基于铝离子掺杂二氧化钛薄膜的染料敏化太阳能电池的光电性能
2012-11-30刘秋平黄慧娟段彦栋孙庆文
刘秋平 黄慧娟 周 洋 段彦栋 孙庆文 林 原,*
(1北京交通大学机械与电子控制工程学院,北京100044;2江西理工大学软件学院,南昌330013;3中国科学院化学研究所光化学重点实验室,北京分子科学国家实验室,北京100190;4九江职业技术学院,江西九江332000)
基于铝离子掺杂二氧化钛薄膜的染料敏化太阳能电池的光电性能
刘秋平1,2,3黄慧娟4周 洋1,*段彦栋3孙庆文3林 原3,*
(1北京交通大学机械与电子控制工程学院,北京100044;2江西理工大学软件学院,南昌330013;3中国科学院化学研究所光化学重点实验室,北京分子科学国家实验室,北京100190;4九江职业技术学院,江西九江332000)
采用水热法制备出Al3+掺杂二氧化钛薄膜,通过玻璃棒涂于导电玻璃上,在450°C的温度下烧结并将其用N3染料敏化制成染料敏化太阳能电池(DSSCs).通过X射线光电子能谱(XPS)、X射线衍射(XRD)、扫描电镜(SEM)及DSSCs测试系统对其进行了测试表征,研究了Al3+掺杂对TiO2晶型及染料敏化太阳能电池的光电性能影响.XPS数据显示Al3+成功掺杂到了TiO2晶格内,由于Al3+的存在,对半导体内电子和空穴的捕获及阻止电子/空穴对的复合发挥重要作用.莫特-肖特基曲线显示掺杂Al3+后二氧化钛平带电位发生正移,并导致电子从染料注入到TiO2的驱动力提高.DSSCs系统测试结果表明,Al3+掺杂的TiO2薄膜光电效率达到6.48%,相对于无掺杂的纯二氧化钛薄膜光电效率(5.58%),其光电效率提高了16.1%,短路光电流密度从16.5 mA·cm-2提高到18.2 mA·cm-2.
二氧化钛;铝掺杂薄膜;水热法;X射线光电子能谱;光电性能;平带电位
1 Introduction
Doped TiO2nanomaterials have been investigated for more than ten years.1-6Doping a metal or nonmetal into TiO2could change the band edge or surface states of TiO2.7Until now, most of the doping for TiO2nanomaterial has been explored for photocatalysis.To the best of our knowledge,there are only a few papers reported in which doped TiO2nanomaterials were used as photoanodes in the dye-sensitized solar cells(DSSCs) (including nitrogen-doped TiO2).8,9For the metal-doped TiO2nanomatrials,Cr-doped,10Yb-doped,8Zn-doped,11and Aldoped12TiO2have been attempted to be applied as the photoanodes of DSSCs.The doping effects,however,do not seem so pronounced by comparison to the corresponding undoped TiO2photoanode.The energy conversion efficiency remained either unchanged or a little improvement.
Here,we report our investigation of introducing Al into TiO2nanocrystals and the fabrication of DSSCs with TiO2photoanodes prepared using Al-doped TiO2nanocrystals.We will study the shift of flat band potential of the TiO2photoanode by doping Al with the method of Mott-Schottky plots and further study electron transport properties by intensity-modulated photocurrent spectroscopy.Above all,we will explore the impact ofAl-doped TiO2on the conversion efficiency of DSSCs.
2 Experimental
2.1 Preparation of Al-doped TiO2and undoped TiO2
Tetrabutyl titanate,butanol,and acetic acid were used without further purification.Distilled water was used with further purification.Al(NO3)3·9H2O and methanol were obtained from Beijing Chemical Reagent Plant.All reagents used were analytical pure.
