橄榄醇分子印迹聚合物的制备及评价
2013-05-08靳亚峰刘润强柏连阳张裕平
靳亚峰, 陈 娜, 刘润强,, 陈 军,, 柏连阳, 张裕平*
(1.河南科技学院应用化学所,河南新乡453003;2.湖南农业大学农药研究所,湖南长沙410128)
5-Alkylresorcinols(ARs),as a group of phenolic lipids,are interesting naturally-occurring compounds,which have been isolated from many plants including the bran fraction of wheat,rye,and barley grains[1,2].They possess a wide variety of biological activities,including fungicidal and bacteriocidal activities against numerous pathogens.This type of compounds can cleave DNA at high concentrations in the presence of copper(II)chloride and oxygen,and are essential components in the synthesis of cannabinoids[3].There were some published papers for alkylresorcinols synthesis but the procedure required expensive reagents,chromatographic purification and determination afterwards[4-9].Another challenge for the analysis of alkylresorcinols is the lack of commercial standards,which is a problem for laboratories wishing to start work on it.Currently,only olivetol(C5)is available but expensive(95% purity,Sigma-Aldrich,St Louis,MO,USA).Previously,C15 was also available from Aldrich at 85%purity,but in order to use this material as a quantitative standard or for biological studies,it needs to be purified.Other options for obtaining standard ARs material is to purify them from raw material(normally rye or wheat bran,or cashew nut shell liquid)using column chromatography or to synthesize them[10].ARs can be analyzed by chromatographic methods such as gas chromatography(GC),high performance liquid chromatography(HPLC)and related technologies[11-15].Linko et al.[16]established a method for the determination of ARs with chain lengths of C15,C17,C19,C21,and C23in human plasma by gas chromatography-mass spectrometry(GCMS).Afterwards,Landberg et al.[17]improved this method by using Oasis MAX SPE cartridges for sample cleanup.A plasma sample of 0.2 mL could be used without pre-incubation with water.Approximately 30 to 50 samples could be analyzed within one day.The improved GC-MS method presented can be used for rapid analysis of ARs in relatively small sample volumes.Compared with the general sorbents used in SPE,molecularly imprinted polymer(MIP)has predetermined selectivity for the template molecule[18-21].The highly cross-linked structure in the polymer can maintain the characters of micro-cavities after the removal of the template molecule and can be used to adsorb the target molecule in the analytical process.Various strategies could be used to prepare MIP for this purpose including precipitation polymerization,bulk polymerization,suspension polymerization,swelling polymerization,miniemulsion polymerization and core-shell emulsion polymerization[22-26].
To the best of our knowledge,there is no published paper using olivetol(AR with chain lengths of C5)as the template to make MIP by bulk polymerization.The obtained MIP was applied as SPE sorbent to pre-concentrate and determine the spiked olivetol and its analogs from wheat bran samples by HPLC.Through the optimization of the sample loading and the desorption conditions,better recoveries of olivetol in three spiked levels were obtained compared with the commercial SPE cartridge.
1 Experimental
1.1 Instruments and chemicals
Chromatographic separation were performed by a HP1100 Series HPLC system(Agilent Technologies,Inc.,Walbronn,Germany)equipped with a quaternary pump,an injector with a 20 μL quantitative tube(Rheodyne 7725i)and a UV detector with changeable wavelength in the range of 190-600 nm.Binding experiments were performed with a UV-2100 spectrophotometer(Beijing Beifen-Ruili Analytical Instrument Co.,Ltd.).Pressure Blowing Concentrator(Supelco,USA),SPE Manifold(Supelco,USA),Quanta 200 scanning electronic microscope(FEI,Hillsboro,Oregon,USA),TENSOR27 infrared spectrometer(Bruker,Germany).
Olivetol(95%)were purchased from Sigma-Aldrich Co.,Ltd.;wheat bran was a present from School of Life Science,Henan Institute of Science and Technology;azobisisobutyronitrile(AIBN)was purchased from Institute of Tianjin Guanfu Fina Chemicals;α-methacrylic acid(MAA)and ethylene dimethacrylate(EDMA)were purchased from Beijing Bailingwei Chemical Reagent Company;phenol,resorcinol,phloroglucinol,m-toluidine,acetic acid,toluene,and dodecanol were purchased from Tianjin Chemical Reagent Company and Beijing Chemical Reagent Company.The monomers were purified by distillation to remove the inhibitors.All other chemicals used are of chromatographically pure and analytical grade.ProElut polystyrene/divinylbenzene(PLS)Glass Cartridge(60 mg/3 mL)was purchased from Dikma Technologies Inc.(CA,USA).
1.2 Preparation of MIP
Some previous reports of the synthesis of MIP about phenol compounds were referred to with great modification including the choice of monomer,porogen and template[27,28].Schematic illustration of olivetol molecular imprinting procedures and its structural analogs for the experiments are shown in Fig.1.Briefly,the molar ratio of the template to the monomer was selected at 1∶4 to ensure the formation of the defined recognition sites with polymer.MIP was synthesized using bulk polymerization method by dissolving 1 mmol olivetol and 4 mmol MAA in a glass tube with 8 mL toluene and 2 mL dodecanol,followed by ultrasonication about 30 min to form stable complex of the imprint molecules and the monomers.The obtained solution was then cooled at room temperature,followed by adding 20 mmol of EDMA,0.040 0 g AIBN.Then,the prepared sample in the tube was degassed and purged with nitrogen before being sealed with a septum.The solution was further saturated with dry nitrogen for 5 min and the glass tube was placed in a water bath at 60℃for 24 h.After polymerization,the tube was broken.The obtained polymer was ground and sieved.The particles with sizes between 47-74 μm were collected and then repeatedly suspended in acetone to remove the small particles.The template was removed by Soxhlet extraction using a two-step procedure,being washed by acetic acid and methanol(1∶9,v/v)about 12 h as the first step,then by methanol for 6 h as the second step.The prepared polymer was dried at 60℃for 24 h under vacuum and stored in desiccator prior to use.Nonimprinted polymer(NIP)was synthesized and treated under the same conditions but without the addition of the template.

Fig.1 (a)Schematic illustrations of the imprint formation and molecular recognition of olivetol and(b)molecular structures of olivetol and structurally-related compounds
1.3 Equilibrium rebinding and binding selectivity of MIP
UV spectrophotometry was used to carry out the steady-state binding experiments at 220 nm.The standard olivetol solutions with different concentrations in the range of 0.1-1.0 mmol/L were prepared with acetonitrile.In a typical experiment,an amount of 0.020 0 g of both polymers(MIP and NIP)was put into 10 mL vials,separately.Then,2 mL of olivetol standard solution was mixed with each polymer.Afterwards,the mixtures were shaken with horizontal shakers for 12 h at room temperature,followed by centrifugation at 3 000 r/min for 5 min.The suspensions were filtered by the filter with 0.22 μm pore size.After the filtrated solutions were diluted to an appropriate con-centration,they were analyzed by UV spectrometry.The amount of olivetol bound to the polymer was calculated by subtracting the amount of the free olivetol from the initial amount added to the mixture.The adsorption quantity(Q)was calculated by equation,
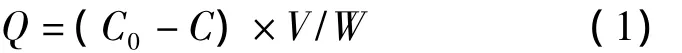
where C0is the concentration of the template olivetol added in the initial solution;C is the concentration of the free template in the solution containing imprinted polymer after shaken for 24 h;V is the volume of the bulk solution;W is the weight of the dry polymer used.
Each experiment was repeated for three times,and the mean value of the static adsorption experiment data was further processed with Scatchard equation to estimate the binding parameters of the MIP and NIP[29,30].Scatchard equation was described as follows,

where Kdand Qmaxare the balance dissociation constant and maximum binding capacity,respectively;C is the balance concentration of the target molecule.The values of Kdand Qmaxcan be calculated from the slope and the intercept of the linear line plotted of Q/C versus Q.
The same experiments were performed to investigate the selectivity using other structurally-related compounds,such as phenol, resorcinol, phloroglucinol and m-toluidine.Firstly,the amounts of 0.020 0 g of both polymers(MIP and NIP)were put into the 10 mL vials,separately.Then,2 mL of olivetol standard solution with a constant concentration of 0.8 mmol/L was mixed with each polymer,separately.The imprinting factor(α)expresses the ratio of specific-to-nonspecific binding for each compound,

In each case,the analyte uptake was normalized against the level of the template olivetol adsorbed and expressed as selectivity index(SI)calculated according to equation,

If SI is equal to 1,there is no difference between MIP and the control polymer.
1.4 Preparation of MI-SPE cartridge
Molecularly imprinted solid phase extraction(MISPE)cartridge was prepared with empty 3 mL cartridge(Dikma)packed by dry method with 0.060 0 g polymer(47-74 μm)and 0.020 0 g diatomite as the filtration aid.Each cartridge was attached with two sieve plates at the bottom and the top ends.The cartridges were subjected to vacuum for 30 s before the insertion of a second frit on top of the sorbent bed.Both MIP and NIP cartridges were firstly conditioned with 5 mL methanol and 5 mL H2O consecutively.Then the samples passed through the cartridge at the constant flow rate of 1 mL/min,followed by 5 mL pure water.The analyte was eluted with 3 mL methanol.The collected solution was dried under N2flow and the residues were diluted with 5 mL acetonitrile for HPLC analysis.

Fig.2 SEM of the prepared MIP and NIP
1.5 Analysis of real samples
The wheat bran was firstly dried in an oven at 105℃ overnight,then 0.500 g dried sample was put into a glass tube,followed by the addition of 10 mL methanol.Afterwards,the mixture was shaken with a horizontal shaker for 24 h at room temperature,followed by centrifugation.The filtrated suspension solution was then filled with methanol to the volume of 50 mL.Subsequently,5 mL wheat bran solution and the related spiked solutions were adopted for the SPE procedure with the spiking concentrations of 2,4,8 mg/L,and used for the chromatographic analysis.
The ProElut PLS Glass Cartridge was processed according to the application manual from Dikma Technologies Inc.It was firstly conditioned with 3 mL methanol and 3 mL H2O consecutively.Then the sample passed through the cartridge at a constant flow rate of 1 mL/min,followed by 3 mL 5%(v/v)methanol aqueous solution.The analyte was eluted by 3 mL methanol.The collected solutions were dried under N2flow and the residues were diluted with a constant volume of 1 mL acetonitrile for HPLC analysis.
2 Results and discussion
2.1 Characterization for MIP and NIP
The morphological structure of the prepared polymer was characterized by scanning electron microscopes(SEM)as shown in Fig.2.The particle sizes of MIP were estimated in the range of 47-74 μm after sieving.Generally,the polymer without template displayed a relatively smooth surface,whilst there were some speckles and cavities appearing on the resulted MIP.The obvious difference between NIP and their related MIP was definitely attributed by imprinting effect.After the olivetol template embedded in the framework of the polymer was removed,more speckles and bigger cavities were formed compared with NIP.
The Fourier transform infrared spectrometer(FTIR)(KBr pellet)spectra of the prepared polymer,the monomer(MAA),the crosslinker(EDMA)and the template(olivetol)coating are discussed(figure not shown).Obvious difference on absorption bands existed in the range of 1 100-1 800 cm-1between the two kinds of polymer(MIP and NIP)and the other three compounds(MAA,EDMA and olivetol).There was a broad absorption band at 3 447 cm-1on the MIP coating corresponding to the stretching vibration of O-H bonds of MAA molecules(functional monomer),the hydrogen bonding and the electrostatic binding interactions between olivetol and MAA.However,for NIP,a weaker absorption peak around 3 444 cm-1was discovered because of the lack of the hydrogen bonding or electrostatic binding interaction between olivetol and MAA.Other absorption peaks matched between MIP and NIP coating were as follows:2 960 or 2 970 cm-1(stretching vibration of C-H bonds on methyl groups),1 731 or 1 733 cm-1(stretching vibration of C=O bonds on carbonyl groups),1 160 cm-1(stretching vibrations of C-O and C-C).
2.2 Equilibrium rebinding study and Scatchard analysis
The data in Fig.3 indicate that the amount of olivetol bound to MIP and NIP was increased in the selective concentration in therangeof 0.1 -1.0 mmol/L.It is easily understood that the MIP exhibited higher capacity of olivetol than that of NIP.However,the amount of olivetol adsorbed on NIP was dramatically lower at high concentration compared with that on MIP.It is concluded that more specific binding sites were generated on MIP at high concentration which was obviously due to the imprinting effect.For example,

Fig.3 Binding isotherm of olivetol on MIP and NIP in acetonitrile
The Scatchard analysis of MIP and NIP was carried out,which showed that the resulted polymer binding sites for olivetol were non-uniform(Fig.4).It is observed that two straight lines were obtained in the case of MIP and NIP in the plot region,which suggests that there were two kinds of binding sites of the high and the low affinities.For the high affinity site of MIP,the linear equation was Q/C=876.55-46.78Q(r=0.999 7).The values of Kdand Qmax,calculated from the slope and the intercept of the linear portion of Scatchard analysis,were 0.021 mmol/L and 18.74 μmol/g,respectively.For the low affinity site,the linear equation wasQ/C=135.55-0.997 6 Q (r=0.992 3),with Kdand Qmaxof 1.002 mmol/L and 135.9 μmol/g,respectively.In contrast,for the high affinity site of NIP,the linear equation was Q/C=390.71-29.60Q,with the values of Kdand Qmaxof 0.039 and 15.26 μmol/g,respectively.For the low affinity site,the linear equation was Q/C=60.95-1.76Q,with Kdand Qmaxof 0.57 mmol/L and 34.74 μmol/g,respectively.
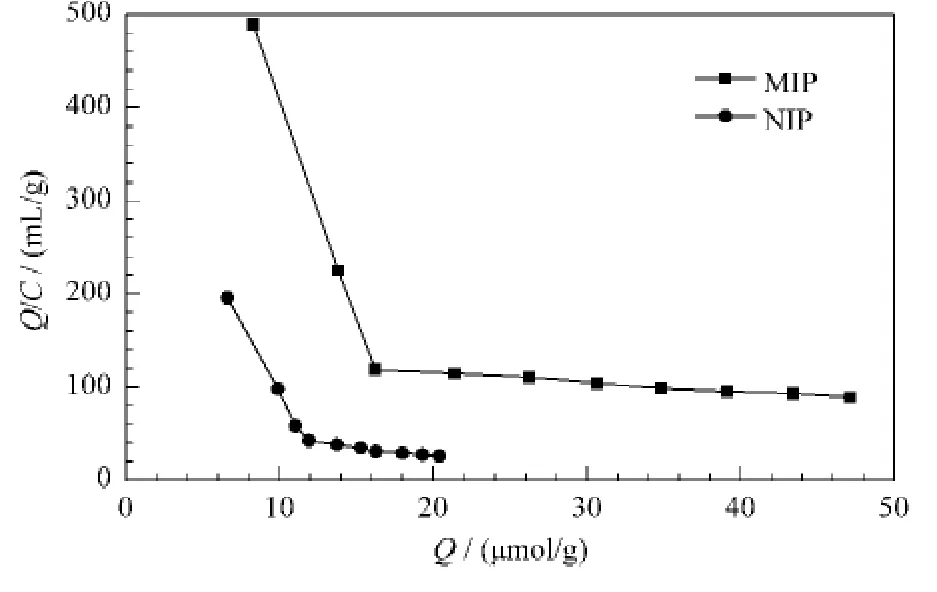
Fig.4 Scatchard plots to estimate the binding nature of olivetol-MIP and olivetol-NIP
2.3 Specificity study of the prepared polymer
Herein,the ability of MIP to discriminate olivetol and the other three structurally related compounds was assessed in single-analyte binding assays.After equilibrium binding experiments were performed according to the foregoing procedures,the related QMIPand QNIPfor each analyte could be determined.As shown in Table 1,the binding amounts of olivetol and its structural analogs on MIP were all higher than those on NIP,indicating the higher specific bindings for the tested compounds on MIP.Generally,the imprinting was primarily based on the interaction of the analyte to the functional groups within polymer cavities,but the combined effects of shape and size complementarity probably affected the imprinting efficiency effectively.It is easily understood that the largest values of α and SI were achieved for the template molecule,compared with the other structurally-related compounds of similar values of α and SI.The α and SI values obtained are comparatively given in Table 1.
