Folate-chitosan-gemcitabine core-shell nanoparticles targeted to pancreatic cancer
2013-01-08JiahuaZhouJunyingWangQianXuShiXuJinWenZeqianYuDetongYang
Jiahua Zhou,Junying Wang,Qian Xu,Shi Xu,Jin Wen,Zeqian Yu,Detong Yang
1Department of General Surgery,Zhongda Hospital,School of Medicine,Southeast University,Nanjing 210029,China; 2Ministry of Education Key Laboratory of Environmental Medicine and Engineering,Southeast University,Nanjing 210009,China
Introduction
Human pancreatic cancer is one of the common clinical malignancies.Its incidence has increased in recent years (1).In more than 80% of patients at diagnosis,local or distant metastasis has occurred (2).It was only about 10% of surgical resection rate and post-operative 5-year survival rate was 15-20%.The survival was 3-6 months and 5-year survival rate is less than 5% in patients with advanced pancreatic cancer (3).Chemotherapy is still one of important means for treating advanced pancreatic cancer,preventing recurrence,prolonging survival time and improving quality of life.
Gemcitabine (Gem) is a standard chemotherapy drug for advanced and postoperative pancreatic cancer.But the effectiveness in pancreatic cancer therapy is unclear.The median survival was only 5.65 months and 1-year survival rate was 18% (2).The main factors that affect the Gem chemotherapy of pancreatic cancer are shown as below: first,short plasma half-life of 8-17 minutes in human (4),for immediately becoming difluorodeoxyuridine by cytidine deaminase after intravenous injection.Therefore,only increasing the dose can achieve effective therapeutic concentrations; second,lack of targeting,so that there were not enough concentrations of Gem in pancreatic tumor tissues meanwhile there is extensive destruction of normal human tissues,causing serious side effects.Nanoparticles are widely used in biomedical research for diagnosis and therapy to improve the half-life and targeting of the drug (5).So we need currently to study a suitable drug delivery system (DDS)with Gem for targeted therapy of pancreatic cancer.
It was showed that the expression level of folate receptor(FR) on human primary or metastatic pancreatic cancer cells was relatively high.Whereas on normal cells,FR expression is significantly lower.Folate (FA) has extremely high affinity for FR,which has been popularly employed as a targeting ligand of various anticancer agents to avoid their non-specific attacks on normal tissues as well as to increase their cellular uptake within target tumor cells via a receptor-mediated endocytosis process (6,7).Therefore,FA-conjugated nanoparticles are an ideal polymeric carrier material for pancreatic tumor-targeted drug delivery.The studies of FR-mediated tumor-targeted drug delivery system focused on FA complexes which modified by FA (8,9),but the studies of the FA-modified nanoparticle drug system packaging small molecule chemotherapy drugs were very limited (10-14).
The folate-chitosan-gemcitabine (FA-Chi-Gem) coreshell nanoparticles that we studied are a tumor-targeted drug delivery carrier,which were made with FA oriented ligands and Chi as a carrier by coaxial electrospray technology,and the particle size was in the range of 200-300 nm (15).Chi connected with FA via amide bond of FA γ-carboxyl conjugating to chitosan amine group,which does not change the conformation of FA and does not affect FR identification (8,16,17).After the nanoparticles were absorbed by cells via FR,chitosan was easy dissolved via acidification of cell endosomes.Gem within FA-Chi-Gem core-shell nanoparticles was quickly and completely released to cytoplasm,resulting in effective concentrations of Gem in pancreatic cancer cells.Moreover,the electrostatic adsorption of nanocarriers with negatively charged lipid layers of the biofilm surface occurs easily because chitosan is a polycation,which can extend Gem retention time at the absorption site and enhance the capacity of passing through the biofilm of drug within the delivery system (18).
This study investigated the FA-Chi-Gem core-shell nanoparticles that were made by coaxial electrospray technology for targeting therapy to pancreatic cancerin vitroandin vivo.