Both undoped and Al-doped TiO2were synthesized by the hydrothermal method.Tetrabutyl titanate(5 mL),butanol(30 mL),acetone(5 mL),and acetic acid(5 mL)were mixed.A mixture of butanol(20 mL)and distilled water(2 mL)was then added into the above solution.After stirring continuously for 1 h,the mixture was transferred into an autoclave for the hydrothermal process at 240°C for 6 h.After cooling to room temperature,the concentrated colloid contained 11%(molar fraction)TiO2.For the case of the Al-doped sample,Al(NO3)3· 9H2O was added into the tetrabutyl titanate(molar ratio of Al and Ti is 1:100)to start the hydrolysis reaction.The obtained sample was denoted asAl-doped TiO2.
2.2 Fabrication of DSSC based on the Al-doped TiO2and undoped TiO2
To prepare the working electrode,a TiO2or Al-doped TiO2slurry was coated onto the fluorine-doped tin oxide glass substrate(FTO,20 Ω·□-1,Hake New Energy Co.Ltd.Harbin)by the doctor blade method,and was then sintered at 450°C for 30 min.Working electrodes with a 2-μm TiO2layer or 2-μm Al-doped TiO2layer were used to test intensity-modulated photocurrent spectroscopy(IMPS)and Mott-Schottky analysis.To fabricate the DSSCs,we used a double-layer structure electrode.A 2-μm thick film of TiO2was first coated onto the FTO and then further coated by an 8-μm thick second layer of Aldoped TiO2.This double-layer structure can retard the electron recombination occurring in the double-layer region.13After cooling to 80°C,the TiO2electrode was immersed into an absolute ethanolic solution of the N3 dye(cis-di(thiocyanato)-N,N-bis(2,2-bipyridyl)-4,4-dicarboxylicacid ruthenium(III))complex for 12 h.The electrolyte was composed of 0.05 mol·L-1iodine(I2),0.5 mol·L-1lithium iodide(LiI),and 0.05 mol·L-1tert-butylpyridine dissolved in 3-methoxypropionitrile.A platinized counter electrode was clipped onto the top of the TiO2electrode to form the test cell.
2.3 Characterization and instruments
X-ray photoelectron spectroscopy data were obtained with an ESCALab220i-XL electron spectrometer(VG Scientific, USA)using 300 W Al Kαradiation.A TR200 was used to measure the film thickness.The XRD patterns of samples were recorded at 40 kV on a Rigaku D/max 2500(Japan)using Cu Kαirradiation.The morphology was examined by scanning electron microscopy(SEM,S-4800,HITACHI,Japan,15 kV).
Mott-Schottky analysis was performed in a three-electrode cell in the dark.TiO2or Al-doped TiO2single film(ca 2 μm) without dyes was used as the electrode,a saturated calomel electrode(SCE)was served as the reference electrode,and platinum wire was used as the counter electrode.The active area was 0.25 cm2.The electrolyte was prepared by dissolving 0.01 mol·L-1I2and 0.1 mol·L-1potassium iodide(KI)in ethylene carbonate and propylene carbonate(1:1 volume ratio).UV-Vis absorption data were measured on a Model U-3010 spectrophotometer(HITACHI,Japan)with an integrating sphere.TiO2or Al-doped TiO2single film(ca 2 μm)adsorbed by dyes was used for the IMPS test.IMPS was performed using a green light emitting diode(λmax=520 nm)driven by a solartron 1255B frequency-response analyzer.The light-emitting diode(LED) provided both the dc and ac components of the illumination. Photoelectrochemical measurements were recorded with a Princeton Applied Research(PAR)potentiostat(Model273)under a light intensity of 100 mW·cm-2at AM1.5 provided by a solar light simulator(Oriel,91160-1000).The active area was 0.2 cm2.
3 Results and discussion
3.1 Characterization of TiO2and Al-doped TiO2
X-ray photoelectron spectroscopy was used to investigate the chemical composition and electronic structure of the Al-doped TiO2.Fig.1a shows the typical full XPS spectrum of Al-doped TiO2.The film is mainly composed of Ti,O,and Al. XPS data indicate that the atomic fraction ratio of Al:Ti is 2.96: 22.72.The photoelectron peak of Al 2p(74.4 eV)can be observed in Fig.1b and indicates the presence of Al3+in the sample.It is clear that Al ions are implanted successfully into the TiO2.
The crystallinity of the Al-doped TiO2film was determined using X-ray diffraction analysis(40 kV on a Rigaku D/max 2500),the XRD patterns of the TiO2and Al-doped TiO2samples sintered at 450°C are shown in Fig.2.The XRD results also indicate that no second phase is detected in both undoped TiO2and Al-doped TiO2films.The structure of the TiO2film did not change much,which confirmed again that the Al3+ions must have been doped into the TiO2lattice successfully.The nanostructures of the porous films were obtained from the SEM image shown in Fig.3.The Al-doped TiO2and pure TiO2samples exhibit uniform porous morphologies.
3.2 Band structure analysis
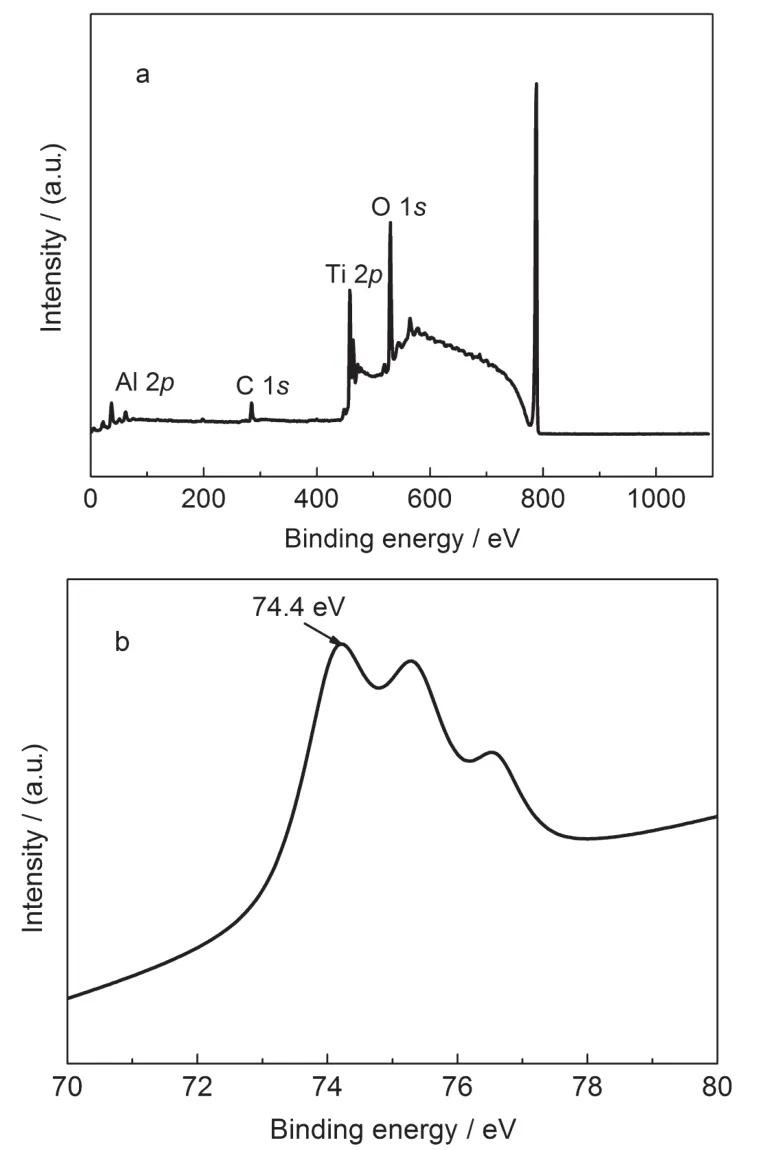
Fig.1 XPS survey spectra ofAl-doped TiO2(a)andAl 2p(b)
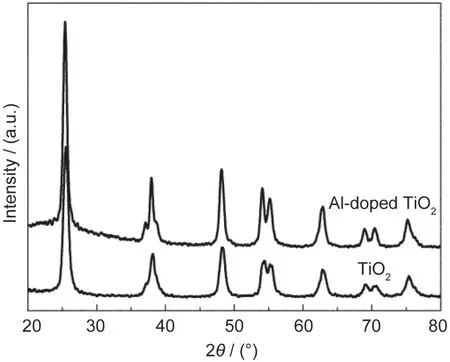
Fig.2 XRD patterns of TiO2andAl-doped TiO2samples

Mott-Schottky analysis is a common tool used to investigate the semiconductor/electrolyte interface.14,15The space charge layer capacitance(Csc)is related to the electron density(ND) and the flat band potential(Efb)of the semiconductor using the Mott-Schottky equation:16where A is the active surface,ε is the relative dielectric constant,taken approximately as 55 for anatase TiO2film,14E is the electrode potential,k is the Boltzmann constant,and T is the absolute temperature.The flat band potential and electron density of the electrode can be calculated from the intercept and slope,respectively.Fig.4 presents typical Mott-Schottky plots for the TiO2and Al-doped TiO2thin film electrodes in the high frequency ranging from 1 to 105Hz.The Efbof the pure TiO2electrode is about-0.7 V(vs SCE),whereas the Efbof the Al-doped TiO2electrode is about-0.5 V(vs SCE).

