilin University, Changchun 130033, China
Laparoscopic versus open resection of gastric gastrointestinal stromal tumors
Zhen-Bo Shu, Li-Bo Sun, Jun-Peng Li, Yong-Chao Li, Da-Yong Ding
Department of Gastrointestinal Surgery, China-Japan Union Hospital, Jilin University, Changchun 130033, China
Corresponding to:Li-Bo Sun, MD. Department of Gastrointestinal Surgery, China-Japan Union Hospital, Jilin University, No. 126, Xiantai Street, Changchun 130033, China. Email: sunlibo0431@sina.com or sunlbjl@126.com.
The aims of this study were to explore whether laparoscopic surgical resections of gastric gastrointestinal stromal tumors (GISTs) would produce better perioperative and similar oncologic outcomes compared with open surgical resection in Chinese patients. Thirty-six gastric GISTs cases were divided into a minimally invasive laparoscopic group and open resection group, depending on the surgical approach that was used. The general preoperative information, operative time, incision length, intraoperative blood loss, postoperative time to first flatulence, postoperative complications, postoperative hospital stay, total hospitalization costs, and such follow-up data as recurrence, metastasis, and mortality rates were compared between two groups. Among the 36 gastric GISTs, 15
Gastrointestinal stromal tumors; laparoscopic resection; open resection
Scan to your mobile device or view this article at:http://www.thecjcr.org/article/view/1543/2478
Introduction
Gastrointestinal stromal tumors (GISTs) are uncommon mesenchymal tumors that arise in the wall of the gastrointestinal (GI) tract and are less than 3% of all GI malignancies (1). GISTs occur most frequently in the stomach (60% to 70%), followed by the small intestine (20% to 30%), colon and rectum (5%), and esophagus (less than 5%). GIST is different from cancer in that its growth is mostly expansive rather than invasive growth and its main metastatic routes are hematogenous and seeding metastasis (2).
Wide resection margins, historically advocated, have not been associated with improved oncologic outcomes when these other tumor factors are considered (3). Simple wedge resection, when feasible, has become the recommended surgical approach. Gastric GIST resection is therefore particularly amenable to a minimally invasive technique, and an increasing number of laparoscopic experiences have been reported demonstrating the feasibility and safety of this approach (4-6). The traditional open resection of GISTshas some inevitable shortcomings, including relatively long surgical incisions, significant postoperative incisional pain, and a tendency toward incision infection, incision dehiscence, incisional hernia, and other complications. In contrast, laparoscopic surgical treatment of GISTs offers the advantages of less trauma, faster postoperative recovery, a short hospital stay, a clearer intraoperative field of view, diagnosis and treatment being conducted at the same time, a more precise operation, and the ability to avoid damage caused by hands, metal retractors, and clogs caused by gauze during the operation (7). With the widespread application of minimally invasive laparoscopic techniques, an increasing number of gastric GIST cases treated with laparoscopic surgery have been reported (4-6,8); however, few studies have compared the laparoscopic and open resection of gastric GISTs (7,9).
The aims of this study were to explore whether laparoscopic surgical resections of gastric GISTs would produce better perioperative and similar oncologic outcomes compared with open surgical resection in Chinese patients.
Patients and methods
Patients
In this study, there were 36 GIST cases in the Department of Gastrointestinal Surgery at the Sino-Japanese Friendship Hospital of Jilin University between January 2010 and February 2012. According to the surgical approach used, the patients were divided into one of two groups: the minimally invasive laparoscopic surgical group: 15 GIST cases (8 males and 7 females; average age 54.21±8.91 years) who received laparoscopic surgical treatment, and the open resection group: 21 GIST cases (11 males and 10 females; average age 52.37±10.13 years) who underwent open resection. For all of the patients, GISTs were confirmed preoperatively using one or more auxiliary examinations, such as abdominal CT, upper gastrointestinal imaging, gastroscopy, and endoscopic ultrasound, and the tumors showed local growth and no distant metastasis.