Materials and methods
Cell culture and animals
Human pancreatic cancer cell lines (COLO357,SW1990,MIA PaCa-2,Capan1,L3.6pl and BxPC3) and other cell lines (Hela,HT29) were cultured in RPMI-1640 (Gibco BRL,China) containing 10% inactivated fetal bovine serum(FBS),100 U/mL penicillin and 100 mg/mL streptomycin(Gibco BRL,China),at 37 ℃ in 5% CO2atmosphere forin vitrogeneration and culture.MIA PaCa-2 and COLO357 cells were provided by Helmut Friess.L3.6pl cell line was presented by Isaiah J Fidler.Capan1,SW1990 and BxPC3 were obtained from the Cell Bank at Chinese Academy of Sciences.
Six- to seven-week-old male nude mice were purchased from Laboratory Animal Center of Academy of Military Medical Sciences (Beijing,China) and housed in specific pathogen free (SPF) animal facility (Southeast university)where is environmentally controlled (22 ℃ and 12 h:12 h light: dark cycle,with the light cycle 08:00-20:00 and the dark cycle 20:00-08:00) withad libitumaccess to standard laboratory chow and water.All of the protocols were approved by the Ethics Committee of Animal Experiments of the Southeast University and the animal study was carried out in accordance the Ethical Guidelines for Animal Use and Care established by Southeast university(Nanjing,China).All surgery was performed under sodium pentobarbital anesthesia,and all efforts were made to minimize suffering.
Immunohistochemistry
The six kinds of pancreatic cancer cells,Hela cells and HT29 cells were seeded in 20 mm sterilized coverslip on the six-well culture plates and cultured in FA-free RPMI-1640(Gibco BRL,China) with 10% inactivated FBS,at 37 ℃in 5% CO2atmosphere.The slides were washed with PBS 3×2 min,fi xed by 4% paraformaldehyde for 30 min,washed with PBS 3×2 min again,incubated for 20 min with 0.5%Triton X-100,washed with PBS 3×2 min and immersed in 3% hydrogen peroxide,incubated for 30 min at room temperature,and washed 3 times with PBS.Subsequently,the slides were incubated in 10% normal blocking goat serum for 30 min,and then incubated with MOV18 (mouse anti-human FR monoclonal antibody,dilution 1:100;ENZO life sciences,USA) at 4 ℃ overnight.The next day,the slides were warmed to room temperature and processed using the labeled horseradish-peroxidase method at 37 °C for 15 min.The slides were washed three times for 5 min each in PBS and stained with 3,3'-diaminobenzidine (DAB).
Five fields of views on each slide were analyzed under high magnification (×400; Leitz microscope,Germany)by two pathologists double-blinded to the goals of the experiment.According to the average of the positive cell number and intensity of immunostaining,cell staining (A value) was graded as 0 (no cells stained),1 (1-10% of the cells stained),2 (11-50% of the cells stained),3 (51-80%of the cells stained) or 4 (81-100% of the cells stained) in accordance with the proportion of stained tumor cells to all tumor cells in slide.Protein expression (B value) was graded as 0 (no staining),1 (weak staining),2 (moderate staining),or 3 (strong staining).The mathematical products of the staining intensity scores and the staining proportion scores served as total assessment scores.The immunohistochemical analysis was classified as HIS value (immunohistochemical score) that was A times B.
Intracellular uptake study
COLO357 cells were cultured in FA-free RPMI-1640 medium containing 10% (V/V) FBS,at 37 ℃ in 5% CO2atmosphere.To determine the extent of nanoparticle uptake in COLO357 cells,the fluorescent nanoparticles were prepared in the same way with Gem replaced by fluorescein isothiocyanate (FITC),and targeted/untargeted fluorescent nanoparticles were prepared with FA-chitosan and chitosan as shell polymer respectively.Firstly,the fluorescent nanoparticles were both incubated with COLO357 cells in complete culture medium with or without 1 mmol/L free FA for 4 h.After incubation,cells were washed three times with PBS and then fixed with 4% paraformaldehyde for 30 min.Finally,the cells were washed three times with PBS and examined under fluorescence microscopy using a 488 nm excitation wavelength.
Cell proliferation assay
Cell proliferation was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium(MTT) method.COLO357 cells (1.5×104/well) were plated into each well of 96-well plates in free-FA RPMI-1640 with 10% FBS and cultured for 24 h.The cells were then treated with Gem or Gem-loaded nanoparticles at the equivalent drug concentration of 0.1,1,10 and 50 μg/mL and incubated for further 72 h.Then,50 μL of MTT solution (2 mg/mL;Sigma) was added and incubated for 2 h.The medium containing MTT solution was removed,and the dark blue crystals were dissolved by adding 100 μL of dimethyl sulfoxide (DMSO).The absorbance was measured with a microplate reader at excitation and reference wavelengths of 550 and 630 nm,respectively.The growth inhibition was shown as the percentage relative to controls treated with DMSO.Each experiment was done at least in triplicate.
Biodistribution study in vivo
The orthotopic pancreatic tumor model was established as described previously (19) under sodium pentobarbital anesthesia and verified by magnetic resonance imaging(MRI; Bruker Biospin,Ettlingen,Germany) scan.The experiment was divided into three groups,each of which has 6 nude mice.The fluorescent nanoparticles were prepared in the same way with Gem replaced by ICGDER02,and untargeted/targeted fluorescent nanoparticles were prepared with chitosan as shell polymer (PEG-ICGDER02-Chi) and FA-Chi (PEG-FA-ICGDER02-Chi) respectively,which were injected to A/B groups that were orthotopic pancreatic tumor groups.The C group was normal nude mice injected with PEG-FA-ICGDER02-Chi nanoparticles.The dose for each mouse was 0.2 mL nanoparticles via caudal vein injection.After 24 h of injection,the mice were sacrificed,and saline infusion was used to avoid the autofluorescence interference in residual blood in organs.Tumors and other vital organs were removed,imaging of isolated organs was performed by near-infrared fluorescence,and data were analyzed via multi-spectral imaging system.
Inhibition study in vivo
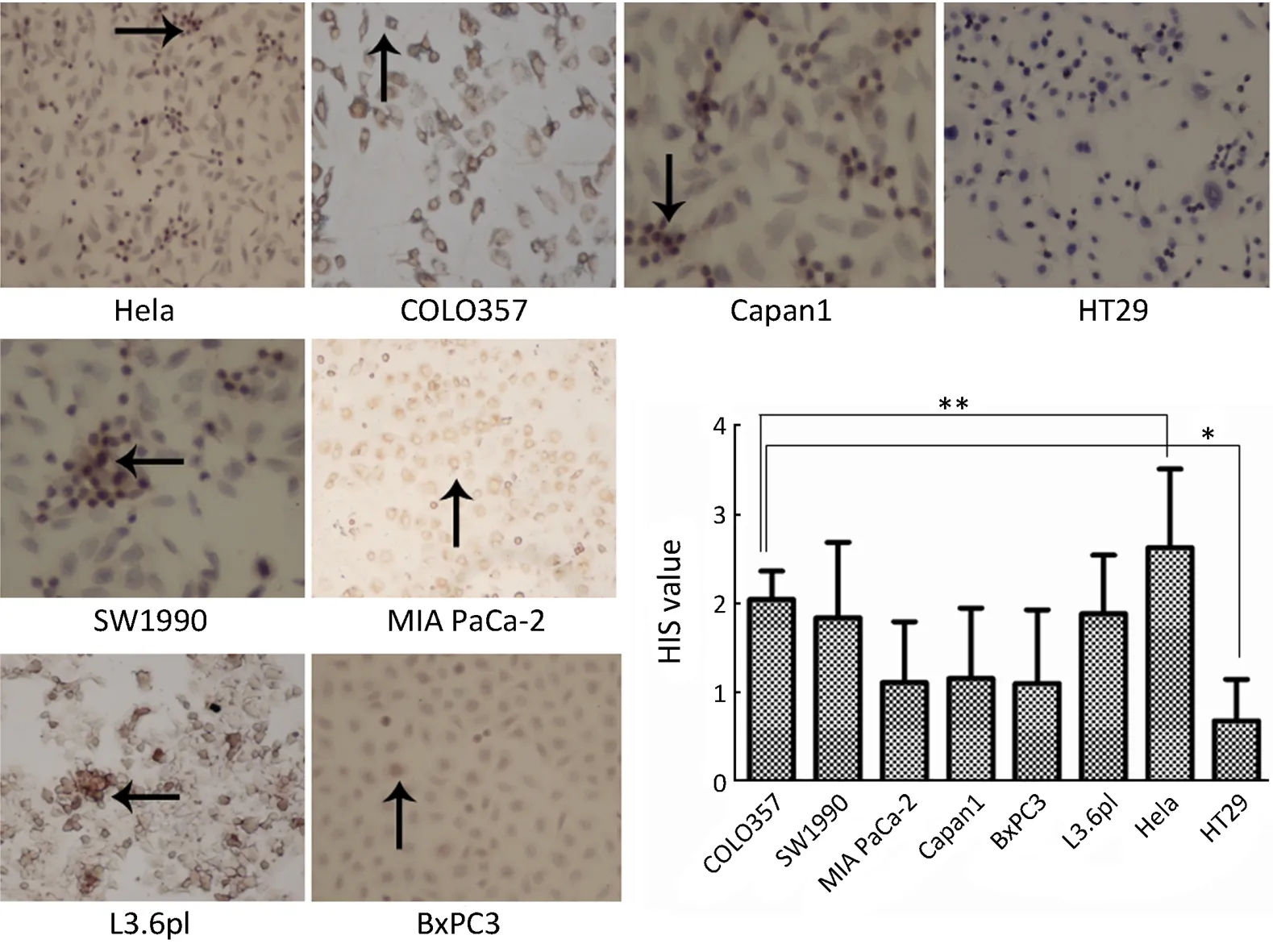
Figure 1 FR expression in Hela,HT29 and human pancreatic cancer cell lines.*,P<0.05.
Thirty-six nude mice for human pancreatic cancer orthotopic xenograft were randomly divided into 6 groups.They were administered with normal saline (NS),PEGFA-Chi,PEG-Chi,PEG-FA-Gem-Chi and PEG-Gem-Chi respectively.Gem concentration was 2.5 mg/mL and Gemloaded nanoparticles were the equivalent drug concentration of 2.5 mg/mL Gem.The nanoparticle solution of non-Gem-loaded was prepared with the same method as the Gem-loaded nanoparticles.
We started to inject the drugs at the 5th day after the orthotopic xenograft of pancreatic cancer in nude mice was performed.Three groups of them were intravenously administered with Gem-loaded nanoparticles with 0.5 mg Gem each mice once every three days and were administered for five times.The nude mice were sacrificed at the 7th day after the end of administration.
Statistical analysis
Data were expressed as,and analyzed with SPSS software (SPSS 17.0; SPSS Inc.,Chicago,IL,USA).Mean difference between groups was compared using one-way analysis of variance (ANOVA).Paired student’st-test was carried out to assess statistical significance.P<0.05 was considered statistically significant.
Results
FR expression of Hela,HT29 and human pancreatic cancer cell lines
The expression of FR in COLO357 was the highest among the six pancreatic cancer cell lines.The expression levels of FR in the six pancreatic cancer cell lines were different.FR was mainly distributed in the pancreatic cancer cell membrane,with a small part in the cytoplasm.HIS score of Hela cells as a control was 2.63±0.89 and higher than pancreatic cancer cell lines (P<0.05),whereas,HIS score of HT29 cells was only 0.68±0.47,which was lower than pancreatic cancer cell lines (P<0.05).HIS scores of COLO357,SW1990 and L3.6pl were higher than those of MIA PaCa-2,Capan1 and BxPC3(P<0.05).But there was no significant difference among COLO357,SW1990 and L3.6pl,and MIA PaCa-2,Capan1 and BxPC3,respectively (Figure 1).
C OLO357 cells uptake targeted/untargeted nanoparticles
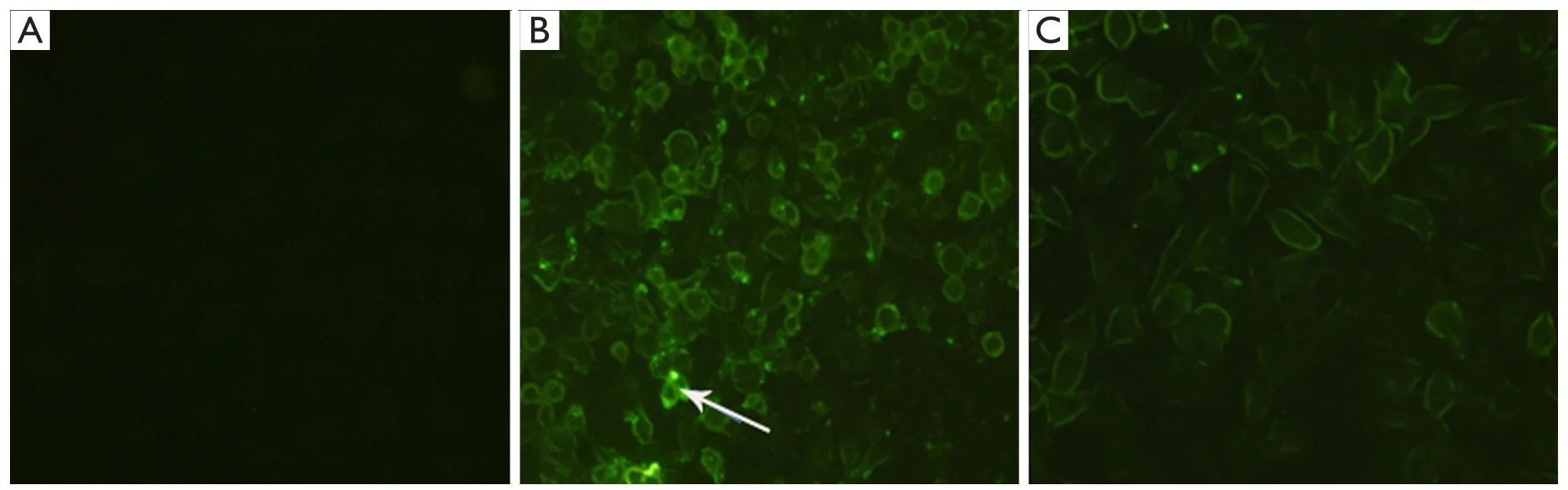
Figure 2 Fluorescent microscopy images of COLO357 cells incubating for 4 h with (A) Chi-FITC,untargeted fluorescent nanoparticles without free FA; (B) FA-Chi-FITC,targeted fluorescent nanoparticles without free FA; (C) FA-Chi-FITC,targeted fluorescent nanoparticles with free FA.
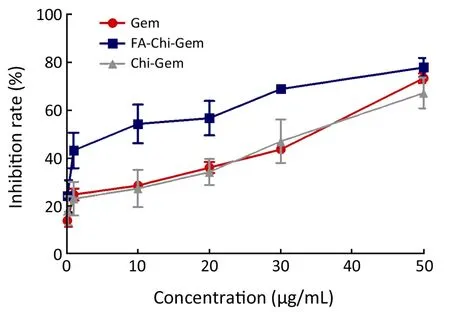
Figure 3 The growth inhibition curve of Gem-loaded nanoparticles on COLO357.
We chose COLO357 to perform the targeted uptake experimentsin vitro.After COLO357 cells were cultured with Chi-FITC nanoparticles for 4 h,the green fluorescence nanoparticles was significantly higher than that of Chi-Gem nanoparticles or Gem in COLO357 (P<0.05).The cell growth inhibition ability of FA-targeted Gem-loaded nanoparticles was significantly higher than that of Chi-Gem (Figure 3).did not significantly appear in membrane and cytoplasm of COLO357.But there was a strong green fluorescence in membrane after COLO357 was cultured with FA-Chi-FITC nanoparticles for 4 h.The green fluorescence was also found in the cytoplasm of COLO357 cells,which indicates that COLO357 can absorb FA-mediated targeting nanoparticles.Because the free FA can competitively bind with FR on cell surface,the competitive inhibition assay demonstrated that the green fluorescence of FA-Chi-FITC uptaken by COLO357 was significantly inhibited by free FA (Figure 2).
Gem-loaded nanoparticles inhibit growth of COLO357 cells
FA-Chi-Gem nanoparticles exhibited non-significant inhibitory effects on COLO357 cells.The inhibition rate of FA-Chi-Gem
Biodistribution of FA-Chi core-shell nanoparticles in vivo
The orthotopic xenograft model was confirmed by MRI and pathology analysis (Figure 4).After 24 h of administration,the mean fluorescence signals of the tumor of isolated organs in B group were twice of the A group by near-infrared fluorescence and were higher than the mean fluorescence signal of normal pancreas in the C group.Moreover the mean fluorescence signal of the liver was the highest among other organs in the A group.The mean fluorescence signal of the liver in group A was about 1.5 times of group B.Because targeted drugs were rapidly metabolized in normal mice,the mean fluorescence signal of each organ in group C was lower than that of groups A and B (Figure 5).
