固相萃取/固相微萃取-气相色谱法测定饮用水中多溴联苯醚
2012-10-28何迎春王正虹周倩如
何迎春,王正虹,李 林,*,周倩如
(1.西南大学食品科学学院,重庆 400716;2.重庆市疾病预防控制中心,重庆 400042)
固相萃取/固相微萃取-气相色谱法测定饮用水中多溴联苯醚
何迎春1,王正虹2,李 林1,*,周倩如2
(1.西南大学食品科学学院,重庆 400716;2.重庆市疾病预防控制中心,重庆 400042)
目的:建立固相萃取/固相微萃取-气相色谱法测定饮用水中多溴联苯醚(BDE-47和BDE-99)含量的新方法。方法:固相萃取-气相色谱直接取水样100mL,过LC-C18柱,经正己烷洗脱,洗脱液于80℃水浴挥干,异辛烷定容至1mL,直接进样1μL测定,该法对BDE-47和BDE-99的检出限分别为0.0008μg/L和0.0009μg/L,回归方程相关系数分别为0.9996和0.9997,RSD(n=6)分别为1.0%~4.9%和0.96%~4.4%;固相微萃取-气相色谱直接取水样10mL置于15mL固相微萃取瓶中,于40℃条件下固相微萃取吸附25min后,纤维头经风干,立即进样测定。该法对BDE-47和BDE-99的检出限分别为0.0000μg/L和0.0044μg/L,回归方程相关系数分别为0.9996和0.9992,RSD (n=6)分别为6.7%~11.4%和6.0%~10.1%。结果:随机抽样某市52个水样进行检测,均未检出BDE-47和BDE-99。结论:建立饮用水中多溴联苯醚固相萃取/固相微萃取-气相色谱检测的新方法,两种方法操作简便、快速,精密度、准确度及回收率均令人满意。
固相萃取;固相微萃取;气相色谱;多溴联苯醚;饮用水
多溴联苯醚(polybrominated diphenyl ethers,PBDEs)是一种性能优良的溴代阻燃剂,目前广泛应用于电子电气产品、塑料制品和室内装饰材料中,因其高检出率、高毒性、持久性和蓄积性成为学者们的研究热点。环境中PBDEs 主要来源于生产和制造PBDEs的工厂、电子产品、工业废水、生活垃圾及医院或其他机构塑料类制品等。PBDEs研究主要集中在土壤或沉积物[1-2]、环境水源[3-4]、生物体[5-6]、人体[7-8]、血液[9]、母乳[10]、牛奶[5,9]、空气[11]等。环境水样前处理方法有固相萃取(solid phase extraction,SPE)[12]、固相微萃取(solid phase microextraction,SPME)[13-14]、分散液相微萃取法(dispersive liquid-liquid microextraction,DLLME)[12,15]、搅拌子吸附萃取法(stir bar sorptive extraction,SBSE)[16]等。PBDEs主要的检测方法有气质联用(gas chromatography-mass spectrometry,GC-MS)法[17-20],该法一般需配备NCI离子源,价格昂贵;高效液相色谱法(high performance liquid chromatography,HPLC)法[15,21]对PBDEs灵敏度不理想。目前我国还没有饮用水中多溴联苯醚的卫生标准和检测标准。本实验拟建立一种操作快速、简便,回收率和精密度较为理想的饮用水中PBDEs检测方法,既为制定国家卫生标准的建立提供参考,也便于各级监测机构对饮用水中PBDEs污染进行调查工作,预防饮用水受到污染而引发的中毒事件。