Surgical methods for the minimally invasive laparoscopic group
The patients were placed in the supine position with general anesthesia and endotracheal intubation. The skin in the surgical area was disinfected with fortified iodine solution and draped with sterile drapes. A small vertical incision was made 3 cm above the umbilicus, a pneumoperitoneum needle was used to puncture the abdominal cavity, and carbon dioxide gas was infused to create pneumoperitoneum with a pressure of 12 to 13 mmHg. The pneumoperitoneum needle was then removed, and a trocar was used to puncture the abdominal cavity. The inner core of the trocar was pulled out and replaced with the laparoscope. Trocars were separately inserted into the left and right sides of the upper abdominal midclavicular line and the anterior axillary line under direct laparoscopic vision, and the necessary surgical equipment was placed. Abdominal exploration was performed to examine the liver, stomach, and abdominal cavity and to identify the tumor position. The surgical approach was determined by the position of the tumor: Laparoscopic gastric wedge resection; Laparoscopic transgastric tumor-everting resection, suitable for tumors located in the posterior wall of the stomach and growing towards the cavity (10); or Laparoscopic proximal or distal gastrectomy, suitable for larger stromal tumors located in the cardia, pylorus, and gastric antrum.
Surgical method for the open resection group
The patients were placed in the supine position with general anesthesia and endotracheal intubation. The skin in the surgical area was disinfected with fortified iodine solution and draped with sterile drapes. An incision was made in the middle of the upper abdomen, and layers were cut to reach the abdominal cavity. Abdominal exploration was performed, and the position of the gastric stromal tumor was identified. The surgical approach was determined by the position of the tumor, and one of the following approaches was used: local excision or wedge resection of the GIST was used for smaller GISTs. The surgical margin was positioned 1 to 2 cm away from the edge of the tumor, and when necessary, the specimens were sent for rapid pathological examination to confirm negative margins; Subtotal gastrectomy was used for larger tumors or tumors located near the cardia or pylorus; Gastric resection was used for larger tumors at the side of the lesser curvature of the stomach.
Histopathological examination and immunohistochemical analysis
The tissues removed during surgery underwent a pathological examination to identify the tumor location andsize, type, cell density, the division phase of the cell nuclei, the risk index based on the Fletcher standard (11), the lymph node metastasis conditions, and the surgical margin conditions. The immunohistochemical analysis included detection of CD117, CD34, smooth muscle actin protein (SMA), S-100, and desmin expression. CD117-positive or CD34-positive results indicated GISTs.
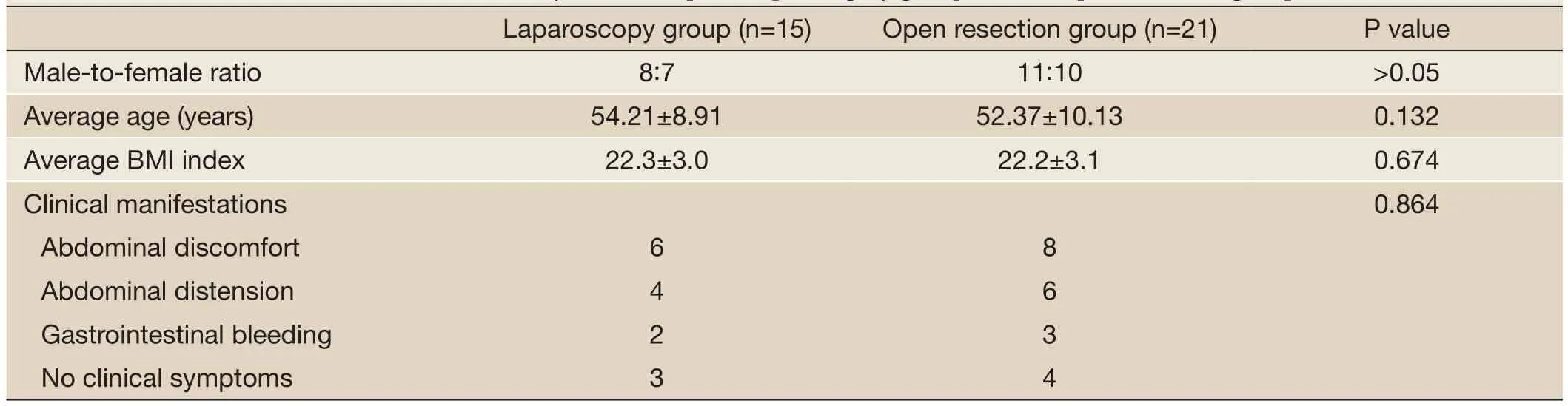
Table 1 Patient’s characteristics of the minimally invasive laparoscopic surgery group and the open resection group
Postoperative data
Postoperative recovery information was from the patients’postoperative disease progress records and included the gastrointestinal function recovery time, postoperative complications, and length of hospital stay. Hospitalization cost information was obtained via lists of patients’ admission and discharge costs. Follow-up data were obtained by telephone follow-up and included information about the follow-up period and such conditions as recurrence, metastasis, and death.
Statistical methods
All data were analyzed using SPSS 17.0 statistical software. Measurement data were processed using t-tests, and ratios were compared using the chi-squared test. P<0.05 indicated statistically significant differences.
Results
Patients’ characteristics
There were 15 GIST cases in the minimally invasive laparoscopic surgery group, with a male-to-female ratio of 8:7. There were 21 GIST cases in the open resection group, with a male-to-female ratio of 11:10. There was no significant difference (P>0.05) between the two groups. There was no significant difference (P>0.05) in the average age of the minimally invasive laparoscopic surgery group and that of the open resection group. There was also no significant difference (P>0.05) in height and body mass index (BMI) between the two groups. The clinical symptoms of the minimally invasive laparoscopic surgery group manifested as abdominal discomfort in six cases, abdominal distension in four cases, and gastrointestinal bleeding in two cases. In addition, three cases had no clinical symptoms. The clinical symptoms of the open resection group manifested as abdominal discomfort in eight cases, abdominal distension in six cases, and gastrointestinal bleeding in three cases. Four cases had no clinical symptoms. There was also no significant difference (P>0.05) in the clinical symptoms between the two groups (Table 1).
Surgical results
In the minimally invasive laparoscopic surgical group, 11 cases underwent laparoscopic wedge resection of the GISTs, and four cases underwent laparoscopic distal subtotal gastrectomy plus Billroth II gastrojejunostomy. In the open resection group, 13 cases underwent wedge resection of the GISTs, and eight cases underwent distal subtotal gastrectomy plus Billroth II gastrojejunostomy. The gross tumors after resection were shown inFigure 1A,B.
The minimally invasive laparoscopic surgery group and the open resection group did not show statistically significant differences in the average operative time (P>0.05). There were statistically significant differences in the incision length between the minimally invasive laparoscopic surgery group and the open resection group(Figure 1C,D) (P<0.05). Intraoperative blood loss did not differ statistically between the minimally invasive laparoscopic surgery group and the open resection group (P>0.05) (Table 2).

Figure 1 Gross specimen of a gastric GIST in laparoscopic surgery (A) and open resection (B); incision after laparoscopic surgery for gastric GISTs (C); incision after open resection for gastric GISTs (D)
Pathological and immunohistochemical results
For the minimally invasive laparoscopic surgery group, four cases were located in the gastric fundus, seven were located in the gastric body, and four were located in the gastric antrum. For the open resection group, six cases were located in the gastric fundus, seven were located in the gastric body, and eight were located in the gastric antrum. There was no significant difference between the two groups (P>0.05). There was no significant difference between the minimally invasive laparoscopic surgery group and the open resection group (P>0.05). GIST properties: For the minimally invasive laparoscopic surgery group, one case had a high malignancy risk, six had a moderate malignancy risk, and eight had a low malignancy risk. For the open resection group, two cases had a high malignancy risk, nine had a moderate malignancy risk, and 10 had a low malignancy risk. There was no statistically significant difference between the two groups (P>0.05). Neither the minimally invasive laparoscopic surgery group nor the open resection group had lymph node metastasis, and the surgical margins were negative in all cases. Cases in both the minimally invasive laparoscopic surgery group and the open resectiongroup were positive for CD117 and CD34, and there was no statistically significant difference between the groups (Table 3).
