Reconstruction of the thoracic tracheal defects with portions of deepithelialized myocutaneous flaps after resection of a large tumor
2013-06-12SushengWangGangLiangZhihuaZhangHangJiChunHouJianxingHeWeiqiangYin
Susheng Wang, Gang Liang, Zhihua Zhang, Hang Ji, Chun Hou, Jianxing He, Weiqiang Yin
1Department of Plastic Surgery,2Department of Cardiothoracic Surgery, The First Affiliated Hospital of Guangzhou Medical University;3Guangzhou Institute of Respiratory Disease, Guangzhou 510120, China
Reconstruction of the thoracic tracheal defects with portions of deepithelialized myocutaneous flaps after resection of a large tumor
Susheng Wang1, Gang Liang1, Zhihua Zhang1, Hang Ji1, Chun Hou1, Jianxing He2,3, Weiqiang Yin2,3
1Department of Plastic Surgery,2Department of Cardiothoracic Surgery, The First Affiliated Hospital of Guangzhou Medical University;3Guangzhou Institute of Respiratory Disease, Guangzhou 510120, China
Corresponding to:Susheng Wang, MD. Department of Plastic Surgery, The First Affiliated Hospital of Guangzhou Medical University, No. 151, Yanjiang Rd, Guangzhou 510120, China. Email: wangssgz@yeah.net.
Objective:To study the possibility of using portions of deepithelialized myocutaneous flaps to the reconstruction of thoracic tracheal defects after resection of a large tumor.
Methods:From June 2007 to June 2012, five cases of defects of the thoracic trachea were reconstructed by applying portions of deepithelialized myocutaneous flaps. The patients were 27-61 years old with 4 male cases and 1 female. The cervical trachea ranged in diameter from 4-8.5 cm with circumferences of approximately 1/3-2/5 of the bronchial circumference.
Results:All five patients with thoracic tracheal defects after resection of a large tumor were cured of portions of deepithelialized myocutaneous flaps, with no tracheal stricture remaining and vomica successfully eliminated. During the first 1 to 3 months after the operation, bronchoscopy showed that the tracheal lumens were smooth, and the visible skin of the musculocutaneous flaps became gray and exhibited a small amount of white discharge.
Conclusions:Despite this being a small series and short follow-up, this thoracic tracheal reconstruction with portions of deepithelialized myocutaneous flaps shows encouraging preliminary results and could be an alternative to other methods for the treatment of carefully selected patients with thoracic tracheal defects.
Deepithelialized; myocutaneous flap; tracheal defect
Scan to your mobile device or view this article at:http://www.thecjcr.org/article/view/1508/2476
Introduction
The thoracic trachea is located in the mediastinum behind the innominate artery and left innominate vein. Due to poor exposure and extension, there is typically insufficient tracheal material that can be used to repair wounds after sleeve resection. The reconstruction of massive thoracic tracheal defect after tumor resection, infections, or trauma remains a challenge in thoracic surgery (1,2). Although primary end-to-end anastomosis has been reported to allow for successful resection of adult trachea up to nearly half of its length, more extensive removal of the tracheal segment usually requires use of artificial materials or autogenous tissues for repair of the resulting defect (3,4). We presented five cases in which reconstruction of a massive airway defect was performed with the portions of deepithelialized myocutaneous flaps after resection of a large tumor.
Patients and methods
Patients
The study group consisted of five cases: 4 males and 1 female ranging in age from 27 to 61 years with an average age of 51 years. These patients suffered from tracheal or bronchial defects resulting from tumor resection. Amongthese patients, four suffered from bronchial lung cancer, and one patient suffered from a tracheal inflammatory myofibroblastic tumor. During the operations, 4 cases required immediate restoration of the defect. The other defect was restored postoperatively. For 3 cases, the defects were located on the right side of the bronchial wall, while the other 2 defects were on the left side of the bronchial wall. The defect circumference ranged from 2/5-1/3 of the bronchial circumference, and the length was between 4-8.5 cm with an average of 5.7 cm.
Flap design and surgical methods
All the wounds were repaired using ipsilateral pedicle island musculocutaneous flaps. The pectoralis major musculocutaneous flap was applied in 3 cases, while the other 2 used the latissimus dorsi musculocutaneous flap. The lesion location was determined using preoperative CT and bronchoscopy. The musculocutaneous flap type was chosen based on the defect location. Lesions in or near the anterior wall of the trachea were usually repaired using the pectoralis major musculocutaneous flap. In other cases, the defects were usually repaired using the latissimus dorsi musculocutaneous flap. The plastic surgeon created a preliminary design for the musculocutaneous flap. The thoracic surgeon exposed the tumor and invaded the trachea by conducting a thoracotomy or used surgical debridement to treat the tracheal fistula and the thoracic empyem. Next, the surgeon remodeled the thoracic cavity and measured the length and width of the tracheal defects. Most importantly, the distance between the distal portion of the tracheal defect and the vascular pedicle of the musculocutaneous flap was determined. Then, the design of the musculocutaneous flap was altered according to the measurements, and the musculocutaneous flap was produced (Figure 1A).
The patients remained in the lateral position while the surgeon cut from the latissimus dorsi musculocutaneous flap. At a distance of 3-5 cm from the ipsilateral axillary, an incision was made in the skin along the thoracodorsal artery. From the outside to the inside, we explored the vessels along the myenteric fascia at the depth of the front margin of the latissimus dorsi muscle. Between 1.5 and 2.5 cm away from the edge of the muscle, the thoracodorsal vascular bundle was explored using a Doppler ultrasound probe. We cut from the pedicle island latissimus dorsi musculocutaneous flap to the remote musculocutaneous flap. Then, the musculocutaneous flap was put in place to repair the tracheal defects along thoracotomy incision. The repair was required to intercept 4 cm of ribs to form the loose vascular pedicle pathway. During the operation, the skin of the musculocutaneous flap had to be in contact with the tracheal defect, making it necessary to confirm and mark specific parts of tracheal defects. Except for the portion of the musculocutaneous flap covering the defect, which must remain the same size, the other parts of the myocutaneous flap became deepithelialized (Figure 1B). After checking that the vascular pedicle was loose, we applied a parachute suture to connect the margins of the epidermal musculocutaneous flap with the tracheal defect (Figure 1C). Then, the dermis of the deepithelialized musculocutaneous flap was sutured with the parietal pleura. Finally, the thoracic surgeon rinsed the thoracic cavity and examined whether the anastomoses had leaked.
The patients remained recumbent while we cut from the pectoralis major musculocutaneous flap. An oblique skin incision was made 3-5 cm below the midpoint of the clavicle, and then the pectoralis major fiber was separated from the pectoralis minor muscle. We explored the pectoralis major thoracic acromial branch artery preoperatively using a Doppler ultrasound probe. We cut from the pedicle pectoralis major musculocutaneous flap to the remote musculocutaneous flap. The musculocutaneous flap was then put in place to repair the tracheal defects using the thoracotomy incision channel or the upper 1-2 intercostal incision pathway. For the same reason as discussed above, it was necessary to intercept 4 cm of ribs to form a loose vascular pedicle pathway. The tracheal defect repair procedures used after this point were almost the same as those used for the latissimus dorsi musculocutaneous flap.
Results
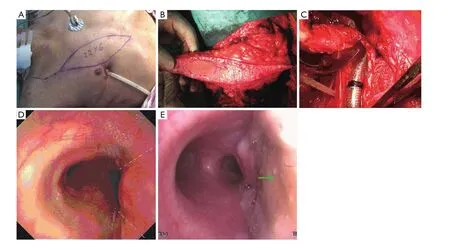
Figure 1 A. Design of the pectoralis major muscle flap; B. Part of the de-epithelialized myocutaneous flap; C. Tracheal defect after tumor excision; D. Bronchoscopy showed multiple tumors involving tracheal rings; E. A bronchoscopy of the repaired trachea with the flap

Table 1 Preoperative and postoperative pulmonary function
The surgical procedures outlined above were performed without incident on 5 patients. The position of the vascular pedicle did not change, and the blood supply to the musculocutaneous flap was sufficient. The wounds healed well in all cases, and the patients were not short of breath during the follow-up period from 3 months to 1 year. During the first 1 to 3 months after the operation, bronchoscopy showed that the tracheal lumens were smooth, and the visible skin of the musculocutaneous flaps became gray and exhibited a small amount of white discharge. The repaired tracheal walls did not swing during breathing. Postoperative pulmonary function tests for time vital capacity (FVC) showed that the forced expiratory volume in the first 1 s (FVE 1) declined and that the FVC decreased more than the FEV1. The respiratory airway resistance and flow rate were both in the normal range compared with standard values (Table 1). All five patients were able to return to their normal lives. Among these five cases, there was a 27-year-old female suffered from a tracheal inflammatory myofibroblastic tumor as observed on a preoperative fiberoptic bronchoscopic biopsy. The tumor was located in the right-middle-lower region of the tracheal mucosa (Figure 1D). The top of the tumor was 3-4 cm away from the tracheal carina. The bottom of the tumor extended to the right main bronchial opening. Intraoperatively, the tumor resection removed 2/5 of the bronchial circumference and extended 8.5 cm in length along the right side of the bronchial wall. The pectoralis major musculocutaneous flap was 12 cm × 6 cm. The postoperative bronchoscopy showed good blood supply to the musculocutaneous flap, a smooth tracheal lumen, and a lack of swinging by the repaired tracheal walls during breathing (Figure 1E).
Discussion
Generally, the length of defects in the cervical trachea is less than 6 cm (1/2 the length of the cervical trachea),and in these cases anastomosis of the defects is effective (5,6). The bronchial length of the anastomosis is related to factors such as the patient’s age, physical condition and local anatomical factors (7). Current methods of repairing bronchial defects consist of tracheal anastomosis, musculo periosteal flap repair, autologous repair by free tissue transplantation, metal-titanium network repair and artificial trachea transplantation (8). However, the clinical applicability of these approaches is limited (9). Thoracic tumors usually involve the trachea, and tracheal defects are a common complication of resection. While minor defects can be repaired by anastomosis, this method is useless for larger defects. Due to the depth of the thoracic trachea and its poor ability to stretch, the length at which re-suturing is effective is highly limited (1,10), while the repair of these defects is a significant problem.
Costantinoet al. considered that the ideal tracheal substitution should possess the following seven characteristics: sufficient strength to withstand the compression of the cervical tissue and avoid collapse during breathing; good flexibility to withstand the neck without transposition or discomfort; air tight without any leaking; a lumen that is continuously covered in a ciliated epithelium, which plays a role in clearing the grume; good blood supply, infection-resistant, and easy-to-heal; applicable to long tracheal defects; good tissue compatibility (11). Tracheal defects caused by tumors are mostly confined to the parietal or parietal-anterior wall. The sectional repair of tracheal defects should also meet these requirements. The pedicle musculocutaneous flap satisfies the basic requirements of tracheal substitution outlined above (12,13). The shape of the musculocutaneous flap is flexible to some extent, enabling it to fit a variety of tracheal defects. The flap has a good blood supply and is strongly resistant to infection. In a short period of time, the edge of the trachea can combine with the dermis to form an air-tight structure. The air flue consisting of the cutaneous face of the flap can withstand the compression of the cervical tissue and remain open during breathing. Postoperative pulmonary function tests and bronchoscopy indicate that the ventilatory function is not adversely affected.
It is important to consider how to choose the flap between pectoralis major musculocutaneous flap and latissimus dorsi musculocutaneous flap (14-17). In this study, we chose the musculocutaneous flap based on the position of the tracheal defect. If the lesion lies in or near the anterior wall of the trachea, we adopt the pectoralis major musculocutaneous flap, while in other cases, we used the latissimus dorsi musculocutaneous flap. When the distance between the defects and the flap is short enough, it is not necessary to use a long pedicle musculocutaneous flap. When the lesion lies in or near the posterior wall of the trachea, we tend to choose the latissimus dorsi musculocutaneous flap. While the distance between the defects and the flap is long, a long pedicle musculocutaneous flap is required. These results demonstrated that using deepithelialized musculocutaneous flaps was a feasible way to repair tracheal defects. This study expands the potential applications of musculocutaneous flaps and provides a new choice for the repair of tracheal defects.
In conclusion, despite this being a small series and short follow-up, this thoracic tracheal reconstruction with portions of deepithelialized myocutaneous flaps shows encouraging preliminary results with low mortality and morbidity, and could be an alternative to other methods for the treatment of carefully selected patients with thoracic tracheal defects, although further study is warranted to verify the clinical benefits.
Acknowledgements
Disclosure:The authors declare no conflict of interest.
1. Yamamoto K, Miyamoto Y, Ohsumi A, et al. Surgical results of carinal reconstruction: an alterative technique for tumors involving the tracheal carina. Ann Thorac Surg 2007;84:216-20.
2. Rashid OM, Nagahashi M, Takabe K. Management of massive soft tissue defects: The use of INTEGRA® artificial skin after necrotizing soft tissue infection of the chest. J Thorac Dis 2012;4:331-5.
3. He J, Xu X, Chen M, et al. Novel method to repair tracheal defect by pectoralis major myocutaneous flap. Ann Thorac Surg 2009;88:288-91.
4. Hata Y, Sakamoto S, Shiraga N, et al. A case of chronic expanding hematoma resulting in fatal hemoptysis. J Thorac Dis 2012;4:508-11.
5. Ridgway E, DeCamp M, Morris D. Bronchopleural fistula repair using combined breast parenchymal and pectoralis major musculocutaneous flap. Ann Thorac Surg 2008;86:1022-5.
6. Mulliken JB, Grillo HC. The limits of tracheal resection with primary anastomosis: further anatomical studies in man. J Thorac Cardiovasc Surg 1968;55:418-21.
7. Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731-9.
8. Allen MS. Surgical anatomy of the trachea. Chest Surg Clin N Am 1996;6:627-35.
9. Mineo TC, Ambrogi V. The diaphragmatic flap: A multiuse material in thoracic surgery. J Thorac Cardiovasc Surg 1999;118:1084-9.
10. Grillo HC. Tracheal replacement: a critical review. Ann Thorac Surg 2002;73:1995-2004.
11. Xu Q, Deng Y, Fu S, et al. A novel tracheobronchial reconstruction for right upper lung carcinoma involving the lower trachea: preliminary results. Ann Thorac Surg 2012;93:1070-4.
12. Costantino PD, Nuss DW, Snyderman CH, et al. Experimental tracheal replacement using a revascularized jejunal autograft with an implantable Dacron mesh tube. Ann Otol Rhinol Laryngol 1992;101:807-14.
13. Urushidate S, Yokoi K, Higuma Y, et al. New way to raise the V-Y advancement flap for reconstruction of the lower lip: bipedicled orbicularis oris musculocutaneous flap technique. J Plast Surg Hand Surg 2011;45:66-71.
14. Wong C, Mojallal A, Bailey SH, et al. The extended transverse musculocutaneous gracilis flap: vascular anatomy and clinical implications. Ann Plast Surg 2011;67:170-7.
15. Molnar JA, Pennington DG. Management of postpneumonectomy bronchopleural-cutaneous fistula with a single free flap. Ann Plast Surg 2002;48:88-91.
16. Taghavi S, Marta GM, Lang G, et al. Bronchial stump coverage with a pedicled pericardial flap: an effective method for prevention of postpneumonectomy bronchopleural fistula. Ann Thorac Surg 2005;79:284-8.
17. Dutra AK, Neto MS, Garcia EB, et al. Patients’satisfaction with immediate breast reconstruction with a latissimus dorsi musculocutaneous flap. J Plast Surg Hand Surg 2012;46:349-53.
Cite this article as:Wang S, Liang G, Zhang Z, Ji H, Hou C, He J, Yin W. Reconstruction of the thoracic tracheal defects with portions of deepithelialized myocutaneous flaps after resection of a large tumor. Chin J Cancer Res 2013;25(2):161-165. doi: 10.3978/j.issn.1000-9604.2013.02.01

10.3978/j.issn.1000-9604.2013.02.01
Submitted Jan 14, 2013. Accepted for publication Feb 19, 2013.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Safety and efficacy of trimodality therapy in patients undergoing extrapleural pneumonectomy
- Treatment of mesothelioma: still a long way to go!
- Role of contrast-enhanced ultrasonography in percutaneous radiofrequency ablation of liver metastases and efficacy evaluation
- Determining the optimal time for bortezomib-based induction chemotherapy followed by autologous hematopoietic stem cell transplant in the treatment of multiple myeloma
- Bevacizumab rescue therapy extends the survival in patients with recurrent malignant glioma
- Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment
