Molecular Dynamic Simulation for HMX/NTO Supramolecular Explosive
2012-07-25LINHe林鹤ZHUShunguan朱顺官ZHANGLin张琳PENGXinhua彭新华LIHongzhen李洪珍CHENYang陈阳
LIN He(林鹤),ZHU Shun-guan(朱顺官),ZHANG Lin(张琳),PENG Xin-hua(彭新华),LI Hong-zhen(李洪珍),CHEN Yang(陈阳)
(1.School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing 210094,Jiangsu,China;2.Institute of Chemical Materials,Chinese Academy of Engineering Physics,Mianyang 621900,Sichuan,China)
Introduction
Explosives are widely used for both civilian and military purposes,but their development is slow.As is well known,the power of explosive is closely related with its crystal density.To increase the crystal density,numerous work has been focused on the synthesis of new explosives.Considering crystal density,sensitivity,stability and cost,few of new explosives can be used practically.In order to improve the properties of the existing explosives, the methods of crystallization[1],coating[2]and PBX[3]are resorted,but they are not effective ways to modify the crystal structure of explosives.
Supramolecular chemistry based on intermolecular interaction is used popularly in the food and pharmaceutical industries.Its host-guest interactions,such as hydrogen and halogen bonds,are the major driving force to form the supramolecules.But only a few papers reported the supramolecular explosive[4-7].However it is the most promising approach to improve the performances of the existing explosives,as it can augment their crystal density[5].Thus,detonation velocities of existing energetic materials will be increased by supramolecuar explosives with desired geometric or topological structure.Besides,their sensitivity will be decreased to a great extent.Compared with the synthesis of new explosives or other methods to improve the performances of existing explosives,the supramolecular explosive is easier,safer and more suitable for largescale production.Usually,it is difficult to obtain the X-ray single crystal diffraction data of supramolecular explosive.Some unconvinced methods,such as X-ray powder diffraction(XRPD),infrared spectroscopy(IR)and differential scanning calorimetry(DSC),are employed to prove the formation of supramolecule.Therefore,some theoretical investigations are necessary to further reveal the supramolecular explosives.
Though HMX,as shown in Fig.1(a),is a powerful explosive,it is not widely used in the propellant and explosive applications for its high mechanical sensitivity.NTO in Fig.1(b)is a potential explosive with high performance and low sensitivity.Large amount of hydrogen atoms or oxygen atoms exist in HMX and NTO.Therefore,the host-guestinteractions,such as hydrogen bonds and electronic effects,between HMX and NTO may exist.The formation of HMX/NTO supramolecular explosive is the most effective method to improve the performances of HMX.Based on the crystal engineering rules[8],a supramolecular explosive composed of HMX and NTO in a 1∶1 molar ratio is designed in this paper.The molecular dynamics simulation for the HMX/NTO supramolecular explosive is performed.In the simulation,the radial distribution function,binding energy,geometry structure and X-ray powder diffraction(XRD)are investigated,to prove the feasibility of the formation of HMX/NTO supramolecular explosive and provide a theoretical basis.
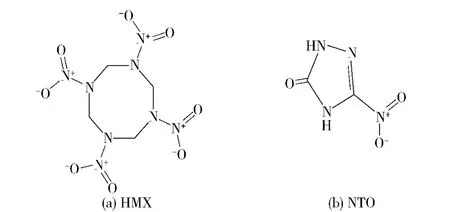
Fig.1 Molecular structure
1 Calculation Methods
1.1 Computational Model
The initial crystal lattices and atomic positions ofβ-HMX can be determined according to its neutron diffraction[9],belong to the space group P21/C with crystal latticea=6.54,b=11.05,c=8.70andβ=124.3°.Based on the Donnay-Harker prediction,the growth rate of the crystal surface is proportional to its attachment energy;therefore,the main crystal morphology can be deduced by AE model.The AE model is used to predict the main stable faces of HMX with different Miller indices(h,k,l).There are two molecules in HMX unit cell.A supercell of 3×3×3 unit cells consisting of 54 molecules can be built.In order to investigate the HMX/NTO supramolecular explosive deeply,six models of HMX/NTO supramolecular explosive are constructed.One of them is half of HMX molecules in the supercell substituted by 27 NTO molecules randomly.In the other five models,the HMX supercell is cleaved,along the main stable surfaces(0 1 1) ,(1 0 0),(1 1),(0 2 0)or(1 0).Then,a vacuum layer with thickness of 30 Å alongccrystallographic axis is added to obtain periodic structure in MD simulation and 27 HMX molecules are substituted by NTO for all five cleaved HMX supercells,respectively.Thus,six models of HMX/NTO supramolecular explosive in 1∶1 molar ratio are established.
1.2 MD Simulation
The molecular dynamic simulation for HMX/NTO supramolecular explosive can be performed by discover module in materials studio[10].Compass force field[11],an initio force field widely used in condensed-phase materials molecular simulation can be applied.After the energy minimizations for each model,the MD simulations can be carried out in the NVT ensemble at 298 K,with Andersen method and random velocities from Boltzman distribution to control the system temperature and initial velocities.In MD simulation,the Coulomb and Van Der Waals interactions are calculated by Ewald[12]and atom-based method[13], respectively.The simulation time is 100 ps with time step of 1fs.The frame output is in every 20 steps.XRD patterns of HMX/NTO supramolecular explosive can be simulated by Reflex code[14]with CoKαirradiation,in scanning range from 5°to 75°and step size of 0.02°,based on the final structure of`HMX/NTO supramolecular explosive.
2 Results and Discussions
2.1 Criteria of System Equilibrium
Two criteria can be applied to judge the system equilibrium state.They are the temperature and energy fluctuations around the averages.Fig.2 shows the fluctuations of temperature and energy in HMX supercell model substituted by NTO randomly in MD simulation.It indicates that the fluctuations of temperature and energy are no more than 10%,which means that the system reaches equilibrium state.The same phenomenon can be found in other five models.
2.2 Crystal Habit of HMX
After the energy minimization of HMX,the main growth surfaces of HMX can be predicted by using AE model in Morphology module,with Compass force field and its charge.In the simulation,the energy can be calculated by Forcite module.Van Der Waals force and eletrostatic actions can be calculated by Atombased and Ewald method.
It can be seen from Tab.1 that there are five main stable surfaces existing in HMX,and the total areas of(0 1 1)and(1 1)surfaces are about 90.99%.The attached energy is mainly composed of Van Der Waals force and eletrostatic actions,and the attached energy Eatt(0 1 1) >Eatt(0 2 0) >Eatt(1 1) >Eatt(1 0) > Eatt(1 0 0).
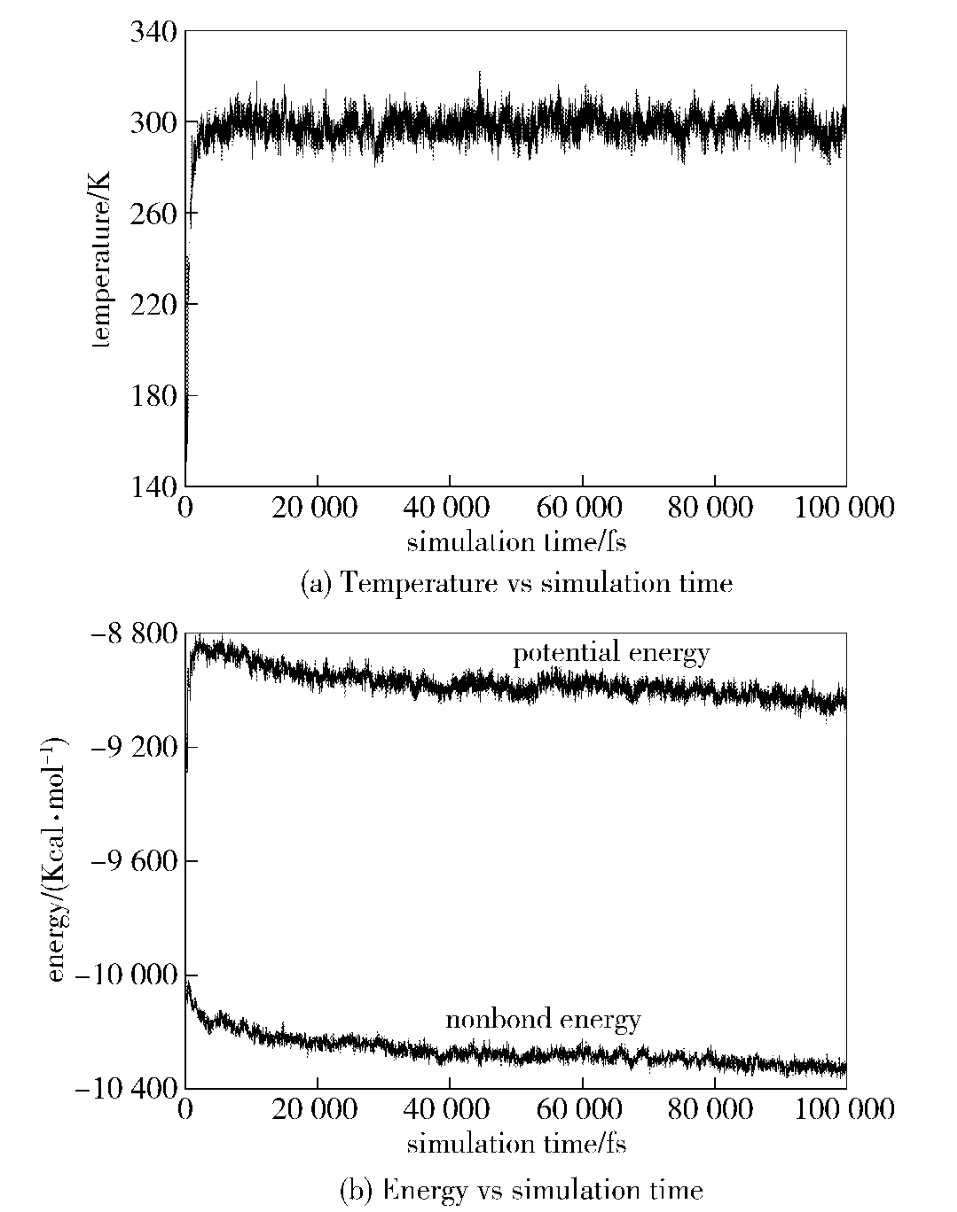
Fig.2 Temperature and energy in HMX supercell model substituted by NTO randomly
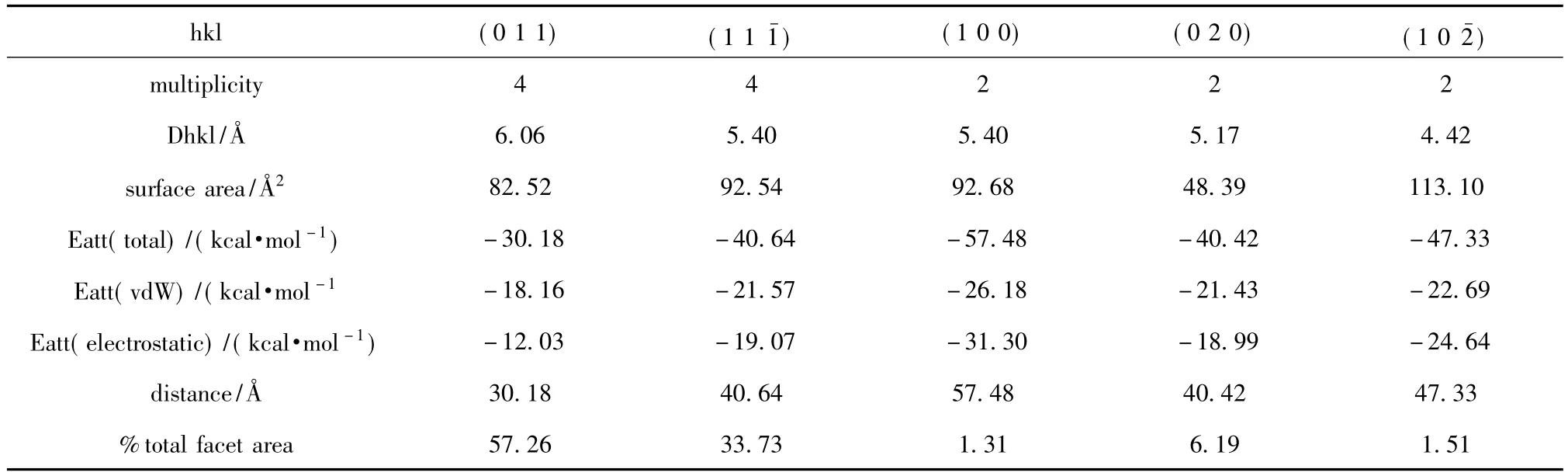
Tab.1 Main stable surfaces of HMX
2.3 Binding Energies
The host-guest interactions exist between HMX and NTO molecules.Their strength can be well described by the binding energy,which is defined as the negative value of the host-guest interactionsEinter,that is

whereEHMX/NTOis the total energy of the HMX/NTO su-pramolecular system,EHMXandENTOare the total energies of HMX and NTO,respectively.
As shown in Tab.2,the strong binding energies exist in six models,and it means that these large subject-object interactions facilitate HMX to combine with NTO.The binding energyEbinding(1 1) >Ebinding(1 0 0)>Ebinding(0 2 0)>Ebinding(random)>Ebinding(1 0 2-) >Ebinding(0 1 1).The more binding energy is,the more easily the supermolecules form.Compared with other five models,the supercell model of HMX cleaved along(1 1)surface is easier to form.
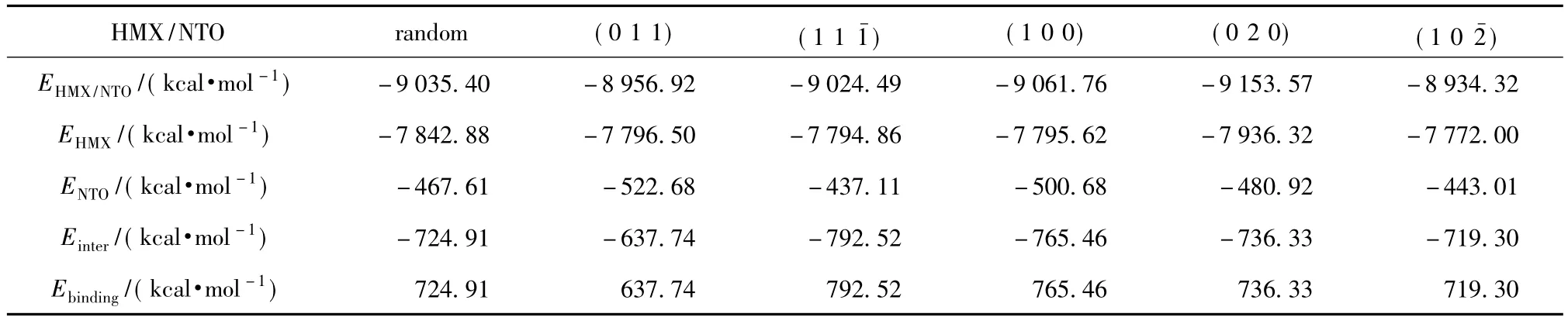
Tab.2 Binding energies between HMX and NTO
2.4 Radial Distribution Function
The radial distribution function(RDF)g(r)is commonly used to describe the degree of atom disorder in the molecule,that is to say,RDF stands for the probability of finding a pair of atoms at a given distancerin a random distribution.Usually,the host-guest interactions range of hydrogen bond and Van Der Waals force are 2.6 - 3.1and 3.1 - 5.0or above 5.0 Å,respectively.The radial distribution functions of six models are shown in Fig.3.AandBare the supercell of HMX substituted by NTO randomly and along(0 1 1)surface.CandDare the supercell of HMX substituted by NTO along(1 1)and(1 0 0)sur-face;EandFrepresent the supercell of HMX substituted by NTO along the stable surfaces(0 2 0)and(1 0),respectively.

Fig.3 Radial distribution function of six models of HMX/NTO supramolecular explosive
To analysis the host-guest interactions clearly,O and H atoms in HMX and NTO can be named as O1,H1,O2and H2,respectively.Obviously,there is a sharp peak inr=2.6,and it means that the strong hydrogen bonds exist between O2and H1;no obvious peaks can be observed in the range of 2.6 -3.1 Å between O1and H2,and it indicates that no hydrogen bonds exist between O1and H2.In the interval of 3.1- 10,several strong and wide peaks can be observed in O1-H2or O2-H1for six models.This phenomenon implies that the strong Van Der Waal forces also exist.In view of hydrogen bonds and Van Der Waals forces,HMX and NTO are assembled mainly via the host-guest interactions of O2-H1.The supercell model of HMX cleaved along(1 1)and(1 0)surfaces are more stable.
2.5 Geometry Structure
The distributions of C-H bond,H-C-H bond angle and the middle dihedral angle in HMX are simulated,as shown in Fig.4.The bond length of C-H bond ranges from 0.975 Å to 1.275 Å.The bond angle of H-CH and the middle dihedral angle fluctuate seriously.It can be seen that HMX geometry structure is distorted in MD simulation.The main reason for this phenomenon is that the host-guest interactions exist in HMX/NTO supramolecular explosive.
2.6 X-ray Powder Diffraction
After MD simulation,the XRD patterns of HMX/NTO supramolecular explosives are calculated.Intermolecular and intramolecular hydrogen bonds are the main driving forces to formation of HMX/NTO supramolecular explosive.Fig.5 shows the XRD patterns of pureβ-HMX(A),pureα-NTO(B)and six models(C to H).It can be seen that the XRD patterns of six models are quite different from pureβ-HMX andα-NTO;they are not the simple combination of the XRD patterns of pureβ-HMX andα-NTO.A new characteristic peak appears in six models when the 2-θranges from 5°to 10°.The characteristic peaks ofβ-HMX andα-NTO disappear in the range of 20°-30°for six models.Any phase has a specificdhkl-I.They all indicate that a new morphology is generated.The structures ofβ-HMX and NTO are damaged by the host-guest inter-actions,and a new supramolecular structure is generated.
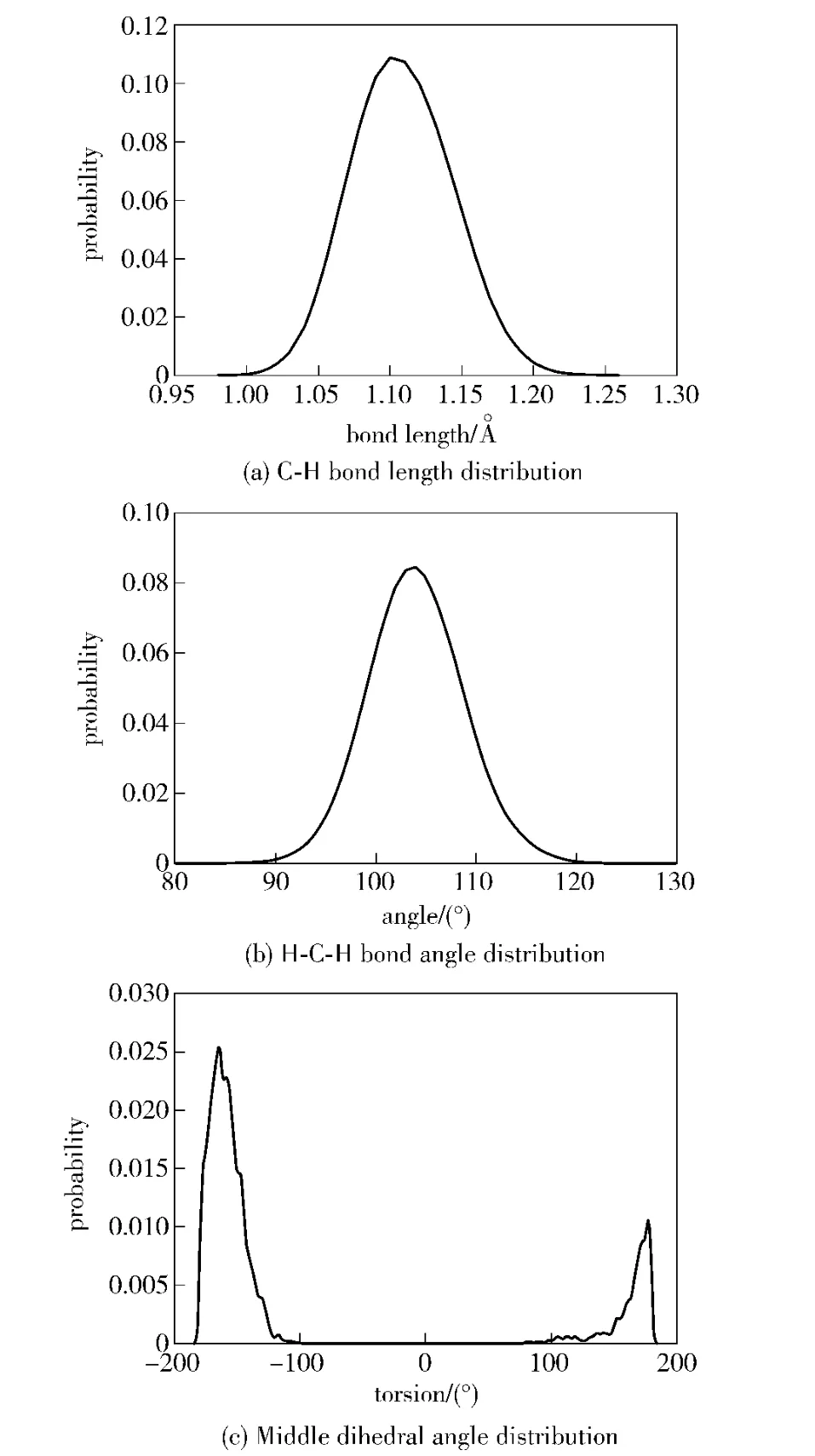
Fig.4 Bond length,bond angle and dihedral angle distribution of HMX

Fig.5 X-ray powder diffraction patterns of HMX/NTO supramolecular explosive
3 Conclusions
In this paper,the formation of HMX/NTO supramolecular explosive is investigated by means of molecular dynamics for six models.The binding energies,RDF,XRD and geometry structure of HMX/NTO supramolecular explosive are studied.The major results can be summarized as follows.
1)The binding energies of HMX/NTO supramolecular explosive are obtained,andEbinding(1 1) >Ebinding(1 0 0)>Ebinding(0 2 0)>Ebinding(random)>Ebinding(1 0 2-) >Ebinding(0 1 1).
2)The radial distribution functiong(r)shows that the strong hydrogen bonds and Van Der Waals forces exist between HMX and NTO.The hydrogen bonds between oxygen atoms in NTO and hydrogen atoms in HMX are the main host-guestinteractions.The supercell models of HMX cleaved along(1 1)and(1 0)surfaces are more stable.
3)The geometry structure of HMX is seriously distorted.The XRD patterns of six models are quite different from pureβ-HMX orα-NTO,indicating a new phase generated.
4)The model of HMX supercell substituted by NTO along(1 1)surface of HMX is easier to form.
[1]Kroeber H,Teipel U.Crystallization of insensitive HMX[J].Propellants Explosives Pyrotechnics,2008,33(1):33-36.
[2]Kim K J,Kim H S.Coating of energetic materials using crystallization[J].Chemical Engineering & Technology,2005,28(8):946-951.
[3]Salazar M R,Kress J D,Lightfoot J M,et al.Experimental study of the oxidative degradation of PBX 9501 and its components[J].Propellants Explosives Pyrotechnics,2008,33(3):182 -202.
[4]Bolton O,Matzger A J.Improved stability and smart-material functionality realized in an energetic cocrystal,angewandte chemie-international edition[J].2011,50(38):8960-8963.
[5]Landenberger K B,Matzger A J.Cocrystal engineering of a prototype energetic material supramolecular chemistry of 2,4,6-Trinitrotoluene[J].Crystal Growth & Design,2011,10(12):5341-5347.
[6]MA P,ZHANG L,ZHU S G,et al.Synthesis,crystal structure and DFT calculation of an energetic perchlorate amine salt[J].Journal of Crystal Growth,2011,335(1):70-74.
[7]SHEN J P,DUAN X H,LUO Q P,et al.Preparation and characterization of a novel cocrystal explosive[J].Crystal Growth& Design,2011,11(5):1759-1765.
[8]Etter M C.Hydrogen bonds as design elements in organic chemistry[J].The Journal of Physical Chemistry,1991,95(12):4601-4610.
[9]Choi C S,Boutin H P.A study of the crystal structure ofcyclotetramethylene tetranitramine by neutron diffraction[J].Acta Crystallographica Section B:Structural Crystallography and Crystal Chemistry,1970,26B:1235 -1240.
[10]Materials Studio 4.0.San Diego,CA:Accelrys Inc.,2006.
[11]Sun H.Compass:an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds[J].The Journal of Physical Chemistry B,1998,102(38):7338-7364.
[12]Ewald P P.Evaluation of optical and electrostatic lattice potentials[J].Annals of Physics,1921,64:253 -287
[13]Tosi M P.Cohesion of ionic solids in the Born model[J].Solid State Physics,1964,16:1-120.
[14]Engel G,Wilke S,Konig O,et al.Powder solve a complete package for crystal structure solution from powder diffraction patterns[J].Journal of Applied Crystallography,1999,32:1169-1179.
猜你喜欢
杂志排行
Defence Technology的其它文章
- Fault Diagnosis Method Based on Fractal Theory and Its Application in Wind Power Systems
- Numerical Simulation of Particle/Matrix Interface Failure in Composite Propellant
- Torque Ripple Suppression Control Strategy for Brushless Integrated Starter/Generator Wound-Field Synchronous Motor
- Monitoring Method for the Electrical Properties of Piezoelectric Transducer
- Research on Microcrack Extension Mechanism of SiCp/Al in the Machining Process
- A Study on Criteria for Barrel Lifetime