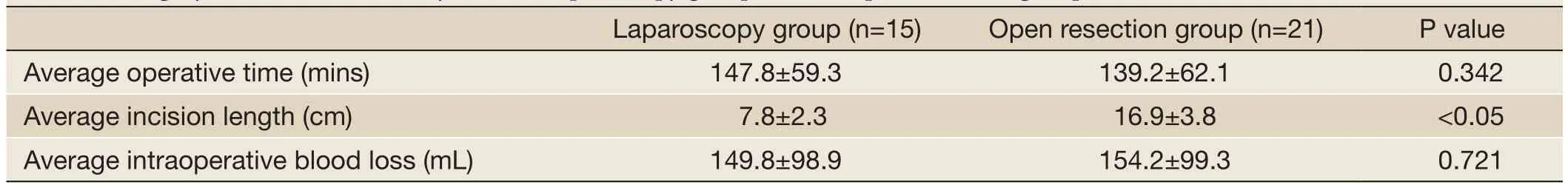
Table 2 Surgery data of the minimally invasive laparoscopy group and the open resection group
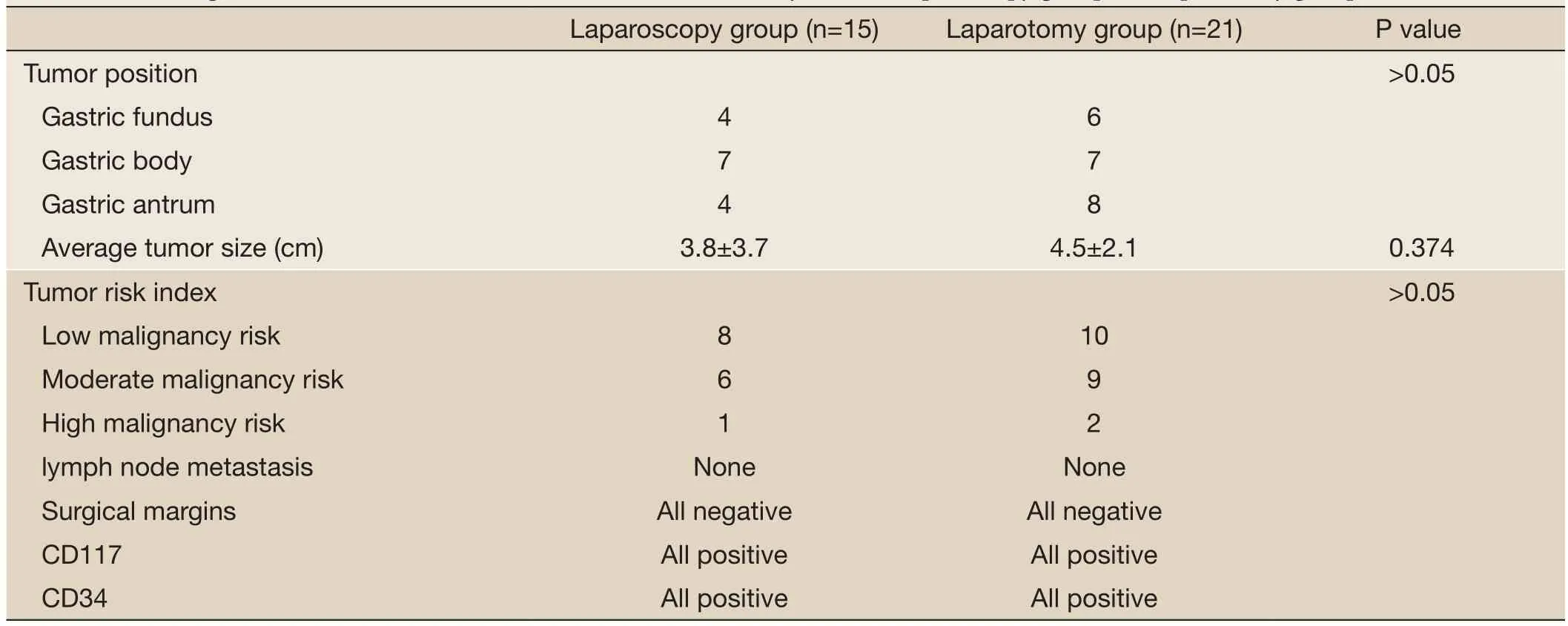
Table 3 Pathological and immunohistochemical data of the minimally invasive laparoscopy group and laparotomy group
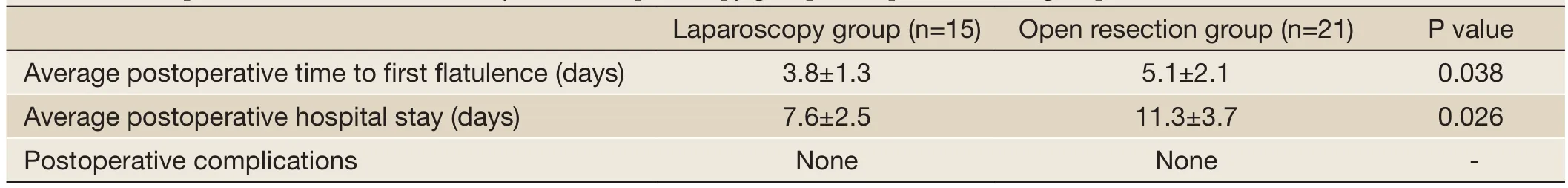
Table 4 Postoperative data of the minimally invasive laparoscopy group and open resection group
Postoperative recovery
The minimally invasive laparoscopic surgery group and the open resection group showed statistically significant differences (P<0.05) in the postoperative time to first flatulence and postoperative hospital stay. Cases in both the minimally invasive laparoscopic surgery group and the open resection group had no postoperative complications, and there was no statistically significant difference between the groups (Table 4).
Discussion
The clinical manifestations of GISTs are not specific, common clinical manifestations include abdominal discomfort, gastrointestinal bleeding, abdominal pain, abdominal mass, anemia, weight loss, belching, abdominal distension, and obstruction (12). When tumors are smaller than 2 cm, there may be no clinical symptoms, and tumors are often found during routine physical examinations. Tumors larger than 5 cm can lead to gastrointestinal bleeding and obstruction. For both benign and malignant gastric stromal tumors, the transfer pathways mainly include hematogenous metastasis and seeding metastasis (13). Lymph node metastasis is rarely observed, thus reducing theneed for large-scale lymph node dissection. The biological properties of GISTs provide an important theoretical basis for the laparoscopic surgical treatment of gastric stromal tumors (14,15). In this study, seven of the 36 gastric GISTs showed clinical symptoms of abdominal discomfort, six cases showed abdominal distension, four cases showed abdominal pain, five cases showed upper gastrointestinal bleeding, seven cases had abdominal masses that were found during physical examinations, and seven cases showed other symptoms. It is difficult to make accurate judgments about the tumors’ properties, size, and location based on the clinical symptoms alone. However, the accurate determination of these factors is the key to successful surgery. Therefore, accurate preoperative assessment of the tumor using abdominal CT, gastroscopy, endoscopic ultrasound, and other auxiliary examinations facilitates the selection of an appropriate surgical approach (16).
Because GISTs are not sensitive to radiotherapy and chemotherapy (17), surgery is the primary treatment approach. Both laparoscopic and open resection of GISTs must adhere to the following principles: extracapsular resection of the intact tumor (18), maximal retention of normal gastric tissue (19), minimal surgical contamination (20), and the avoidance of postoperative gastrointestinal tract stenosis (5). Compared with open resection, laparoscopic surgery for GIST treatment offers the advantages of less trauma, a clearer operating field of view, simultaneous diagnosis and treatment, faster postoperative recovery, shorter hospital stay, reduced incidence of intestinal obstruction, and a smaller laparoscopic surgical incision, which also effectively reduces postoperative incisional pain, the incidence of incision infection, and such serious complications as incision dehiscence and incisional hernia (7). Lukaszczyket al. first reported the surgical method of resecting GISTs under laparoscopy (21). They proved that laparoscopic techniques were sufficient to achieve the range of resection, would not damage the tumor, and could reduce the local tumor recurrence rate. Nguyenet al. performed a clinical followup and controlled study of the surgical treatment of gastric GISTs; they proved that the laparoscopic resection of gastric stromal tumors is safe and feasible and leads to faster recovery, shorter postoperative hospital stay, faster recovery of gastrointestinal function, and less pain compared with open resection (19). The results of this study showed that there were no statistically significant differences between the laparoscopy and open resection groups in terms of the patients’ general data, including gender, age, height, and BMI; clinical symptoms; the incidence of postoperative complications; and pathology reports, including the location, size, and risk index of the GISTs. In addition, there were also no statistically significant differences between the two groups in terms of operation time, intraoperative blood loss, and the postoperative recurrence rate. The average incision length for the laparoscopy group was significantly smaller than that of the open resection group, and the difference was statistically significant. The postoperative time to first flatulence and the postoperative hospital stay of the laparoscopy group were shorter than those of the open resection group, and the differences were statistically significant. These results are consistent with recent clinical reports, proving that laparoscopic surgical treatment of gastric stromal tumors is safe and feasible and leads to less trauma and more rapid recovery (7,9,22).
Compared with open resection, the laparoscopic surgical treatment of GISTs results in less trauma and faster postoperative recovery. It also offers the following advantages: the operating field of view is clearer, diagnosis and treatment can occur simultaneously, and the operation can be more precise. During laparoscopic surgery, the lens can rotate flexibly within the abdominal cavity, providing a wider and broader field of view. The primary lesion and other lesions can be identified and treated during the surgery. Because the laparoscopic images can be enlarged and the instrument is lightweight and flexible, a more precise operation can be achieved. Larger injuries caused by the hands, metal retractors, and clogs caused by gauze can be avoided. During open resection, it is necessary to pull the lesions out through the incision to facilitate resection, and damage to tissues and organs caused by hands, metal retractors, and clogs caused by gauze are unavoidable. Laparotomic surgery leads to a high incidence of postoperative intestinal obstruction, and it can result in the tearing of the splenic capsule and bleeding. During the laparoscopic surgical procedure, the in situ operation avoids unnecessary pulling, decreases tissue damage to a minimal level, and reduces the incidence of postoperative intestinal obstruction. The application of laparoscopic staplers also reduces compression on the tumor and avoids potential tumor rupture after compression. Removing the tumor after placing it in the specimen bag also avoids tumor seeding near incision sites, thus improving compliance with tumorfree operation standards.
One of the disadvantages of the laparoscopic technique is that the average total hospitalization cost for the laparoscopy group was slightly higher than that of the open resection group. Surgeons’ improved operation skills andthe increased popularity of laparoscopic surgery will further shorten operation times, postoperative recovery times, and hospital stays, further reducing total hospitalization costs and easing patients’ economic burden.