1 材料与方法
1.1 材料与试剂
多溴联苯醚标准品(BDE-47、BDE-99,50μg/mL)美国Accustandard公司;异辛烷(色谱纯) 美国Tedia公司;二氯甲烷(色谱纯) 美国Sigma公司;甲醇、乙腈、正己烷(色谱纯) 天津市四友精细化学品有限公司;环己烷(色谱纯) 天津市光复精细化工研究所;丙酮(色谱纯) 天津市科密欧化学试剂有限公司;氯化钠(优级纯) 天津光复科技发展有限公司。
1.2 仪器与设备
7890A气相色谱仪[配有微池电子捕获检测器(μECD)]美国Agilent公司;固相萃取仪、SupelcleanTMLC-C18柱(6mL,500g) 固相萃取柱、PDMS(厚度100μm) 美国Supelco公司;数字型磁力加热搅拌装置(PC-420D) 美国Corning公司;HWS28型电热恒温水浴锅 上海一恒科学仪器有限公司。
1.3 方法
1.3.1 样品处理
随机抽取某市自来水样品17个、二次供水样品17个、市售矿泉水样品14个、桶装水样品2个、管材浸泡水样品2个共52个样品,其中自来水和二次供水采样于某市区部分宾馆、酒店、饭店、血站;矿泉水购自某市区超市;桶装水为实验室自制三级水;管材浸泡水为水样于实验室利用PPR管浸泡24h后测定。
1.3.2 色谱条件
SPE-GC色谱条件:色谱柱:J&W HP-5毛细管柱(30m×0.32mm,0.25μm);升温程序:150℃保持1min,以40℃/min升至280℃,保持3min;进样口温度:280℃;载气(N2)流速:2.5mL/min;进样量:1μL;不分流进样;检测器温度:290℃;尾吹气流量(N2):30mL/min。
SPME-GC色谱条件:解吸温度:270℃;手动进样;其他同SPE-GC色谱条件。
1.3.3 样品前处理
SPE-GC前处理:水样直接取100mL,过LC-C18柱,正己烷洗脱,洗脱液于80℃水浴挥干,异辛烷定容至1mL,直接进样1μL测定;SPME-GC前处理:水样直接取10mL置于15mL固相微萃取瓶中,于40℃条件下SPME吸附25min后,纤维头经风干,立即进样测定。
1.3.4 标准曲线(工作曲线)的绘制
SPE-GC:分别准确取BDE-47和BDE-99 50μg/mL标准溶液适量配制成0.0、1.0、10.0、50.0、100.0、500.0、1000.0μg/L,每个质量浓度平行测定3次,以质量浓度为X轴,峰面积均值为Y轴,绘制标准曲线;SPME-GC:分别准确取BDE-47和BDE-99 50μg/mL标准溶液适量配制成0.0、10.0、20.0、50.0、100.0、500.0、1000.0ng/L,每个质量浓度平行测定3次,以质量浓度为X轴,峰面积平均值为Y轴,绘制工作曲线。
2 结果与分析
2.1 SPE样品前处理条件优化
2.1.1 洗脱剂种类与体积选择
PBDEs极性介于非极性到中等极性之间,根据相似相容原理,选择从非极性到极性7种不同洗脱剂,即环己烷、正己烷、二氯甲烷-正己烷、二氯甲烷、丙酮、乙腈、甲醇,该条件下采用二氯甲烷2mL+甲醇-水(1:1,V/V)5mL活化;流速约20滴/min进行加标回收率实验,结果表明正己烷作为洗脱剂洗脱效率高于其他6种洗脱剂。
从1~5mL检验洗脱剂的洗脱效果,结果显示2mL洗脱剂就能将吸附于SPE柱填料上的目标物洗脱。
2.1.2 活化剂种类与体积选择
以正己烷为洗脱剂,流速约20滴/min,对二氯甲烷2mL+甲醇-水(1:1)5mL、甲醇7mL、二氯甲烷2mL+甲醇-水(2:1) 5mL、二氯甲烷2mL+甲醇-水(3:2) 5mL、甲醇-水(2:1)7mL、甲醇-水(3:2)7mL、二氯甲烷7mL、二氯甲烷2mL+甲醇5mL 8种活化剂配比进行选择,结果表明:二氯甲烷+甲醇回收率效果优于其他7种活化剂,因此选择二氯甲烷(2mL)+甲醇(5mL)作为活化剂。
活化剂一般用量在10mL以下,过多将降低回收率。本实验分别以二氯甲烷1mL+甲醇3mL、二氯甲烷2mL+甲醇5mL和二氯甲烷3mL+甲醇7mL 3种混合比例对SPE柱活化。结果显示,二氯甲烷2mL+甲醇5mL活化效果最好。
2.1.3 SPE柱流速优化
流速直接影响待测物洗脱效率,流速过快目标物与SPE填充材料没有充分作用,洗脱效果较差;流速过慢,导致作用时间过长,无法洗脱待测物,所以必须选择合适的流速来保证最佳洗脱效果。采用Supelco固相萃取仪来进行SPE实验,由于流速不能全自动控制,只能手动控制流速。实验表明,约30滴/min流速对BDE-47和BDE-99洗脱效果最佳。
2.2 SPME前处理条件优化
2.2.1 SPME解吸温度及时间优化
固相微萃取解吸温度直接影响纤维头吸附的目标物解吸是否彻底,温度过低解吸不完全,过高可能超过纤维头的最高温度,导致纤维萃取头使用寿命大大缩短。选择250~280℃,根据峰面积与解吸温度的相关性来选择解吸温度,SPME其他条件:萃取温度常温(21℃)、萃取时间20min、转速1150r/min、解吸时间3min。从250~270℃BDE-47和BDE-99灵敏度显著升高,270~275℃ BDE-47和BDE-99灵敏度没有显著变化,至280℃达最高值。另外PDMS萃取头最高承受温度为280℃,如果纤维头温度过高,将显著缩短萃取头使用寿命,所以应该选择低于280℃的解吸温度。因此选择270℃作为解吸温度。
PBDEs解吸一般在前3min完成。本研究选择1~5min范围,根据BDE-47和BDE-99在此范围的峰面积来选择最短最适合的解吸时间,其他条件:解吸温度270℃、萃取温度常温(21℃)、萃取时间20min、转速1150r/min。在3min左右目标物解吸已经比较完全,在此后4~5min峰面积没有显著增加,因此本实验选择3min解吸。
2.2.2 SPME萃取温度及时间优化
固相微萃取与温度之间存在明显正相关,一般随萃取温度升高,待测物峰面积都有升高。本实验选择常温(21℃)、30、40、50℃检验BDE-47和BDE-99峰面积随萃取温度变化情况。SPME其他条件:解吸温度270℃、解吸时间3min、萃取时间20min、转速1150r/min;随温度的升高,BDE-47和BDE-99灵敏度显著升高,在40~50℃达到较高的响应值,介于在50℃时没有明显高于40℃时BDE-47和BDE-99的峰面积,因此选择萃取温度为40℃。
SPME是一个动态萃取过程,随纤维萃取头毛细管浸入试样中,试样中目标待测物不断萃取到纤维头中。选择0~35min来考察纤维头的萃取效果,其他条件:解吸温度270℃、解吸时间3min、萃取温度40℃、转速1150r/min、在0~25min范围内BDE-47和BDE-99峰面积显著升高,随时间进一步增加至30、35min它们各自峰面积没有明显变化。综合考虑,选择萃取时间为25min。
2.2.3 SPME转速优化
通常搅拌子转速对待测物峰面积有极为显著的影响,选择转速0~1150r/min来优化最佳转速,其他条件:解吸温度270℃、解吸时间3min、萃取温度40℃、萃取时间25min。在转速500r/min时BDE-47和BDE-99灵敏度十分低,随转速的增加它们各自的峰面积灵敏度明显提升,至1150r/min达到最大值。由于选择的磁力搅拌器最大转速为1150r/min,并有研究证明,转速高于1500r/min重复性不理想,故本实验转速选择1150r/min。
2.2.4 SPME离子强度(盐)影响实验
固相微萃取通常在加入盐的情况下有利于待测物的萃取。有研究显示,NaCl添加量在4%以下对萃取效率没有影响[13]。本研究选择0~30% NaCl加入量来考察PBDEs萃取效率(峰面积变化)是否受离子强度的影响。随离子强度剂(NaCl)加入量的增加,BDE-47和BDE-99峰面积明显降低,即NaCl的加入会大大降低BDE-47和BDE-99的响应,具体机理还待进一步研究。本实验过程中选择不加盐(NaCl)。
2.2.5 助溶剂甲醇加入量对萃取效率的影响

表1 多溴联苯醚的线性范围、回归方程、相关系数、检出限和RSD值Table 1 Linear range, regression equation, correlation coefficient, limit of detection (LOD) and relative standard deviation (RSD) for BDE-47 and BDE-99
有研究显示甲醇的加入会使萃取效率明显提高,本实验选择从0~30%分别加入不同量甲醇,根据BDE-47和BDE-99峰面积与甲醇加入量的关系来确定甲醇加入量。随甲醇量加入的增加,BDE-47和BDE-99峰面积明显降低,与已有研究结果不符,所以本实验选择不加助溶剂。
2.3 标准品的色谱测定
采用SPE-GC法、SPME-GC法分离测定BDE-47和BDE-99,标准样品色谱见图1。
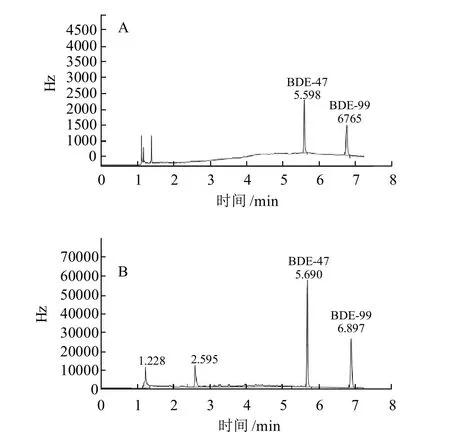
图1 BDE-47和BDE-99标准SPE-GC(A)和SPME-GC色谱图(B)Fig.1 SPE-GC and SPME-GC chromatograms of mixed BDE-47 and BDE-99 standards
2.4 标准曲线(工作曲线)、回归方程、相关系数、检出限、RSD
在最佳条件下,对加标样品进行测定,绘制标准曲线结果见表1。
SPE-GC法测定结果表明:BDE-47和BDE-99在0~1000.0μg/L质量浓度范围内呈良好的线性关系,线性相关系数分别为0.9996、0.9997;检出限(水样取100mL)分别为0.0008、0.0009μg/L(RSN=6);RSD(n=6)分别为1.0%~4.9%、0.96%~4.4%。
SPME-GC法测定结果表明: BDE-47和BDE-99在0~1.0μg/L质量浓度范围内呈良好的线性关系,线性相关系数分别为0.9996、0.9992;检出限(水样取10mL)分别为0.0008、0.0044μg/L (RSN=3);RSD(n=6)分别为6.7%~11.4%、6.0%~10.1%。
2.5 加标回收实验