Gem-loaded nanoparticles inhibition effect in vivo
There were a good inhibition effect on tumors in group Gem,PEG-FA-Gem-Chi and PEG-Gem-Chi.Moreover the inhibition effect on tumor in group PEG-FA-Gem-Chi was better than that in groups Gem and PEG-Gem-Chi (P<0.05).There was no significant inhibition effect in groups NS,PEG-Chi and PEG-FA-Chi (Figure 6).
Discussion
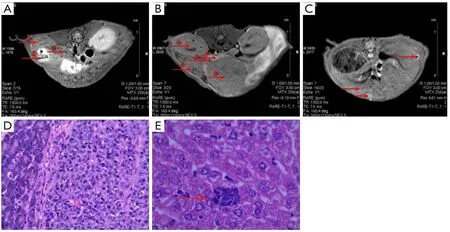
Figure 4 Orthotopic xenograft tumor of human pancreatic cancer.(A) MRI in nomal Balb/c mouse; (B) MRI in orthotopic xenograft tumor of human pancreatic cancer; (C) MRI in liver metastasis of orthotopic xenograft tumor of human pancreatic cancer; (D) Hematoxylin and eosin (HE) staining in orthotopic xenograft tumor of human pancreatic cancer; E.HE staining in liver metastasis of orthotopic xenograft tumor of human pancreatic cancer.
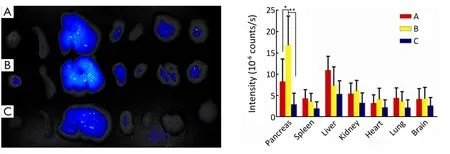
Figure 5 Biodistribution of nanoparticles in vivo.(A) PEG-ICGDER02-Chi in orthotopic xenograft tumor of human pancreatic cancer; (B)PEG-FA-ICGDER02-Chi in orthotopic xenograft tumor of human pancreatic cancer; (C) PEG-FA-ICGDER02-Chi in nomal Balb/c mouse.*,P<0.05; **,P<0.05.
FR is a membrane glycoprotein connected to glycosylated phosphatidylinositol (GPI).α-FR and β-FR are membraneassociated proteins,which are anchored to the cell membrane by GPI.α-FR expression located in luminal surface of polarized epithelial cells of normal adult tissues and basolateral membrane of retinal pigment epithelial cells.High expression of α-FR and β-FR exists in many malignant tumors.
Nikki’s study demonstrated FR expression level was positively correlated with the invasion of pancreatic cancer and cancer stage (20).The tumor tissue and adjacent normal pancreatic tissue in 76 pancreatic cancer patients were detected by Zhen (21).He found that there was positive FR expression in tumor tissue of pancreatic cancer patients while no FR expression was found in normal pancreas.Moreover,the expression sites were mainly in cell membrane,partially visible in cytoplasm.FR expression levels were correlated with lymph node metastasis in pancreatic cancer.We examined the FR expression in six human pancreatic cancer cell lines.In accordance with that report,our results also demonstrated that there were different expression levels of FR in six pancreatic cancer cell lines and FR was mainly distributed in pancreatic cancer cell membrane.
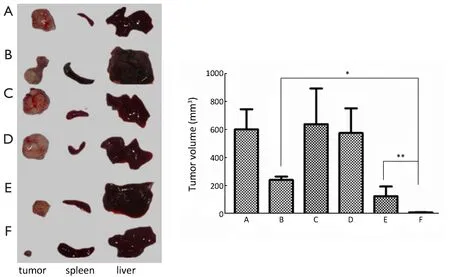
Figure 6 The inhibitory effect of different drugs on orthotopic xenograft tumor of human pancreatic cancer.(A) NS; (B) Gem; (C) PEGFA-Chi; (D) PEG-Chi; (E) PEG-Gem-Chi; (F) PEG-FA-Gem-Chi.*,P<0.01; **,P<0.01.
Because the FA targeting effect depends on a sufficient amount of FR expression on cell surfaces or different FR expression levels of the different differentiation of cancer cells in the same tumor tissues,we chose COLO357 to perform experimentsin vitroandin vivobecause of its high expression level of FR.
Currently,the studies focus on extending the half-life of Gem by directly modified Gem or microscale effect of nanoparticles to avoid the disadvantage of the short halflife and serious systemic toxic side effects of Gem.Vandana used the PEG-modified Gem (22).The contact chance of Gem with deoxycytosine deaminase was reduced due to the shielding effect of PEG,and thus Gem decomposition by deoxycytidine deaminase was reduced.It was found that IC50of Gem was 1.90 times than that of PEG-Gem for the extended residence time of PEG-Gem in the extracellular environment in pancreatic cancer.FA-Chi-Gem core-shell nanoparticles displayed the higher toxicity to pancreatic cancer cells as well as FA targeted advantages with extended half-life of Gem.The results suggested this kind of nanoparticles may be used as a high efficiency drug delivery system with the advantages of strong targeting and high toxicity to pancreatic cancer cells.
Our previous studies showed that the bareload-Gem FA-Chi nanoparticles have no significant cytotoxicity (15).IC50of FA-Chi-Gem nanoparticles to COLO357 was 13.51 μg/mL while IC50of Gem was 35.01 μg/mL and IC50of Chi-Gem nanoparticles was 33.27 μg/mL.The results suggested that the inhibition rate of FA-Chi-Gem nanoparticles was significantly higher than Chi-Gem nanoparticles or Gem single in COLO357.Moreover,the cell killing efficiency of FA-Chi-Gem nanoparticles was Gem-dose- and time-dependent in pancreatic cancer.
In the targeted group,the mean fluorescence signal of tumor tissue was strong and the mean fluorescence signal of liver was lower than that in the untargeted group.It indicated that active target can reduce the nanoparticles be swallowed by the reticuloendothelial system.The reticuloendothelial system can quickly identify the injected nanoparticles and uptake most of them as foreign substances (23).That is also the main reason in this study that fluorescence signal of liver tissue was higher than that of other organs.There was a well biodistribution of PEG-FA-ICGDER02-Chi in tumor tissue of orthotopic xenografts because of the active targeting of PEG-FA-Gem-Chi in tumor,and consequently,the inhibition effect was enhanced.It was consistent with the results of intracellular uptake studies and biodistribution ofin vivo.We observed relatively extensive lymphocytic infiltration of liver and lungs in group Gem while not in groups PEG-FA-Gem-Chi and PEG-Gem-Chi.On the other hand,the nanoparticles gathered in tumor tissue with imperfect vascular structures because nanoparticles have enhanced permeability and retention (EPR) effect.Furthermore,the accumulation of drugs in tumor was enhanced via FA modification,which further can reduce the cell killing effects in normal tissues.
FA-Gem-Chi core-shell nanoparticles had a good antitumor effectin vitroandin vivo.Firstly,the PEG-FAGem-Chi with core-shell structure was prepared by the coaxial electrospray.Through connecting FA with FR on cell membrane in pancreatic cancer,the nanoparticles were uptaken into cells via endocytosis.Secondly,after the connection with the FR of cell membrane in pancreatic cancer reaches saturation,the nanoparticles can be uptaken into intracellular endosomes via non-specific endocytosis,and endosomes release Gem.Thirdly,the active targeting uptake of FA-modified nanoparticles can reduce the reticuloendothelial system swallow.Finally,Chi polypositive particles can bind to anionic group on the cell surface,which enhanced drug-loaded nanoparticle retention hysteresis effect,and thus PEG-FA-Gem-Chi produced strong inhibitory effect.
Summarily,the development of FA-Chi-Gem core-shell nanoparticles realized truly targeted chemotherapy of FR-positive pancreatic cancer.It can enhance the therapeutic effect,minimize the side effects,and avoid damages to normal tissues,and thus can greatly improve the prognosis of pancreatic cancer patients.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No.81071967 and 30872500) and the Natural Science Foundation of Jiangsu province (Project No: BK2010242).
Disclosure:The authors declare no conflict of interest.
1.Siegel R,Naishadham D,Jemal A,et al.Cancer Statistics,2013.CA Cancer J Clin 2013;63:11-30.
2.Zhang DS,Wang DS,Wang ZQ,et al.Phase I/II study of albumin-bound nab-paclitaxel plus gemcitabine administered to Chinese patients with advanced pancreatic cancer.Cancer Chemother Pharmacol 2013;71:1065-72.
3.Xu B,Zheng WY,Jin DY,et al.Treatment of pancreatic cancer using an oncolytic virus harboring the lipocalin-2 gene.Cancer 2012;118:5217-26.
4.Reid JM,Qu W,Safgren SL,et al.Phase I trial and pharmacokinetics of Gemcitabine in children with advanced solid tumors.J Clin Oncol 2004;22:2445-51.
5.Wang YX.Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application.Quant Imaging Med Surg 2011;1:35-40.
6.Leamon CP,Reddy JA.Folate-targeted chemotherapy.Adv Drug Deliv Rev 2004;56:1127-41.
7.Zhao H,Yung LY.Selectivity of folate conjugated polymer micelles against different tumor cells.Int J Pharm 2008;349:256-68.
8.Low PS,Henne WA,Doorneweerd DD.Discovery and development of folic-acid-based receptor targeting for Imaging and therapy of cancer and inflammatory diseases.Accounts Chem Res 2008;41:120-9.
9.Low PS,Kularatne SA.Folate-targeted therapeutic and imaging agents for cancer.Curr Opin Chem Biol 2009;13:256-62.
10.Zhao HZ,Yung LY.Addition of TPGS to folateconjugated polymer micelles for selective tumor targeting.J Biomed Mater Res A 2009;91A:505-18.
11.You J,Li X,de Cui F,et al.Folate-conjugated polymer micelles for active targeting to cancer cells: preparation,in vitro evaluation of targeting ability and cytotoxicity.Nanotechnology 2008;19:045102.
12.Han X,Liu J,Liu M,et al.9-NC-loaded folate-conjugated polymer micelles as tumor targeted drug delivery system: Preparation and evaluation in vitro.Int J Pharm 2009;372:125-31.
13.Liu Y,Li K,Pan J,et al.Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of Docetaxel.Biomaterials 2010;31:330-8.
14.Benepal TS,Judson I.ZD9331: discovery to clinical development.Anti-cancer Drugs 2005;16:1-9.
15.Xu S,Xu Q,Zhou J,et al.Preparation and characterization of folate-chitosan -gemcitabine core-shell nanoparticles for potential tumor-targeted drug delivery.J Nanosci Nanotechnol 2013;13:129-38.
16.Sudimack J,Lee RJ.Targeted drug delivery via the folate receptor.Adv Drug Deliv Rev 2000;41:147-62.
17.Hilgenbrink AR,Low PS.Folate receptor-mediated drug targeting: From therapeutics to diagnostics.J Pharm Sci 2005;94:2135-64.
18.Agnihotri SA,Mallikarjuna NN,Aminabhavi TM.Recent advances on chitosan-based micro- and nanoparticles in drug delivery.J Controlled Release 2004;100:5-28.
19.Zhou J,Yu Z,Zhao S,et al.Lentivirus-based DsRed-2-transfected pancreatic cancer cells for deep in vivo imaging of metastatic disease.J Sur Res 2009;157:63-70.
20.Parker N,Turk MJ,Westrik E,et al.Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay.Anal Biochem 2005;338:284-93.
21.Zheng HM.Expression of molealar imaging marker folate receptor alpha in pancreatic cancer and its significance.J Shandong Univ (Health Sciences) 2010;48:83-5.
22.Vandana M,Sahoo SK.Long circulation and cytotoxicity of PEGylated gemcitabine and its potential for the treatment of pancreatic cancer.Biomaterials 2010;31:9340-56.
23.Bergman AM,Adema AD,Balzarini J,et al.Antiproliferative activity,mechanism of action and oral antitumor activity of CP-4126,a fatty acid derivative of Gemcitabine,in vitro and in vivo tumor models.Invest New Drugs 2011;29:456-66.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Construction of PR domain eukaryotic expression vector and its inhibitory effect on esophageal cancer cells
- Use of 18F-FDG PET/CT to locate primary malignancies in patients with hepatic cirrhosis and malignant ascites
- Synergistic inhibitory effect of wogonin and low-dose paclitaxel on gastric cancer cells and tumor xenografts
- shRNA-mediated Slc38a1 silencing inhibits migration,but not invasiveness of human pancreatic cancer cells
- Effects of salidroside on glioma formation and growth inhibition together with improvement of tumor microenvironment
- Antioxidant and anticancer activity of Artemisia princeps var.orientalis extract in HepG2 and Hep3B hepatocellular carcinoma cells