The intraoperative positioning of the GISTs is particularly relevant to the surgery. In our opinion, the best approach is preoperative positioning by applying titanium clips and methylene blue near the tumor under the gastroscopy. When applying methylene blue, smaller injection volumes should be favored because more methylene blue can be added later if the initial injection volume is not enough, whereas excessive methylene blue will stain a large area of the stomach wall and increase the range of resection. If the tumors are small and difficult to locate during surgery, intraoperative gastroscopic positioning should be applied to produce accurate positioning without wasting time. In addition, the ability to identify the anatomic structures in a good laparoscopic surgical visual field is essential for surgeons to operate in an accurate and standardized way; it is also a key to the success of laparoscopic surgery and is of vital significance for the entire surgery. Such factors as viewing angle and visual range as well as good cooperation among the surgeons and assistants are particularly important.
In terms of the surgical safety issues, in addition to avoiding intraoperative tumor rupture (23), it is necessary to ensure that the surgical margins are pathologically negative. Complete tumor resection with negative margins generally requires that the edge of the resection range be at least 2 to 3 cm away from the tumor (24). Other study reported that the distance could be as small as 1 to 2 cm. However, this determination of the distance should be adjusted for different surgical sites (25). In our cases, the surgical margins were all negative during the postoperative pathological examination, and the distances from the tumor to the surgical margin were larger than 2 cm. The patients were followed for 6 to 24 months, and no recurrence was reported.
In summary, laparoscopic surgical treatment for GISTs has numerous merits, including less trauma, faster postoperative recovery, shorter hospital stay, a clearer intraoperative visual field, simultaneous diagnosis and treatment, a more precise operation. This method can avoid large injuries caused by hands, metal retractors, and clogs caused by gauze during the operation and can reduce compression on the tumor and decrease the risk of compression-induced tumor rupture; thus, it is believed to comply with the tumor-free operation standards better than open resection does. Therefore, the laparoscopic surgical treatment of GISTs is safe, feasible, and effective. The increased popularity of laparoscopic surgery and surgeons’improved operation skills will further reduce operation times, speed postoperative recovery, and shorten hospital stays, thus decreasing hospitalization costs and reducing patients’ economic burden.
Acknowledgements
Disclosure:The authors declare no conflict of interest.
1. Zhao X, Yue C. Gastrointestinal stromal tumor. J Gastrointest Oncol 2012;3:189-208.
2. Kumar K, Rowsell C, Law C, et al. Coexistence of gastrointestinal stromal tumour and colorectal adenocarcinoma: Two case reports. J Gastrointest Oncol 2011;2:50-4.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8.
4. Novitsky YW, Kercher KW, Sing RF, et al. Longterm outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006;243:738-45; discussion 745-7.
5. Hodges K, Kennedy L, Meng F, et al. Mast cells, disease and gastrointestinal cancer: A comprehensive review of recent findings. Transl Gastrointest Cancer 2012;1:138-150.
6. Sasaki A, Koeda K, Obuchi T, et al. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery 2010;147:516-20.
7. Karakousis GC, Singer S, Zheng J, et al. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol 2011;18:1599-605.
8. Berindoague R, Targarona EM, Feliu X, et al. Laparoscopic resection of clinically suspected gastric stromal tumors. Surg Innov 2006;13:231-7.
9. Wan P, Yan C, Li C, et al. Choices of surgical approaches for gastrointestinal stromal tumors of the stomach: laparoscopic versus open resection. Dig Surg 2012;29:243-50.
10. Song KY, Kim SN, Park CH. Tailored-approach of laparoscopic wedge resection for treatment of submucosal tumor near the esophagogastric junction. Surg Endosc 2007;21:2272-6.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis ofgastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65.
12. Gong JS, Kang WY, Liu T, et al. CT findings of a gastrointestinal stromal tumor arising from small bowel. Quant Imaging Med Surg 2012;2:57-8.
13. Aparicio T, Boige V, Sabourin JC, et al. Prognostic factors after surgery of primary resectable gastrointestinal stromal tumours. Eur J Surg Oncol 2004;30:1098-103.
14. Joensuu H, Fletcher C, Dimitrijevic S, et al. Management of malignant gastrointestinal stromal tumours. Lancet Oncol 2002;3:655-64.
15. Rossi CR, Mocellin S, Mencarelli R, et al. Gastrointestinal stromal tumors: from a surgical to a molecular approach. Int J Cancer 2003;107:171-6.
16. Privette A, McCahill L, Borrazzo E, et al. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc 2008;22:487-94.
17. Halpern J, Kim YJ, Sultana R, et al. Effectiveness of radiation therapy in GIST: A case report. J Gastrointest Oncol 2012;3:143-6.
18. Everett M, Gutman H. Surgical management of gastrointestinal stromal tumors: analysis of outcome with respect to surgical margins and technique. J Surg Oncol 2008;98:588-93.
19. Nguyen SQ, Divino CM, Wang JL, et al. Laparoscopic management of gastrointestinal stromal tumors. Surg Endosc 2006;20:713-6.
20. Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg 2006;244:176-84.
21. Lukaszczyk JJ, Preletz RJ Jr. Laparoscopic resection of benign stromal tumor of the stomach J Laparoendosc Surg 1992;2:331-4.
22. Melstrom LG, Phillips JD, Bentrem DJ, et al. Laparoscopic versus open resection of gastric gastrointestinal stromal tumors. Am J Clin Oncol 2012;35:451-4.
23. Sokolich J, Galanopoulos C, Dunn E, et al. Expanding the indications for laparoscopic gastric resection for gastrointestinal stromal tumors. JSLS 2009;13:165-9.
24. Nishimura J, Nakajima K, Omori T, et al. Surgical strategy for gastric gastrointestinal stromal tumors: laparoscopic vs. open resection. Surg Endosc 2007;21:875-8.
25. Pucci MJ, Berger AC, Lim PW, et al. Laparoscopic approaches to gastric gastrointestinal stromal tumors: an institutional review of 57 cases. Surg Endosc 2012;26:3509-14.
Cite this article as:Shu ZB, Sun LB, Li JP, Li YC, Ding DY. Laparoscopic versus open resection of gastric gastrointestinal stromal tumors. Chin J Cancer Res 2013;25(2):175-182. doi: 10.3978/j.issn.1000-9604.2013.02.03

10.3978/j.issn.1000-9604.2013.02.03
laparoscopic surgical treatment (laparoscopy group, n=15), and 21 received routine open resection treatment (open resection group, n=21). The laparoscopy group and the open resection group showed statistically significant differences (P<0.05) in incision length (7.8±2.3vs. 16.9±3.8 cm), postoperative time to first flatulence (3.8±1.3vs. 5.1±2.1 d), postoperative hospitalization time (7.6±2.5vs. 11.3±3.7 d), and total cost of hospitalization (RMB 28,239±5,521vs. RMB 23,761±5,362). There were no statistically significant differences (P>0.05) between the laparoscopy group and the open resection group in operative time (147.8±59.3vs. 139.2±62.1 min) and intraoperative blood loss (149.8±98.9vs. 154.2±99.3 mL). Both groups had no postoperative complications, no recurrence and metastasis, and no postoperative mortality. There were no statistically significant differences between the two groups in postoperative complications, postoperative recurrence and metastasis, and postoperative mortality. In conclusion, compared with open resection, the laparoscopic resection of gastric GISTs offers the advantages of less trauma, faster recovery, and shorter hospital stay.
Submitted Jan 14, 2013. Accepted for publication Feb 19, 2013.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Safety and efficacy of trimodality therapy in patients undergoing extrapleural pneumonectomy
- Treatment of mesothelioma: still a long way to go!
- Role of contrast-enhanced ultrasonography in percutaneous radiofrequency ablation of liver metastases and efficacy evaluation
- Determining the optimal time for bortezomib-based induction chemotherapy followed by autologous hematopoietic stem cell transplant in the treatment of multiple myeloma
- Bevacizumab rescue therapy extends the survival in patients with recurrent malignant glioma
- Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment