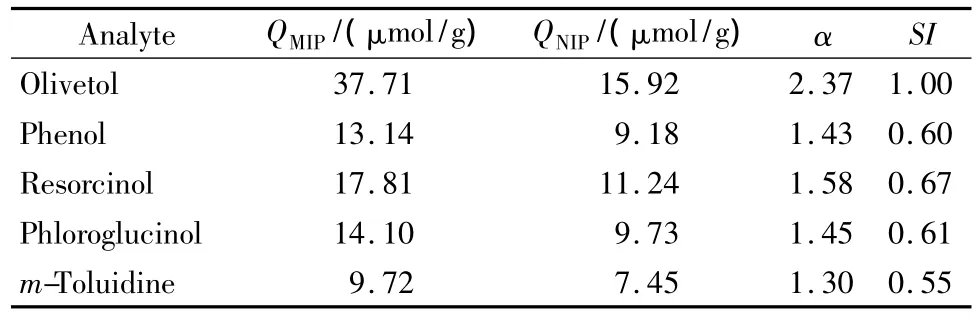
Table 1 Maximum binding amounts(Qmax),imprinting factors(α)and standardized selectivity indexes(SI)for different analytes
2.4 Optimization of MI-SPE cartridge
The same steps used in a common SPE procedure such as conditioning,sample loading,washing,and elution should be optimized during the operation of MISPE.Some important factors that probably influenced the extraction process were investigated in detail.
The loading solvent needs to be carefully selected to promote the rebinding of the substrate to the specific sites.In our experiments,when methanol,acetonitrile,and ethyl acetate were selected as the loading solvents separately,more than 80%leakage of olivetol occurred on the MI-SPE cartridge.When dichloromethane and hexane were selected,slight swelling of the packed adsorbent was caused.Since most the environmental samples were in aqueous solutions,5 mL aqueous solution with 10 mg/L olivetol was thus chosen as the loading solvent.In this case,almost all of the olivetol could be retained on the MI-SPE cartridge,revealing good affinity of the prepared MIP to the target olivetol.Different flow rates of the loading solvents were investigated in the range of 1.0 to 5.0 mL/min.The results showed that the recoveries of olivetol decreased abruptly as the flow rate changing to 2.0 mL/min(Fig.5).Considering the compromise between the larger recovery of olivetol and the total analysis time,the flow rate of 1.0 mL/min was used for the next optimization.
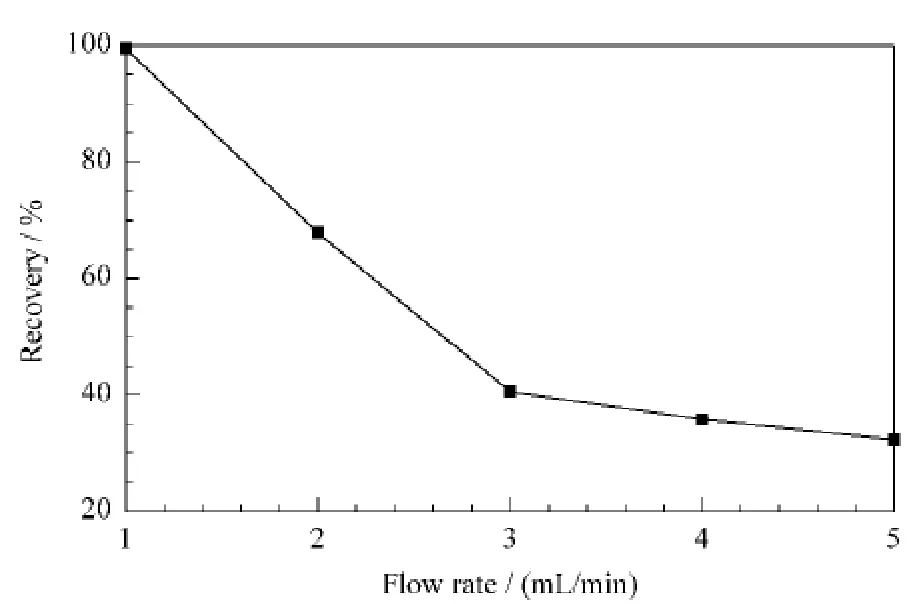
Fig.5 Optimization of flow rate of loading solvent
After loading the spiked aqueous solution on the cartridge,the target analyte should be retained on the SPE cartridge by both specific and nonspecific interactions.A subsequent washing process was required to remove the nonspecifically bound compounds,and consequently to achieve selective pre-concentration of olivetol.The solutions(5 mL)of different methanol volume percentages of 0%,10%,30%and 50%were tested for washing conditions.The results showed that the recovery of olivetol decreased obviously with the increase of the methanol content.The pure water possessed the highest elution strength,which resulted in the largest retention of olivetol on the cartridge.Thus,water was chosen as the washing solution in the experiments.
The solutions of different volume ratios(10∶0,9∶1,8∶2,7∶3,6∶4)of methanol and acetic acid were investigated for their elution efficiencies.The solution of methanol and acetic acid with different percentages showed relatively low eluting ability for the target molecules.Most of the target molecules could not be eluted even with more than 15 mL elution solvent,whilst only 3 mL pure methanol could elute the target molecules effectively.Therefore,3 mL methanol was used for the elution in all the experiments.
The measurement of the breakthrough volume is important for SPE because the breakthrough volume represents the sample volume that can be pre-concentrated without loss of the analyte during elution.The evaluation of the cartridge capacity was carried out by consecutively passing 1 mL standard olivetol solution with a constant concentration of 10 mg/L through MI-SPE.The increasing step volume(1 mL)of the olivetol solution was kept throughout the experiment.The results showed that olivetol could not be detected after 89 mL standard solution passing through the MI-SPE cartridge.The largest cartridge capacity was calculated to be 0.081 mmol/g.
2.5 Validation of MI-SPE-HPLC method
The calibration curve was constructed using the areas of the chromatographic peaks measured at ten increasing spiked levels ranging from 0.1 to 100 mg/L.Good linearity was observed for three analytes throughout the concentration range.The regression equations and the limits of detection(LOD,S/N=3)were as follows:yolivetol=66.14x+54.28(r=0.999 8,LOD=0.062 mg/L);yphenol=42.61x+147(r=0.997 7,LOD=0.058 mg/L);ytoluidine=88.65x+257.73(r=0.998 3,LOD=0.031 mg/L).Here y refers to the peak area and x refers to the mass concentration(mg/L).
To further investigate the effect of the sample matrix on the selectivity and the accuracy of the MI-SPEHPLC method for real analysis,the recovery experiments were carried out by spiking three different levels of the target molecules(olivetol,phenol and m-toluidine)into the wheat bran samples.The accuracy and precision of the method were assessed by performing analyses of the spiked samples in three replicates.As shown in Table 2,the relative standard deviations(RSDs)for all the recoveries were in the range of 1.4%-5.0%.The high recoveries(97.8%-98.8%)and the clear chromatograms of MI-SPE-HPLC method demonstrated that MIP was an effective sorbent to enrich olivetol in the real samples.This method overcomes the drawbacks of the template leakage of MIP in real analysis.Under the same experimental conditions,57.2%-60.1%analytes were eluted in the washing step with NIP as the sorbent,which demonstrated that the higher affinity of MIP toward olivetol resulted from the specific recognition sites formed by the imprinting effect.ProElut PLS was highly cross-linked polystyrene-divinylbenzene(PS-DVB)copolymer used for the extraction of polar analytes in aqueous solvents including phenols,polycyclic aromatic hydrocarbons,etc.Here,the commercial ProElut PLS cartridge provided general recoveries of olivetol(90.2%-92.0%),which was due to its low affinities to the template molecule.It should be noted that PLS cartridge,as a general SPE sorbent,possessed high non-specific interaction to phenol and m-toluidine with high recoveries in the range of 89.0%-90.9%.

Table 2 Recoveries of the model compounds in spiked samples on MIP,NIP and PLS cartridges(n=5)
2.6 Analysis of wheat bran sample
5-Alkylresorcinol is found in high amounts(0.03%-0.15%of the whole kernel weight)in the bran of wheat and rye.It consists of a phenolic ring with two hydroxyl groups at the meta position,and an odd-numbered alkyl chain at position 5.The alkyl chains in cereal alkylresorcinols varies from 15-to 25-carbon long[10].The blank wheat bran sample was investigated as a reference.The peak of olivetol with the alkyl chain of 5-carbon long was not observed in the chromatogram.As shown in Fig.6,there was a typical impurity peak for the original sample.It’s retention time was between those of m-toluidine and olivetol.The spiked sample was then analyzed with NIP,MIP and PLS cartridges,separately.The chromatograms indicated that the interference was significantly reduced after SPE treatment,whilst the original impurity peak still appeared after PLS treatment.Moreover,the largest peak area for the olivetol template was obtained with MIP cartridge,which revealed that MIP had the highest affinity and selectivity for olivetol in the aqueous solution.
3 Conclusions
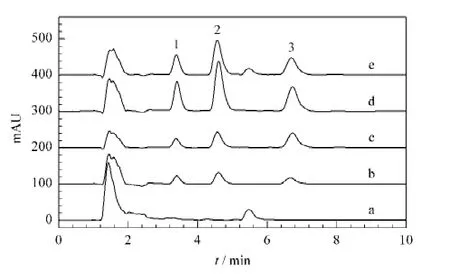
Fig.6 Comparative chromatograms of the target olivetol and its structurally-related compounds in wheat bran samples(a)original sample without pretreatment;(b)spiked sample with the same concentration of 8 mg/L for three compounds pretreated on NIP cartridge;(c)spiked sample with the same concentration of 8 mg/L for three compounds pretreated on MIP cartridge;(d)mixed standard solution with the same concentration of 8 mg/L for three compounds;(e)spiked sample with the same concentration of 8 mg/L for three compounds pretreated on PLS cartridge.Experimental conditions:stationary phase,YMC C18(150 mm ×4.6 mm i.d.,5 μm);mobile phase,acetonitrile-H2O(48∶52,v/v);flow rate,0.8 mL/min;UV wavelength,224 nm;injection volume,20 μL;room temperature.Peaks:1.phenol;2.m-toluidine;3.olivetol.
In this study,MIP coupled with HPLC was used for the selective determination of olivetol from the wheat bran.Good precision and accuracy of the MI-SPE cartridge for olivetol and the related compounds in wheat bran demonstrated the potential feasibility of the prepared MIP for extraction of alkylresorcinols.
[1] Ross A B,Shepherd M,Schüpphaus M,et al.J Agric Food Chem,2003,51:4111
[2] Shewry P R,Charmet G,Branlard G,et al.Trends Food Sci Tech,2012,25:70
[3] Zhu Y,Conklin D R,Chen H,et al.Bioorg Med Chem,2011,19:3973
[4] Ross A B,Shepherd M J,Bach Knudsen K E,et al.Brit J Nutr,2003,90:787
[5] Cocotl-Yanez M,Sampieri A,Moreno S,et al.Microbiology,2011,157:1685
[6] Miku M,Yohei K,Nobutaka F,et al.Phytochemistry,2010,71:1059
[7] Brandt J C,Wirth T.Beilstein J Org Chem,2009,5:22
[8] Daniel S,Odon V,Yanet R,et al.J Bacteriol,2009,191:3142
[9] Masanori F,Nobutaka F,Sueharu H.J Biol Chem,2008,283:13983
[10] Ross AB,Åman P,Andersson R,et al.J Chromatogr A,2004,1054:157
[11] Landberg R,Andersson A M A,Åman P,et al.Food Chem,2009,113:1363
[12] Anja K,Anna-Maria L P,Perttu H,et al.Clin Chem,2007,53:1380
[13] Ross A B,Kochhar S.J Agric Food Chem,2009,57:5187
[14] Marklund M,Landberg R,Åman P,et al.J Chromatogr B,2011,879:647
[15] Marklund M,Landberg R,Åman P,et al.J Chromatogr B,2004,878:888
[16] Linko A-M,Parikka K,Wähälä K,et al.Anal Biochem,2002,308:307
[17] Landberg R,Åman P,Kamal-Eldin A.Anal Biochem,2009,385:7
[18] Xu A,Fang G,Wang S.Food Chem,2010,119:845
[19] Yu J C C,Lai E P C.Food Chem,2007,105:301
[20] Quesada-Molina C,Claude B,García-Campaía A M,et al.Food Chem,2012,135:775
[21] Cirillo G,Curcio M,Parisi O I,et al.Food Chem,2011,125:1058
[22] Turiel E,Martín-Esteban A.Anal Chim Acta,2010,668:87
[23] Yan H Y,Cheng X L,Yang G L.J Agric Food Chem,2012,60:5524
[24] Jiang X,Jiang N,Zhang H,et al.Anal Bioanal Chem,2007,389:355
[25] Nong S Y,Lin F H,Huang X J,et al.Chinese Journal of Chromatography,2012,30:1133
[26] Wang X Y,Tan H R,Qi K Z,et al.Chinese Journal of Chromatography,2010,28:1107
[27] Yuan Z G,Liu Y Z,An F Q.Microchim Acta,2011,172:89
[28] Son L T,Katagawa K,Kobayashi T.J Membr Sci,2011,375:295
[29] Shaikh H,Memon N,Khan H,et al.J Chromatogr A,2012,1247:125
[30] Puoci F,Garreffa C,Lemma F,et al.Food Chem,2005,93:349