Fig.3 SEM images of TiO2(a)andAl-doped TiO2(b)samples
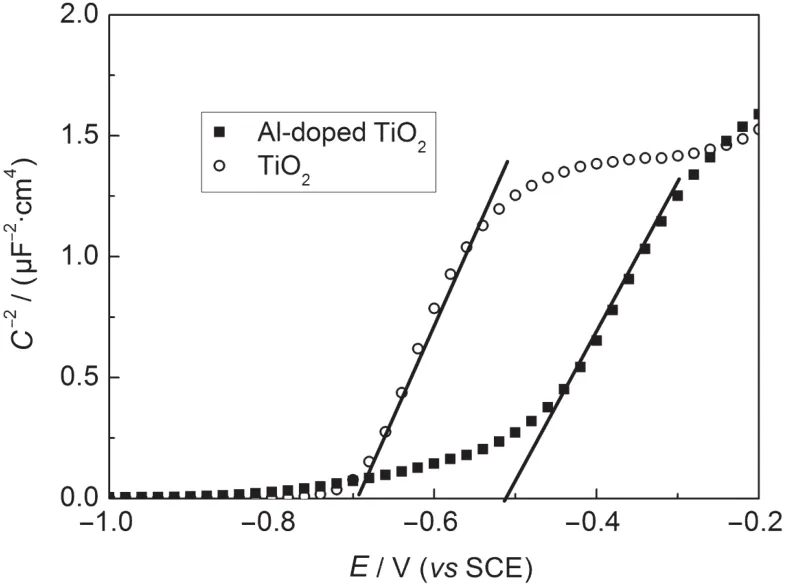
Fig.4 Mott-Schottky plots for TiO2andAl-doped TiO2
The increase of the Efbincreases the energy gap between the LUMO of dye and conduction band of TiO2,which results in an increased injection driving force of electrons and then improves the electron injection efficiency from the LUMO of the dye to the TiO2conduction band.The estimated value of the electron density NDof Al-doped TiO2is 1.79×1019cm-3,which is higher than that for TiO2(1.56×1018cm-3).The increase of NDoriginated mainly from doping with Al,indicating that less charge transfer occurred and it impeded the charge transfer more effectively than the latter.The UV-Vis absorption spectra of the films are shown in Fig.5.As reported,11the UV absorption of the films can be assigned to the absorption caused by the excitation of electrons from the band to band or band defect transitions.Comparing the variation in the optical absorption behavior of Al-doped TiO2and TiO2films without N3 dye, both the films exhibit maximum absorption peak at 360 nm. From the curve,it can be seen that the pure TiO2film shows a weak absorption in the region,and has an absorption onset at 390 nm.By doping with the Al,the as-formed films have a red-shift absorption.
3.3 Effect of the charge transport
Intensity modulated photocurrent spectroscopy is a useful method to study charge transport.Fig.6 shows a complex plane plot of the IMPS spectrum for the TiO2and Al-doped TiO2thin films.The response appears in the fourth quadrant of the complex plane and displays one semicircle,i.e.,it is a single time constant process in IMPS measurements where the frequency at the apex of the semicircle is related to the time constant of the process.
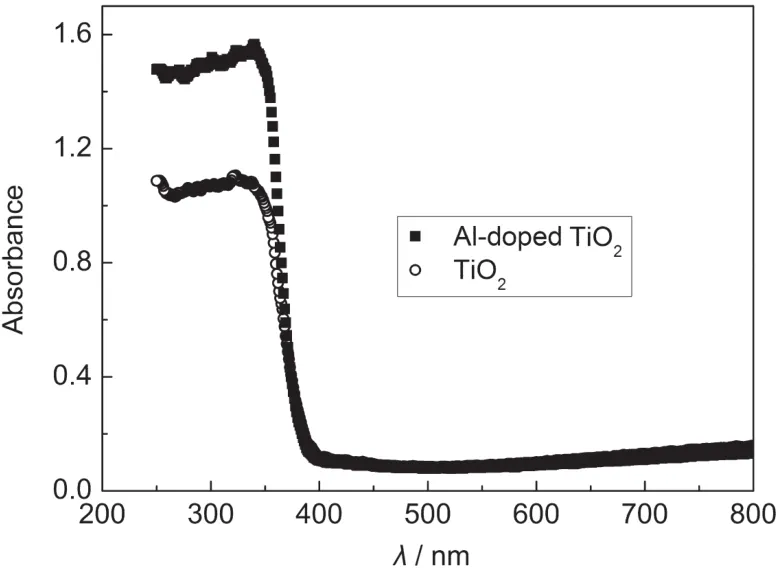
Fig.5 UV-Vis absorption spectra for the pure TiO2and the Al-doped TiO2films
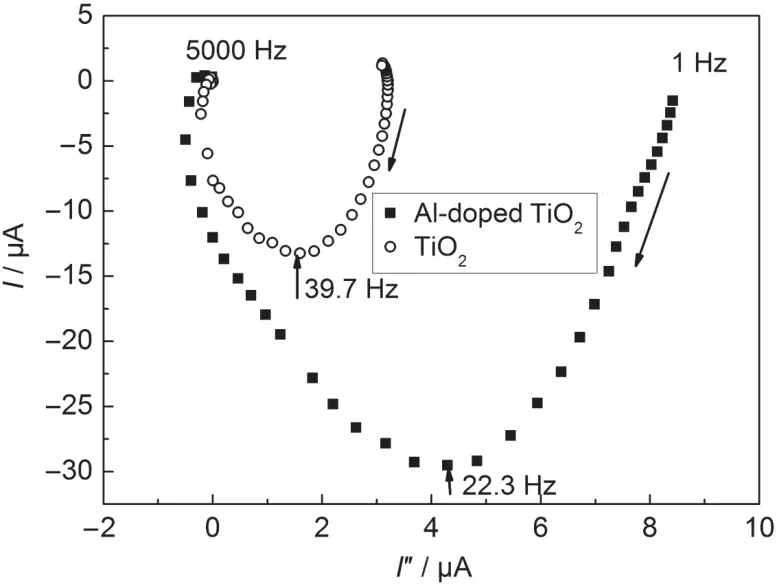
Fig.6 Complex plane plots of the TiO2and theAl-doped TiO2 cells obtained from IMPS measurements
The time constant(electron transit time)can be estimated from τD=(2πfmax)-1,where fmaxis the minimum characteristic frequency of the IMPS at the imaginary plots,which gives an estimate of the average time that photoinjected electrons need to reach the back contact.For films with comparable film thickness and dye loading,such as those investigated here,the electron transit time should enable a valid comparison of the electron transport in the films.17-19The electron transit time for the Al-doped TiO2and TiO2electrodes is 4.01 and 7.14 ms,respectively.From the measured τDvalue,one can estimate that the electron transport properties of the Al-doped TiO2thin films are different from those of the TiO2thin films.The fast electron transport in the Al-doped TiO2film is quite remarkable. Fast electron transport can improve charge-collection efficiency and thus increase photocurrent density.The reason that the Al-doped TiO2thin film has faster electron transport in the film may be related to the increased electron density.
3.4 Photovoltaic performance
The photocurrent density-voltage curves of the DSSCs based on the TiO2and Al-doped TiO2films are shown in Fig.7.The photovoltaic parameters are summarized in Table 1.Under light intensity of 100 mW·cm-2atAM1.5,the short-circuit photocurrent densities of TiO2andAl-doped TiO2films are 16.5 and 18.2 mA·cm-2,with Vocvalues of 605 and 575 mV and FF values of 0.61 and 0.59,respectively.A photovoltaic efficiency of 6.48%for Al-doped TiO2thin film is obtained,which is higher than the undoped TiO2thin film.
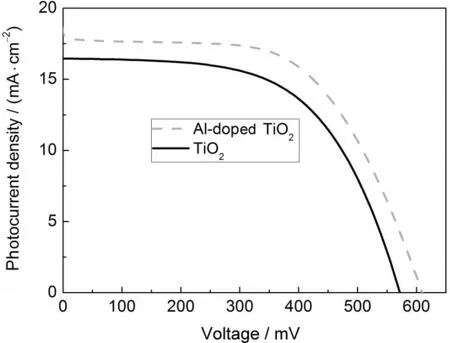
Fig.7 Photocurrent density-voltage curves of DSSCs based on TiO2andAl-doped TiO2photoanodes underAM1.5 illumination
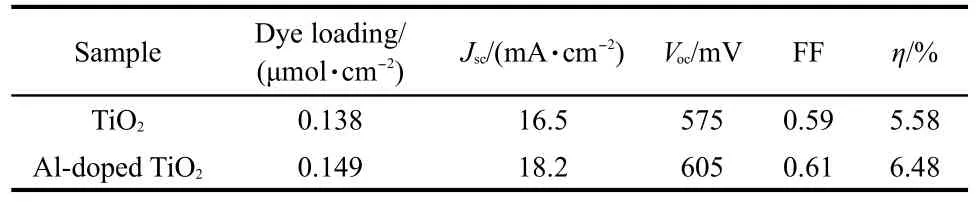
Table 1 Photovoltaic characteristics of the DSSCs based on TiO2 andAl-doped TiO2photoanodes
The dye-loading amount is similar for both of the films,indicating that the enhancement of photocurrent for Al-doped TiO2is not due to the increase of the dye adsorption.Therefore,the enhanced efficiency is mainly ascribed to the increased Jsc, which is improved by 10.3%from 16.5 to 18.2 mA·cm-2.The increased Jscis due to competitive reactions including:(1)the injection must be faster than the relaxation of the excited state of the sensitizer dye;and(2)the transport of electrons must be faster than the reaction with molecules in the solution.The more positive flat band potential of the Al-doped TiO2is the result of the effective driving force for the photoelectron,which can lead to a larger electron injection efficiency from the LUMO of dye into the conduction band of the semiconductor.The NDof Al-doped TiO2is higher than that of TiO2.The result of the IMPS gives the exact values of the electron transit time of Al-doped TiO2(4.01 ms)and TiO2(7.14 ms).Therefore,it favors charge transport in Al-doped TiO2film.Doping can create a donor level,which increases the concentration of the carriers and reduces the film resistance.20-25
4 Conclusions
Al-doped TiO2film was prepared by the hydrothermal method to construct a DSSC.The present results demonstrate the improvement in the photoelectric performance of DSSC by introducing the Al3+ions into the TiO2films.Experimental results showed that Al-doped TiO2films resulted in flat band potential to shift positively by-0.2 compared with the undoped TiO2. The supplementary charge incorporated in the doped TiO2with Al3+ions was found to increase the concentration of carriers.Both the positive shift of flat band potential and the faster charge transport produce an increase of Jscby up to 1.7 mA· cm-2for DSSC.The photovoltaic efficiency of DSSC based on Al-doped TiO2thin film reaches 6.48%,which is improved by 16.1%.This finding shows the feasibility of TiO2with Al-doping in a dye-sensitized solar cell and provides an effective way to improve the Jscfor solar cells.