由表2可见,对矿泉水样进行加标0.02、0.1、0.5μg/L的SPE-GC法加标实验,各测定6次,该法BDE-47和BDE-99回收率分别为91.5%~103.0%、90.7%~102.0%。对矿泉水样进行加标1、10、50μg/L的SPMEGC法加标实验,各测定6次,该法BDE-47和BDE-99回收率分别为81.1%~91.8%、82.0%~94.8%。
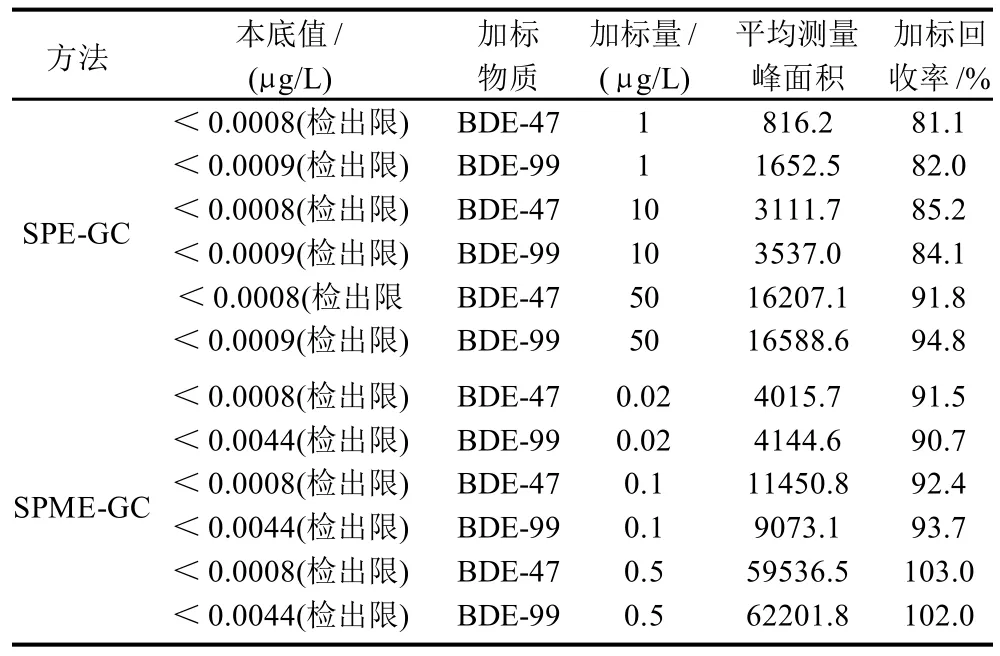
表2 回收率实验Table 2 Results of recovery tests
2.6 实样测定
随机抽取52个水样按照SPE前处理和SPME前处理方法进行处理后,分别采用SPE-GC和SPME-GC检测BDE-47和BDE-99含量,两种方法均没有检出BDE-47和BDE-99。
3 结 论
3.1 本实验采用SPE/SPME-GC-μECD测定饮用水中BDE-47和BDE-99含量,两种方法均具有良好线性、回收率和精密度,干扰实验结果显示不存在干扰。
3.2 采样嘉陵江上、中、下游源水,利用两种方法测定其中PBDEs含量,干扰实验结果显示不存在其他杂质干扰,且均未检出BDE-47和BDE-99,表明嘉陵江水源目前没有受到PBDEs污染。加标回收率结果显示良好回收率,因此两种方法均可用于源水中BDE-47和BDE-99的测定。
3.3 实验发现玻璃容器对PBDEs存在显著吸附作用。
[1] WANG Jing, MA Yunjuan, CHEN Shejun, et al. Brominated flame retardants in house dust from e-waste recycling and urban areas in South China: implications on human exposure[J]. Environment International, 2010, 36(6): 535-541.
[2] YUSA V, PARDO O, PASTOR A, et al. Optimization of a microwaveassisted extraction large-volume injection and gas chromatography-ion trap mass spectrometry procedure for the determination of polybrominated diphenyl ethers, polybrominated biphenyls and polychlorinated naphthalenes in sediments[J]. Analytica Chimica Acta, 2006, 557(1/2): 304-313.
[3] FONTANA A R, MARIA F, SILVA M F, et al. Determination of polybrominated diphenyl ethers in water and soil samples by cloud point extraction-ultrasound-assisted back-extraction-gas chromatography-mass spectrometry[J]. Journal of Chromatography A, 2009, 1216(20): 4339-4346.
[4] FONTANALS N, BARRI T, BERGSTROM S, et al. Determination of polybrominated diphenyl ethers at trace levels in environmental waters using hollow-fiber microporous membrane liquid-liquid extraction and gas chromatography-mass spectrometry[J]. Journal of Chromatography A, 2006, 1133(1/2): 41-48.
[5] GOMARA B, GARCIARUIZ C, GONZALEZ M J, et al. Fractionation of chlorinated and brominated persistent organic pollutants in several food samples by pyrenyl-silica liquid chromatography prior to GC-MS determination[J]. Analytica Chimica Acta, 2006, 565(2): 208-213.
[6] FUJITA H, HONDA K, HAMADA N, et al. Validation of high-throughput measurement system with microwave-assisted extraction, fully automated sample preparation device, and gas chromatography-electron capture detector for determination of polychlorinated biphenyls in whale blubber[J]. Chemosphere, 2009, 74(8): 1069-1078.
[7] GOMARA B, HERRERO L, GONZALEZ M J. Feasibility of electron impact and electron capture negative ionization mass spectrometry for the trace determination of tri- todeca-brominated diphenyl ethers in human samples[J]. Analytica Chimica Acta, 2007, 597(1): 121-128.
[8] COVACI A, VOORSPOELS S. Optimization of the determination of polybrominated diphenyl ethers in human serum using solid-phase extraction and gaschromatography-electron capture negative ionization mass spectrometry[J]. Journal of Chromatography B, 2005, 827(2): 216-223.
[9] TAKASUGA T, SENTHILKUMAR K, TAKEMORI H, et al. Impact of fermented brown rice with Aspergillus oryzae (FEBRA) intake and concentrations of polybrominated diphenylethers (PBDEs) in blood of humans from Japan[J]. Chemosphere, 2004, 57(8): 795-811.
[10] BORDAJANDI L, ABAD E, JOSEGONZALEZ M. Occurrence of PCBs, PCDD/Fs, PBDEs and DDTs in Spanish breast milk: enantiomeric fraction of chiral PCBs[J]. Chemosphere, 2008, 70(4): 567-575.
[11] SJTKLIN A, CARLSSON H, THURESSON K, et a1. Flame retardants in indoor air at an elec tronics recycling plant and at other work environments [J]. Environmental Science and Technology, 2001, 35(3): 448-454.
[12] LIU Xiujuan, LI Jianwang , ZHAO Zhixu, et al. Solid-phase extraction combined with dispersive liquid-liquid microextraction for the determination for polybrominated diphenyl ethers in different environmental matrices[J]. Journal of Chromatography A, 2009, 1216(12): 2220-2226.
[13] WANG Junxia, JIANG Dongqing, GU Zhiyuan, et al. Multiwalled carbon nanotubes coated fibers for solid-phase microextraction of polybrominated diphenyl ethers in waterand milk samples before gas chromatography withelectron-capture detection[J]. Journal of Chromatography A, 2006, 1137(1): 8-14.
[14] MONTES R, RODNIGUES I, RUBI E, et al. Suitability of polydimethylsiloxane rods for the headspace sorptive extraction of polybrominated diphenyl ethers from water samples[J] Journal of Chromatography A, 2007, 1143(1/2): 41-47.
[15] LI Yanyan, WEI Guohui, HU Jia, et al. Dispersive liquid-liquid microextraction followed by reversed phase-high performance liquid chromatography for the determination of polybrominated diphenyl ethers at trace levels in landfill leachate and environmental water samples[J]. Analytica Chimica Acta, 2008, 615(1): 96-103.
[16] LLORCAPORCEL J, MARTINEZSANCHEZ G, LVAREZ B, et al. Analysis of nine polybrominated diphenyl ethers in water samples by means of stir bar sorptive extraction-thermal desorption-gas chromatography-mass spectrometry[J]. Chim Acta, 2006, 569(1/2): 113-118.
[17] LCPEZ P, BRANDSMA S A, LEONARDS P E G, et al. Methods for the determination of phenolic brominated flame retardants, and byproducts, formulation intermediates and decomposition products of brominated flame retardants in water[J]. Journal of Chromatography A, 2009, 1216(3): 334-345.
[18] TOLLBACK P, BJORKLUND J, OSTMAN C. Large-volume programmed-temperature vaporiser injection for fast gas chromatography with electron capture and mass spectrometric detection of polybrominated diphenyl ethers[J]. Journal of Chromatography A, 2003, 991(2): 241-253.
[19] KORYTAR P, COVACI A, LEONARDS P E, et al. Comprehensive two-dimensional gas chromatography of polybrominated diphenyl ethers [J]. Journal of Chromatography A, 2005, 1100(2): 200-207.
[20] SANCHEZ A J, BONET J, VELASCO G, et al. Determination and occurrence of phthalates, alkylphenols, bisphenol A, PBDEs, PCBs and PAHs in an industrial sewage grid discharging to a municipal wastewater treatment plant[J]. Science of the Total Environment, 2009, 407(13): 4157-4167.
[21] FRANSCICO V, AMPARO R G, SIGBRITT K. Microwave-assisted extraction for qualitative and quantitative determination of brominated flame retardants in styrenic plastic fractions from waste electricaland electronic equipment (WEEE)[J]. Talanta, 2009, 78(1): 33-39.
Determination of Polybrominated Diphenyl Ethers in Drinking Water by Solid Phase Extraction or Solid-phase Micro-extraction Combined with Gas Chromatography
HE Ying-chun1,WANG Zheng-hong2,LI Lin1,*,ZHOU Qian-ru2
(1. College of Food Science, Southwest University, Chongqing 400716, China;
2. Chongqing Center for Disease Prevention and Control, Chongqing 400042, China)
Objective: To establish a novel method for determining the contents of polybrominated diphenyl ethers (BDE-47 and BDE-99) in drinking water by solid phase extraction (SPE) or solid phase micro extraction (SPME) combined with gas chromatography (GC). Methods: In the SPE-GC method, 100 mL of water sample was purified by LC-C18 SPE column chromatography through elution with hexane. The eluent was evaporated to dryness in water bath at 80℃, and the remaining residue was re-dissolved with isooctane and made up to 1 mL. Finally, 1μL of the solution was injected into GC for the determination. The limits of detection (LOD) for BDE-47 and BDE-99 were 0.0008μg/L and 0.0009μg/L (RSN= 6), respectively, the regression equations showed good linearity with a correlation coefficient of 0.9996 and 0.9997, respectively, and the RSDs for 6 replicate determinations were in the range of 1.0%-4.9% and 0.96%-4.4%, respectively. In the SPME-GC method, 10 mL of water sample was placed into a 15-mL SPME bottle and adsorbed by solid-phase micro-extraction for 25 min at 40 ℃ with a rotation speed of 1150 r/min. Then, the fiber was air-dried, immediately followed by GC analysis. The LODs of the SPME-GC method for BDE-47 and BDE-99 were 0.0000μg/L and 0.0044μg/L (RSN = 6), respectively, the linear correlation coefficients were 0.9996 and 09992, respectively, and the RSDs for 6 replicate determinations varied in the range of 6.7%-11.4% and 6.0%-10.1%, respectively. Results: BDE-47 and BDE-99 were undetected in 52 samples randomly collected from a certain city. Conclusion: Both analytical methods are characterized by simplicity, rapidity, high precision, good accuracy and satisfactory recovery rate.
solid phase extraction;solid phase micro-extraction;gas chromatography;polybrominated diphenyl ethers;drinking water
R282.2
A
1002-6630(2012)08-0236-05
2011-04-24
何迎春(1985—),男,硕士,研究方向为食品安全与质量控制。E-mail:jl2641@126.com
*通信作者:李林(1957—),男,研究员,本科,研究方向为食品安全与质量。E-mail:lilinlqc@163.com