(1) Choi,W.;Termin,A.;Hoffmann,M.J.Phys.Chem.1994,98, 13669.
(2)Asahi,R.;Morikawa,T.;Ohwaki,T.;Aoki,K.;Taga,Y.Science 2001,293,269.
(3) Ishii,T.;Kato,H.;Kudo,A.J.Photochem.Photobiol.A:Chem. 2004,163,181.
(4) OʹRegan,B.;Grätzel,M.Nature 1991,353,737.
(5) Hagfeldt,A.;Boschloo,G.;Sun,L.;Kloo,L.;Pettersson,H. Chem.Rev.2010,110,6595.
(6)Wang,Z.S.;Yanagida,M.;Sayama,K.;Sugihara,H.Chem. Mater.2006,18,2912.
(7) Imahori,H.;Hayashi,S.;Umeyama,T.;Eu,S.;Oguro,A.; Kang,S.;Matano,Y.;Shishido,T.;Ngamsinlapasathian,S.; Yoshikawa,S.Langmuir 2006,22,11405.
(8) Ma,T.;Akiyama,M.;Abe,E.;Imai,I.Nano Lett.2005,5,2543.
(9) Tian,H.;Hu,L.;Zhang,C.;Liu,W.;Huang,Y.;Mo,L.;Guo, L.;Sheng,J.;Dai,S.J.Phys.Chem.B 2010,114,1627.
(10)Kim,C.;Kim,K.;Kim,H.;Han,Y.J.Mater.Chem.2008,18, 5809.
(11) Xu,W.;Dai,S.;Hu,L.;Liang,L.;Wang,K.Phys.Lett.2006, 23,2288.
(12) Ko,K.H.;Lee,Y.C.;Jung,Y.J.J.Colloid Interface Sci.2005, 283,482.
(13)Wang,K.P.;Teng,H.Phys.Chem.Chem.Phys.2009,11,9489.
(14) Krol,R.;Goossens,A.;Schoonman,J.J.Electrochem.Soc. 1997,14,1723.
(15) Liu,B.S.;Zhao,X.J.Surf.Sci.2005,595,203.
(16) Randeniya,L.K.;Bendavid,A.;Martin,P.J.;Preston,E.W. J.Phys.Chem.C 2007,111,18334.
(17) Zhu,K.;Neale,N.;Miedaner,A.;Frank,J.Nano Lett.2007,7, 69.
(18) Zhang,D.;Toshida,T.;Oekermann,T.;Furuta,K.;Minoura,H. Adv.Funct.Mater.2006,16,1228.
(19) Baiju,K.;Shajush,P.;Wunderlich,W.;Mukundan,P.;Kumar, S.;Warrier,K.J.Mol.Catal.A:Chem.2007,276,41.
(20) Furubayashi,Y.;Hitosugi,T.;Yamamoto,Y.;Inaba,K.;Kinoda, G.;Hirose,Y.;Shimada,T.;Hasegawa,T.Appl.Phys.Lett. 2005,86,252101.
(21) Shi,J.F.;Xu,G.;Miao,L.;Xu,X.Q.Acta Phys.-Chim.Sin. 2011,27,1287.[史继富,徐 刚,苗 蕾,徐雪青.物理化学学报,2011,27,1287.]
(22) Li,J.;Kong,F.T.;Wu,G.H.;Zhang,C.N.;Dai,S.Y.Acta Phys.-Chim.Sin.2011,27,881. [李 洁,孔凡太,武国华,张昌能,戴松元.物理化学学报,2011,27,881.]
(23) Lu,X.;Mou,X.;Wu,J.;Zhang,D.;Zhang,L.;Huang,F.;Fu, F.;Huang,S.Adv.Funct.Mater.2010,20,509.
(24) Feng,X.;Shankar,K.;Paulose,M.;Grimes,C.Angew.Chem. Int.Edit.2009,48,8095.
(25) Henglein,A.Chem.Rev.1989,89,1861.
October 26,2011;Revised:December 13,2011;Published on Web:December 16,2011.
Photovoltaic Performance of Dye-Sensitized Solar Cells Based on Al-Doped TiO2Thin Films
LIU Qiu-Ping1,2,3HUANG Hui-Juan4ZHOU Yang1,*DUAN Yan-Dong3SUN Qing-Wen3LIN Yuan3,*
(1School of Mechanical,Electronic and Control Engineering,Beijing Jiaotong University,Beijing 100044,P.R.China;2College of Software,Jiangxi University of Science and Technology,Nanchang 330013,P.R.China;3Beijing National Laboratory for Molecular Sciences,Key Laboratory of Photochemistry,Institute of Chemistry,Chinese Academy of Sciences,Beijing 100190, P.R.China;4Jiujiang Vocational&Technical College,Jiujing 332000,Jiangxi Province,P.R.China)
Al-doped TiO2thin films were synthesized by the hydrothermal method.To prepare a working electrode,a TiO2or AlTiO2slurry was coated onto a fluorine-doped tin oxide glass substrate by the doctor blade method and the coated substrate was sintered at 450°C.TiO2and Al-doped TiO2films were characterized by X-ray photoelectron spectroscopy(XPS),X-ray diffraction(XRD),scanning electron microscopy(SEM),and tested by the dye-sensitized solar cell(DSSCs)system.The influences of Al-doping on TiO2crystal form and the photovoltaic performance of DSSCs were investigated.X-ray photoelectron spectroscopy(XPS)data indicate that the doped Al ions exist in the form of Al3+,and these ions play a role as e-or h+traps and reduce the e-/h+pair recombination rate.The corresponding Mott-Schottky plot indicates that the Al-doped TiO2photoanode shifts the flat band potential positively.The positive shift of the flat band potential improves the driving force of injected electrons from the LUMO of the dye to the conduction band of TiO2.The Al-doped TiO2thin film shows a photovoltaic efficiency of 6.48%, which is higher than that of the undoped TiO2thin film(5.58%)and the short-circuit photocurrent density increases from 16.5 to 18.2 mA·cm-2.
Titanium dioxide;Al-doped film;Hydrothermal method;X-ray photoelectron spectroscopy;Photovoltaic performance;Flat band potential
10.3866/PKU.WHXB201112161
O646
∗Corresponding authors.ZHOU Yang,Email:Yzhou@bjtu.edu.cn;Tel:+86-10-51685554.LIN Yuan,Email:liuyuan@iccas.ac.cn;
Tel:+86-10-82615031.
The project was supported by the National Key Basic Research Program of China(973)(2006CB202605),National High-Tech Research and Development Program of China(863)(2007AA05Z439),and National Natural Science Foundation of China(20973183).
国家重点基础研究发展规划项目(973)(2006CB202605),国家高技术研究发展计划项目(863)(2007AA05Z439)及国家自然科学基金项目(20973183)资助
